- 1Department of Respiratory, Children’s Hospital of Chongqing Medical University, National Clinical Research Center for Child Health and Disorders, Ministry of Education Key Laboratory of Child Development and Disorders, Chongqing, China
- 2Children's Hospital of Chongqing Medical University, Chongqing Key Laboratory of Pediatrics, Chongqing, China
- 3Department of Respiratory Medicine, Shanghai Children’s Medical Center, Shanghai Jiaotong University School of Medicine, Shanghai, China
- 4Department of Respiratory and Critical Care Medicine, West China Hospital of Sichuan University, Sichuan, China
Objectives: Prediction of the efficacy of continuous positive airway pressure (CPAP) on bronchiolitis is necessary for timely treatment. This study aims to establish a nomogram for efficacy of CPAP on bronchiolitis, and compares accuracy with Pediatric Risk of Mortality III (PRISM III), Brighton Pediatric Early Warning Score (Brighton PEWS) and Pediatric Critical Illness Score (PCIS).
Methods: From February 2014 to December 2020, data on children diagnosed with bronchiolitis and treated with CPAP in Chongqing was collected. The nomogram was evaluated by using multivariate logistic regression analysis. We compared the predictive value of model with PRISM III, PEWS and PCIS.
Results: A total of 510 children were included. The nomogram prediction model including fever, APTT, white blood cells, serum potassium concentration, lactic acid, immunodeficiency, atelectasis, lung consolidation, congenital airway dysplasia and congenital heart disease was established. The AUC of the nomogram was 0.919 in the training set and 0.947 in the validating set. The model fitted well, as evidenced by the calibration curve and Hosmer-Lemeshow goodness-of-fit test. We discovered that the nomogram significantly performed better than PRISM III, PCIS and PEWS.
Conclusions: A nomogram including ten factors for predicting the efficacy of CPAP on bronchiolitis was established. It had higher performance than the PRISM III, PCIS, and PEWS in terms of clinical benefits.
Introduction
Bronchiolitis is a respiratory disease specific to children under 2 years old, which peaks in the fall and winter (1). It is a major cause of illness and hospitalization in infants and children. Hospital costs in the US exceed $734 million (2).
Studies have demonstrated that continuous positive airway pressure (CPAP) and high-flow nasal cannula (HFNC) can enhance physiologic and clinical outcomes associated with respiratory distress and failure due to bronchiolitis (3–5). However, mechanical ventilation is more appropriate for infants who have worsening severe distress despite non-invasive ventilation. Several studies suggest that CPAP improves ventilation and avoids endotracheal intubation (6, 7). Therefore, predicting the efficacy of CPAP on bronchiolitis will help improve physiologic and clinical outcomes. To our knowledge, bronchiolitis can cause symptoms such as fever, cough and wheezing. A domestic study found that P/F after 2 h of CPAP treatment, atelectasis and cardiac insufficiency can predict CPAP treatment failure in children with bronchiolitis (8). In general, Combining clinical manifestations, laboratory indicators and complications can be used to predict the efficacy of CPAP on bronchiolitis. Besides, the Pediatric Risk of Mortality III (PRISM III), Brighton Pediatric Early Warning Score (Brighton PEWS), and Pediatric Critical Illness Score (PCIS) are used to assess the condition of critically ill children. There is a lack of application of a scoring system in studies of CPAP efficacy prediction for bronchiolitis.
We aim to develop a predictive tool for CPAP treatment failure in children with bronchiolitis. We also compare the predictive value of model with PRISM III, PEWS and PCIS.
Materials and methods
Data source
According to the inclusion and exclusion criteria, a case-control study was conducted from February 2014 to December 2020 at the Respiratory Department, Children's Hospital of Chongqing Medical University. It was used to develop the prediction model. The validation data set comprised children with bronchiolitis between January 2021 and June 2022 in the Children's Hospital of Chongqing Medical University. All participants' guardians have signed the “Basic Informed Consent of Inpatients”. Our study complies with medical ethics standards.
Study population
Inclusion criteria: (1) children who were clinically diagnosed with bronchiolitis younger than 2 years old, (2) Grading of severity: moderate to severe (9), (3) Nasal prongs CPAP therapy was supplied after admission.
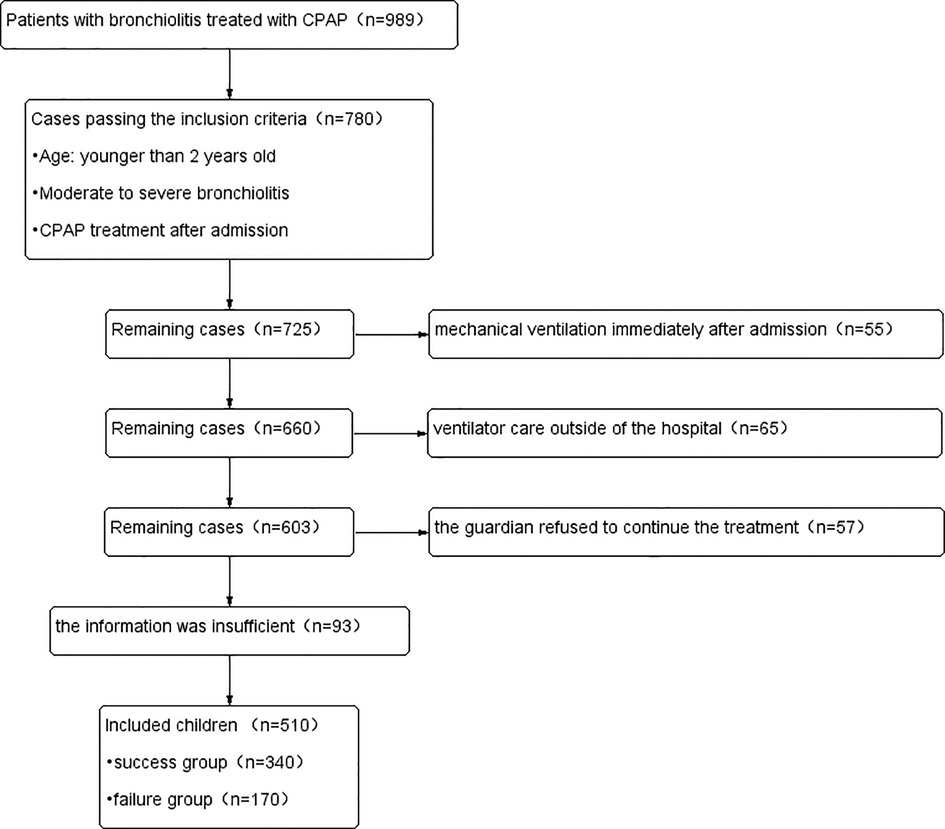
Exclusion criteria: (1) mechanical ventilation immediately after admission, (2) ventilator care outside of the hospital, (3) the information was insufficient, (4) the guardian refused to continue the treatment.
Children who were weaned within 48 h after CPAP treatment were included in the success group, while children who needed to take endotracheal intubation within 48 h after CPAP treatment were included in the failure group. The positive continuous pressure of CPAP almost ranged from 2 to 6 cmH20. The fraction of oxygen in the gas flowing in the system was subsequently adjusted to maintain a SpO2 of 95% or more. In the first 48 h after admission, we would give drugs such as budesonide and bronchodilators for symptomatic treatment. In addition, the drug treatment plan would be adjusted according to the examination results of patients.
Data extraction
Two people independently collected the clinical symptoms, laboratory values and radiological data of hospitalized children. Within 24 h of admission, two individuals independently documented PRISM III, PCIS, and PEWS-related variables. If some indicators occurred more than once within 24 h, the most recent data before CPAP use was chosen. ALL data used for prediction were extracted before commencement of CPAP.
Definitions
The PRISM III includes 17 physiological parameters such as heart rate, temperature, white blood cells (WBC) and so on (10). The higher the score, the more severe the disease. The PCIS includes 11 items including respiration, PaO2 and PH. The lower the score, the more severe the disease (11). The Brighton PEWS includes three aspects: consciousness, cardiovascular and respiration (12). The severity of the disease is correlated with the score. Malnutrition was defined as weight loss and subcutaneous fat loss. Liver damage was defined as the elevation of enzymology or bilirubin. Myocardial damage was diagnosed through clinical symptoms, signs and laboratory tests. Usually, children with myocardial damage will show shortness of breath, long sighs, and pale complexion. Laboratory tests showed that the serum and myocardial enzymes were elevated in the acute phase. Renal insufficiency was defined as the decline of renal function with or without oliguria or anuria.
Statistical analysis
The sample size was determined using the PASS15.0 application. Normally distributed continuous data were compared by independent samples t-test and were expressed as mean ± standard deviation (M ± SD). Nonnormally distributed continuous data were compared using the Mann-Whitney U test and expressed as median (upper and lower quartiles) [P50(P25-P75)]. Categorical data were analyzed by the χ2 test or Fisher's exact test and were presented as numbers (n) and percentages (%). The factors influencing the efficacy of CPAP on children with bronchiolitis were investigated using logistic regression analysis. When p-value < 0.05, the difference was statistically significant. The prediction value of three scoring systems was assessed using the receiver operating characteristic (ROC) curve.
The picked predictors were presented with a nomogram in a training set and then validated in another data set. To verify the accuracy of nomogram, the area under the curve (AUC) of ROC was employed. The calibration curve and the Hosmer-Lemeshow test were used to assess the predictive performance of the nomogram. All data analyses were performed using SPSS statistics V.25.0 and R V.3.6.1 software.
Results
Clinical characteristics of included children
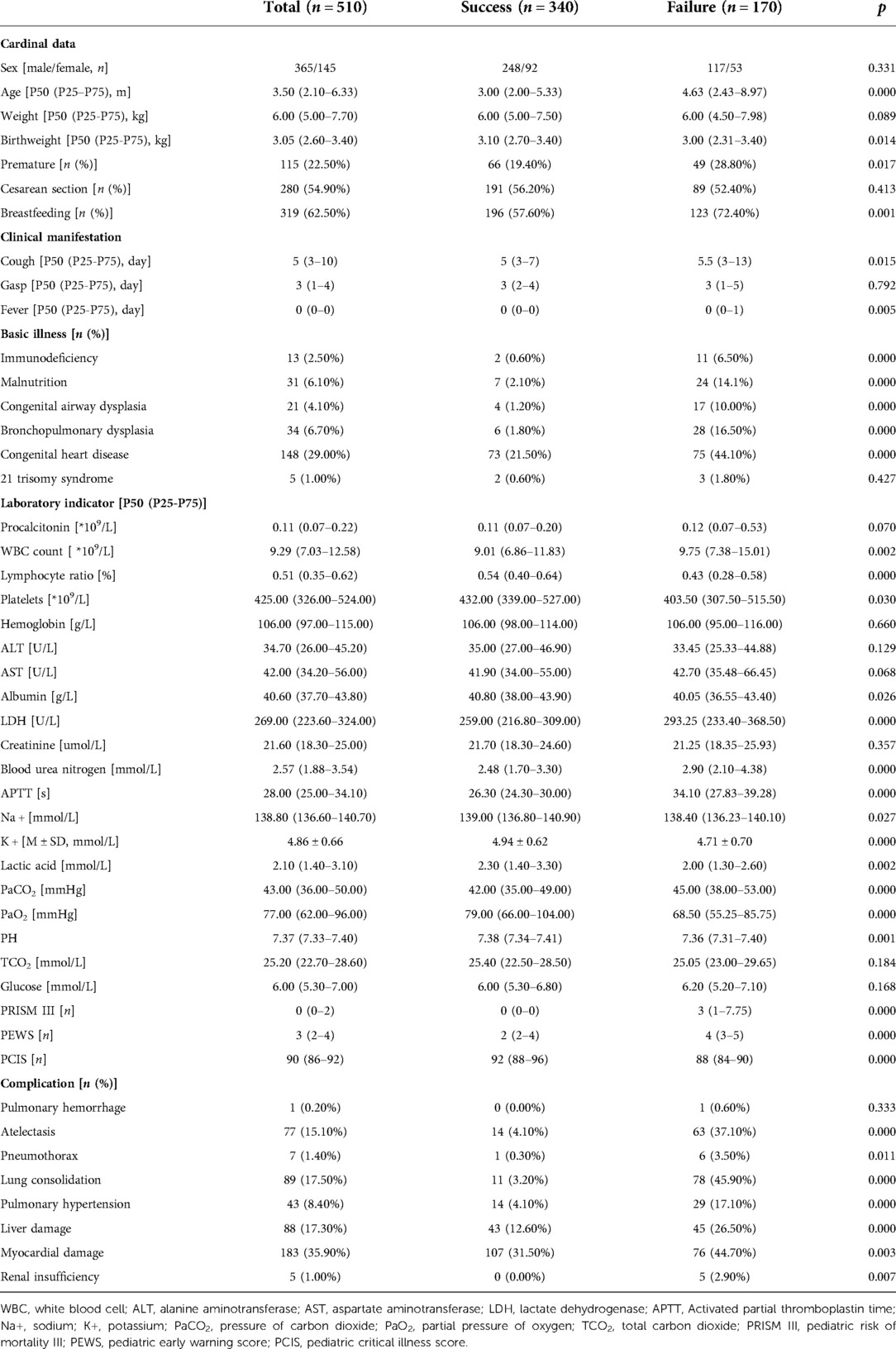
510 children with bronchiolitis were eventually included in this study based on the inclusion and exclusion criteria, as well as the results of the sample size calculated by the PASS 15.0 software (Figure 1). The success group had 340 cases and the failure group had 170 cases in the training set. 250 children were divided into the validating set. The success group had 170 cases and the failure group had 80 cases in the validating set. Children in failure group were older than those in success group (p = 0.000). Birthweight, cough, fever, L, Plt, ALB, Na+, K+, lactic acid (Lac), PaO2, PH, PCIS were significantly lower, while procalcitonin, WBC, LDH, blood urea nitrogen, APTT, PaCO2, PRISM III, PEWS were significantly higher in the failure group than the success group. Premature, breastfeeding, myocardial damage were lower in failure group. Immunodeficiency, malnutrition, congenital airway dysplasia (CAD), bronchopulmonary dysplasia, congenital heart disease, atelectasis, pneumothorax, lung consolidation, pulmonary hypertension, liver damage, renal insufficiency were different in two groups. Demographic, clinical characteristics, laboratory findings and complications of the patients are summarized in Table 1.
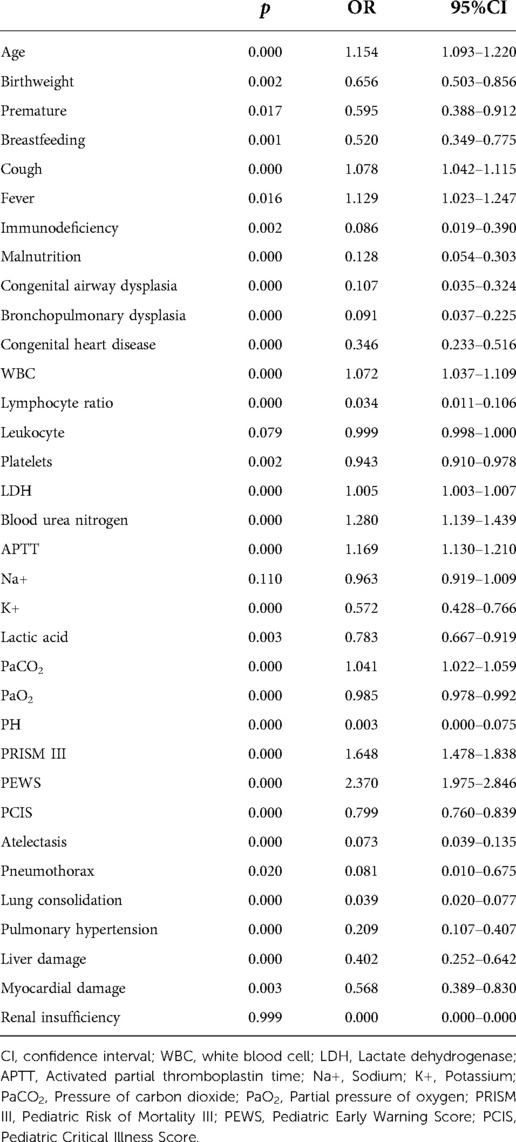
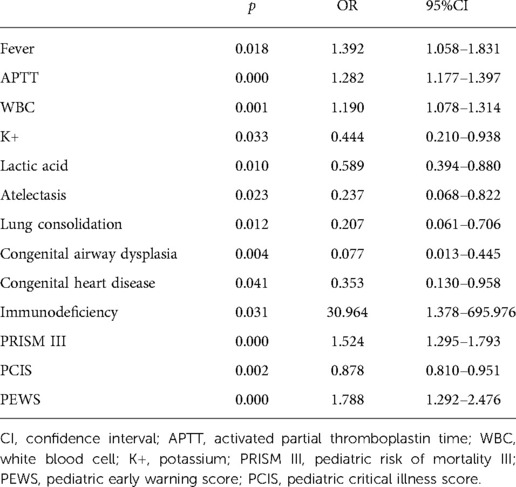
In univariate and multivariate logistic regression analysis, fever, APTT, WBC, serum potassium concentration, Lac, immunodeficiency, atelectasis, lung consolidation, CAD, congenital heart disease (CHD), PRISM III, PCIS, and PEWS were the influencing factors of the efficacy of CPAP on bronchiolitis (Tables 2, 3).
Developing a nomogram in the training set
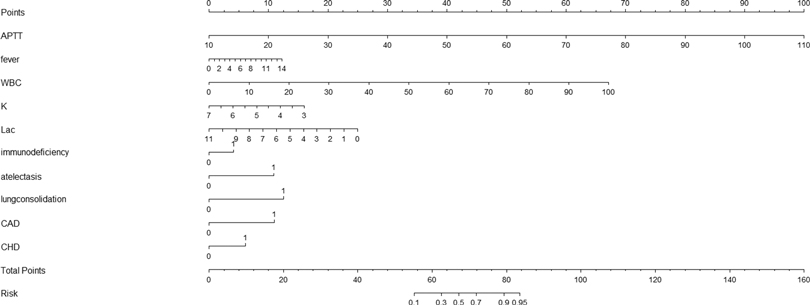
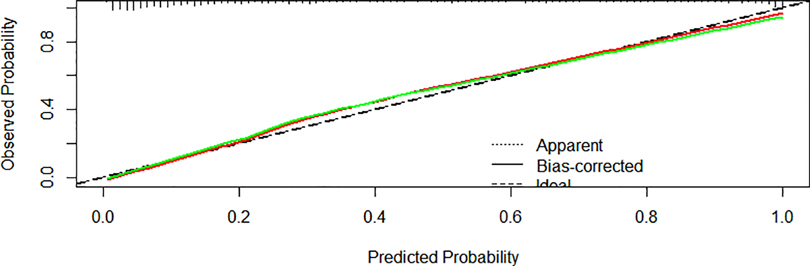
We created a nomogram that included statistically significant influencing factors (Figure 2). The nomogram revealed that immunodeficiency, atelectasis, CAD, lung consolidation and CHD had a risk score of 4.5, 12, 12, 13, 6. The duration of fever, APTT and WBC risk score all increased with the actual value of treatment failure in children with bronchiolitis. In contrast, the risk scores for potassium and lactate increased with decreasing actual value of treatment failure in patients. In the training set's nomogram, the AUC for the probability of CPAP treatment failure in children with bronchiolitis was 0.919 (0.895–0.943) (Figure 3). The calibration curve for the model revealed high agreement between prediction and observation in the training cohort (Figure 4). The p-value of Hosmer-Lemeshow test was 0.2707, which indicated that the model fitted well.
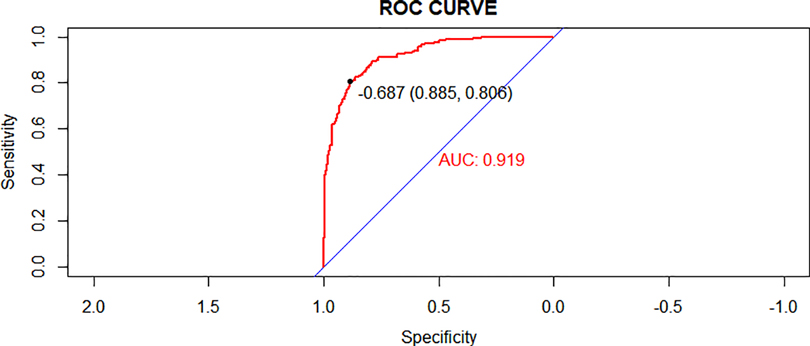
Figure 3. Receiver operating characteristic (ROC) curves of the nomogram model in the training cohort.
Validation of the nomogram
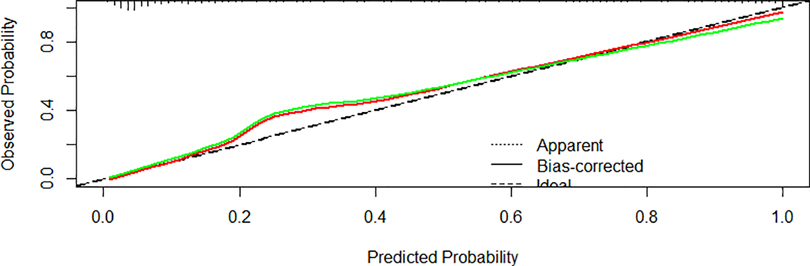
In the validating set, the AUC for the probability of CPAP treatment failure for bronchiolitis was 0.947 (0.921–0.974) (Figure 5). Hosmer-Lemeshow goodness-of-fit tests revealed no significant difference between observed and predicted events (p = 0.8606) (Figure 6).
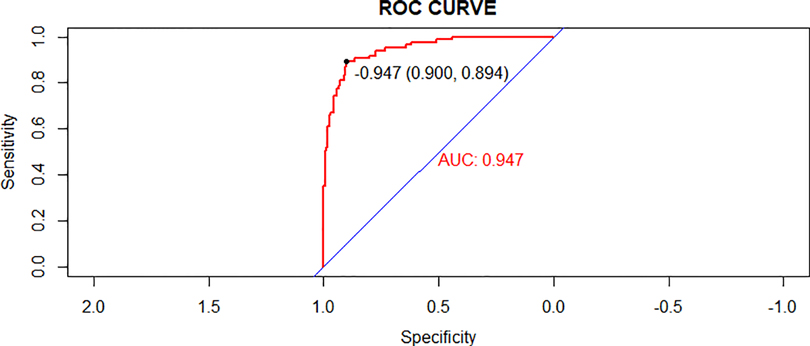
Figure 5. Receiver operating characteristic (ROC) curves of the nomogram model in the validation cohort.
Comparison with three critical scores
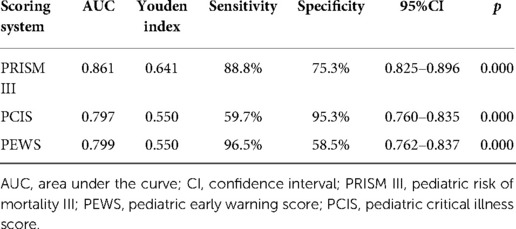
For all included children, PRISM III, PCIS, and PEWS had an AUC of the probability of CPAP treatment failure on bronchiolitis of 0.861 (0.825–0.896), 0.797 (0.760–0.835), 0.799 (0.762–0.837) (Figure 7, Table 4). The results indicated that three scores can predict the efficacy of CPAP on bronchiolitis to a certain extent. To clarify the clinical utility of nomogram, we compared the AUC of nomogram with the curves of three scoring systems. The predictive power was comparable to PEWS and PCIS (p = 0.937), but none of them was as accurate as PRISM III (p < 0.05). The AUC of nomogram was significantly bigger than PRISM III, PCIS, and PEWS (all p < 0.05).
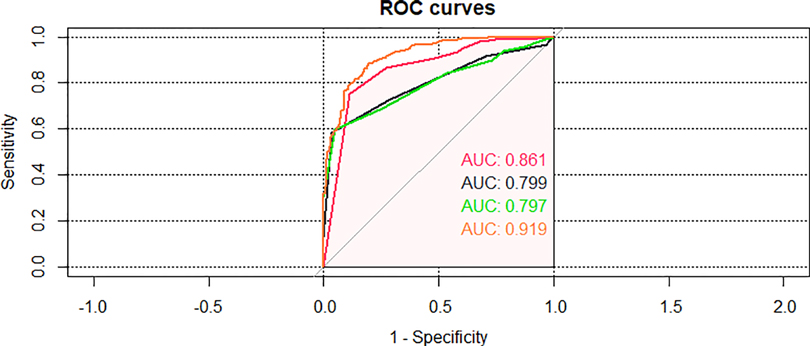
Figure 7. Area under the curve (AUC) of comparison of the nomogram and the three critical scores in the validating set.
Discussion
We developed and validated a predictive model to predict CPAP treatment for bronchiolitis. The prediction model incorporates fever, APTT, WBC, serum potassium concentration, Lac, immunodeficiency, atelectasis, lung consolidation, CAD and CHD. It shows good accuracy, which indicates that nomogram may has good utility in clinical practice. This nomogram was more accurate than PRISM III, PEWS and PCIS.
CPAP is a valuable non-invasive ventilation technique that can reduce work of breathing and improve gas exchange (13). Jat et al.'s study discovered CPAP can reduce respiratory rate when compared to standard treatment (3). Furthermore, CPAP has been shown in certain trials to significantly improve respiratory symptoms (6, 14). In general, it's important to investigate factors that contribute to CPAP treatment failure on bronchiolitis.
In this study, fever, APTT, WBC, serum potassium concentration, Lac, immunodeficiency, atelectasis, lung consolidation, CAD, CHD, PRISM III, PEWS, PCIS were the influencing factors of CPAP treatment failure on bronchiolitis. The findings may have implications for clinical practice. Fever and WBC count both represent the inflammatory reaction of the body. The clinical manifestations of bronchiolitis include cough, wheezing and fever (1). Prolonged fever indicates that patients are suffering from severe disease with a long course. The WBC count will increase when there is infection and inflammatory reaction is intense, which is a defensive reaction of the body. Studies have shown that WBC counts can be used to predict and diagnose the severity of bronchiolitis (15). The rise of APTT indicates that the blood is in a hypercoagulable state, which is caused by the release of various inflammatory factors and clotting substances during infection (16). The hypercoagulable state of blood will cause pulmonary microvascular contraction and blood block, which leads to aggravation of pulmonary and respiratory diseases. The study (17) showed that hypokalemia was a common electrolyte abnormality in pneumonia. Hypokalemia can cause damage to the body, such as abdominal distension, arrhythmia, dyspnea, etc. Changes in Lac can lead to imbalance in patient's internal environment. Bronchiolitis will cause changes in serum metabolism of patients. The study discovered that the risk factors for prolonged hospitalization of children with bronchiolitis were cardiac abnormalities, airway abnormalities and immune abnormalities (18, 19). Atelectasis and lung consolidation are well-recognized factors for severity of disease with bronchiolitis. They are clinically established indicators of disease severity. When patients are complicated by CAD or CHD, immunodeficiency, atelectasis, or lung consolidation, airway function will be impaired. Therefore, close monitoring risk factors, comprehensive treatment measures and oxygen supply after maintaining airway patency are the key to treatment success.
PRISM III is the most widely used pediatric critical care score. Kaur's study proved that it is capable of accurately determining the severity of the disease and predicting prognosis (20). Mayordomo-Colunga et al.'s study found that higher PRISM III was associated with non-invasive ventilation treatment failure (21). Our findings were similar to theirs. Furthermore, the result revealed that PRISM III had high sensitivity and specificity. This could be because PRISM III includes important physiological parameters from multiple systems. However, it is impractical to dynamically monitor all physiological parameters in clinical practice.
PEWS is simple and convenient, and it can serve as a useful tool for clinicians to evaluate the condition of children effectively. Therefore, it was suggested in the UK's 2015 guidelines on bronchiolitis (22). Our study also confirmed that PEWS is useful to predict the efficiency of CPAP on bronchiolitis. But the specificity of PEWS was low, which may be the limited scoring content.
PCIS is a pediatric critical care score that is most frequently used in China. Secondary hospitals can acquire pertinent data for scoring. A study suggested that PCIS might be utilized to evaluate the severity of disease and forecast prognosis, which is similar to our study (23). In addition, the results demonstrated that PCIS's predictive value is inferior to PRISM's, which was consistent with the findings of earlier investigations (24). This suggests that PCIS has low sensitivity in predicting CPAP treatment failure on bronchiolitis.
In our study, the performance of the prediction model established is significantly better than three scoring systems. We have to admit there is a fundamental bias in our research. We are comparing 3 all-comers (including any disease or disorder) scoring systems in a disease-specific study population with a disease-specific nomogram that we have formulated from the disease-specific data of that specific study group. We take into account factors including pulmonary problems and the length of the illness to build a nomogram. The nomogram model can assist us in intuitively comprehending the impact of numerous influencing factors at various levels on the effectiveness of CPAP on bronchiolitis. Furthermore, data of the predictive model are available for clinical use and can be applied to hospitals at different levels.
This study had several strengths. It's the first time to build a prediction model for CPAP treatment failure on bronchiolitis. There is a lack of tools about CPAP treatment on bronchiolitis. In practical clinic physicians need a prediction model for CPAP treatment failure on bronchiolitis. Three scoring systems for assessing the effectiveness of CPAP on bronchiolitis has also never been studied before. It is encouraging that three scoring systems can also predict the efficacy of CPAP on bronchiolitis. The nomogram performed better in predicting efficiency. This study is useful for clinical work because it identifies influencing factors that contribute to CPAP treatment failure on bronchiolitis. Although the scoring method might be challenging to implement in actual work settings, the nomogram is rather straightforward and might gain popularity over time. The limitation of this study is that it is a single-center case-control study, which may lead to heterogeneity of results. The physiological characteristics and outcomes may change as bronchiolitis in children progresses under various situations. The numerical variation of nomogram may be statistically relevant, but in real clinical practice would not be so different. Therefore, more research is needed in the future to find more indicators applicable to clinical practice.
Conclusion
In this study, we found that fever, APTT, WBC, serum potassium concentration, Lac, immunodeficiency, atelectasis, lung consolidation, CAD, CHD, PRISM III, PCIS, and PEWS were the influencing factors of the efficacy of CPAP on bronchiolitis. Compared with the three scoring systems, nomogram composed of the remaining independent risk factors had higher predictive power.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by Children's hospital of Chongqing medical university. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
QS is responsible for conception, data search, inclusion and exclusion of studies, data extraction, assessment of methodological quality, data analysis and writing the manuscript. ZZ is responsible for conception, data search, inclusion and exclusion of studies, data extraction, assessment of methodological quality, data analysis. JL and YZ is responsible for data extraction and data analysis. JD is responsible for supervision, interpretation of results and checking the first and the final versions of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Dalziel SR, Haskell L, O'Brien S, Borland ML, Plin AC, Babl FE, et al. Bronchiolitis. Lancet. (2022) 400:392–406. doi: 10.1016/S0140-6736(22)01016-9
2. Fujiogi M, Goto T, Yasunaga H, Fujishiro J, Mansbach JM, Camargo CA, et al. Trends in bronchiolitis hospitalizations in the United States: 2000–2016. Pediatrics. (2019) 144(6):e20192614. doi: 10.1542/peds.2019-2614
3. Jat KR, Dsouza JM, Mathew JL. Continuous positive airway pressure (CPAP) for acute bronchiolitis in children. Cochrane Database Syst Rev. (2022) 4:Cd010473. doi: 10.1002/14651858.CD010473.pub4
4. Tang G, Lin J, Zhang Y, Shi Q. The effects and safety of continuous positive airway pressure in children with bronchiolitis: a systematic review and meta-analysis. J Trop Pediatr. (2021) 67(2):fmaa128. doi: 10.1093/tropej/fmaa128
5. Lin J, Zhang Y, Xiong L, Liu S, Gong C, Dai J. High-flow nasal cannula therapy for children with bronchiolitis: a systematic review and meta-analysis. Arch Dis Child. (2019) 104:564–76. doi: 10.1136/archdischild-2018-315846
6. Lal SN, Kaur J, Anthwal P, Goyal K, Bahl P, Puliyel JM. Nasal continuous positive airway pressure in bronchiolitis: a randomized controlled trial. Indian Pediatr. (2018) 55:27–30. doi: 10.1007/s13312-018-1222-7
7. Milési C, Matecki S, Jaber S, Mura T, Jacquot A, Pidoux O, et al. 6 cmH2O continuous positive airway pressure versus conventional oxygen therapy in severe viral bronchiolitis: a randomized trial. Pediatr Pulmonol. (2013) 48:45–51. doi: 10.1002/ppul.22533
8. Luo SY, Wu Y, Yi Q, Wang ZL, Tang Y, Zhang GL, et al. Predictive factors for failure of continuous positive airway pressure treatment in infants with bronchiolitis. Zhongguo Dang Dai Er Ke Za Zhi. (2020) 22(4):339–45. doi: 10.7499/j.issn.1008-8830.1910026
9. Editorial Committee of Chinese Journal of Pediatrics, Respiratory Group of Pediatrics Branch of Chinese Medical Association. Expert consensus on diagnosis, treatment and prevention of bronchiolitis. Chin J Pediatr. (2015) 53(3):168–71.
10. Pollack MM, Patel KM, Ruttimann UE. PRISM III: an updated pediatric risk of mortality score. Crit Care Med. (1996) 24:743–52. doi: 10.1097/00003246-199605000-00004
11. Ren XX, Song GW. Pediatric risk of mortality III score and pediatric CriticalIllness score. Appl Clin Pediatr. (2006) 21:382–4.
12. Agulnik A, Méndez Aceituno A, Mora Robles LN, Forbes PW, Soberanis Vasquez DJ, Mack R, et al. Validation of a pediatric early warning system for hospitalized pediatric oncology patients in a resource-limited setting. Cancer. (2017) 123:4903–13. doi: 10.1002/cncr.30951
14. Thia LP, McKenzie SA, Blyth TP, Minasian CC, Kozlowska WJ, Carr SB. Randomised controlled trial of nasal continuous positive airways pressure (CPAP) in bronchiolitis. Arch Dis Child. (2008) 93:45–7. doi: 10.1136/adc.2005.091231
15. Doğan M, Köse M, Öztürk MA, Hangül M, Aslaner H. The relationship between immature platelet fraction and severity of acute bronchiolitis. Turk J Pediatr. (2021) 63(6):1056–63. doi: 10.24953/turkjped.2021.06.014
16. Xin T. Changes and clinical significance of serum levels of strem-1, fibrinogen and d-dimer in patients with severe pneumonia. J Clin Pulm. (2018) 23:155–8. doi: 10.3969/j.issn.1009-6663.2018.09.037
17. Alfano G, Ferrari A, Fontana F, Perrone R, Mori G, Ascione E, et al. Hypokalemia in patients with COVID-19. Clin Exp Nephrol. (2021) 25(4):401–9. doi: 10.1007/s10157-020-01996-4
18. Ghazaly M, Nadel S. Characteristics of children admitted to intensive care with acute bronchiolitis. Eur J Pediatr. (2018) 177(6):913–20. doi: 10.1007/s00431-018-3138-6
19. Mecklin M, Heikkilä P, Korppi M. Low age, low birthweight and congenital heart disease are risk factors for intensive care in infants with bronchiolitis. Acta Paediatr. (2017) 106(12):2004–10. doi: 10.1111/apa.14021
20. Kaur A, Kaur G, Dhir SK, Rai S, Sethi A, Brar A, et al. Pediatric risk of mortality III score – predictor of mortality and hospital stay in pediatric intensive care unit. J Emerg Trauma Shock. (2020) 13:146–50. doi: 10.4103/JETS.JETS_89_19
21. Mayordomo-Colunga J, Rey C, Medina A, Martínez-Camblor P, Vivanco-Allende A, Concha A. Helmet versus nasal-prong CPAP in infants with acute bronchiolitis. Respir Care. (2018) 63:455–63. doi: 10.4187/respcare.05840
22. NICE. Bronchiolitis in children: Diagnosis and management. London: National Institute for Health and Care Excellence (NICE) (2015).
23. Ying J, Zhang Z, Lu G. Application of pediatric critical case score and third-generation pediatric death risk score in critically ill children. Chin J Evidence Based Pediat. (2018):185–9.
Keywords: continuous positive airway pressure, bronchiolitis, prediction model, children, treatment
Citation: Shi Q, Zhao Z, Lin J, Zhang Y and Dai J (2022) A prediction model for the efficacy of continuous positive airway pressure on bronchiolitis. Front. Pediatr. 10:1033992. doi: 10.3389/fped.2022.1033992
Received: 1 September 2022; Accepted: 27 October 2022;
Published: 29 November 2022.
Edited by:
Hong-Ren Yu, Kaohsiung Chang Gung Memorial Hospital, TaiwanReviewed by:
Kent Thorburn, Alder Hey Children's Hospital, United KingdomDaniela Perrotta, Bambino Gesù Children's Hospital (IRCCS), Italy
© 2022 Shi, Zhao, Lin, Zhang and Dai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jihong Dai MjUxMDIzNDQ2NUBxcS5jb20=
Specialty Section: This article was submitted to Pediatric Pulmonology, a section of the journal Frontiers in Pediatrics
 Qingxia Shi
Qingxia Shi