
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 18 October 2022
Sec. Pediatric Gastroenterology, Hepatology and Nutrition
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.1033570
 Silvio Veraldi1,2*†
Silvio Veraldi1,2*† Andrea Pietrobattista1,†
Andrea Pietrobattista1,† Giovanna Soglia3
Giovanna Soglia3 Lidia Monti3
Lidia Monti3 Tommaso Alterio1
Tommaso Alterio1 Antonella Mosca1
Antonella Mosca1 Daniela Liccardo1
Daniela Liccardo1 Maria Sole Basso1
Maria Sole Basso1 Claudia Della Corte1
Claudia Della Corte1 Luca Russo4
Luca Russo4 Manila Candusso1
Manila Candusso1 Fabrizio Chiusolo5
Fabrizio Chiusolo5 Francesca Tortora5
Francesca Tortora5 Marco Spada6
Marco Spada6 Giuseppe Maggiore1
Giuseppe Maggiore1
Sarcopenia is a clinical condition characterized by a reduction in muscle mass, which typically affects adult patients; however, it has recently been recognized in pediatric literature. Few studies in children with chronic liver disease (CLD) undergoing liver transplantation (LT) have investigated the role of sarcopenia, with controversial results. The aim of our study was to assess the prevalence and impact of sarcopenia among children with CLD who are candidates for LT. We conducted a retrospective, single-center study at Bambino Gesù Children's Hospital (Rome, Italy) from July 2016 to July 2021, evaluating all children (0–16 years old) with CLD listed for LT with an abdomen computed tomography imaging available before LT. The total psoas muscle surface area (t-PMSA) was defined as the sum of left and right psoas muscle surface area measured at L4–L5 on axial images. The t-PMSA z-score was calculated according to reference data, and sarcopenia was defined as a t-PMSA z-score of ≤−2 (1–16 years) or a psoas muscle index [PMI; PMI = t-PMSA/(100 × BSA)] of <50th percentile of the population examined (<1 year). Clinical, laboratory, and LT outcome data were collected from all the patients with CLD. 27 out 48 (56%) of the patients aged 1–16 years were sarcopenic. No differences were noted in anthropometrics, nutritional support, liver function tests, model for ESLD (MELD), or pediatric ESLD (PELD) scores between patients with and without sarcopenia. The former showed a higher prevalence of respiratory complications (66.7% vs. 42.1%) and need for inotropes (40.7% vs. 10.8%) after LT. Among patients aged 0–1 years (n: 36), those with reduced muscle mass (50%) had a longer hospitalization time (44 vs. 24 days) and higher incidences of multi-organ failure syndrome (38.9% vs. 0%) and intensive care unit-related infections (61.1% vs. 27.8%) compared to those with greater muscle mass. t-PMSA and PMI were statistically significant predictors of LT outcomes. Sarcopenia is a reliable index of frailty in children with CLD, as its presence is associated with the risk of a more challenging LT. Future studies will have to investigate the functional aspects of sarcopenia and conceive preventive measures of muscle wasting in CLD patients.
Chronic liver disease (CLD) in pediatric patients is associated with progressive structural and functional deterioration of the liver due to fibrosis, cholestasis, and subsequent hepatocellular necrosis (1). The nutritional state is a key element in pediatric patients with CLD, as it can affect the quality of life, morbidity, and mortality in both the pre- and post-transplant periods (2). Sarcopenia is a clinical condition characterized by the reduction of muscle mass and function and has been shown to play a relevant role in malnutrition (3, 4). Sarcopenia typically affects older or adult patients with chronic diseases, but it has recently been recognized in the pediatric literature with a growing interest, especially in CLD (5–7). Overall, knowledge on sarcopenia is limited by the lack of uniform definitions and similarities of principles with malnutrition, which could lead to the co-occurrence of both clinical conditions with overlapping risk in children with growth failure, neurodevelopmental delay, and postoperative outcome (8, 9). Recently, the North American Working Group on Sarcopenia in Liver Transplantation claimed that most children are too young to perform functional tests assessing frailty, which could lead to an underestimation of the impact of this condition on this vulnerable population (10). Thus, muscle mass assessment is a cornerstone of sarcopenia evaluation in this vulnerable population, and cross-sectional imaging such as computed tomography (CT) to evaluate the psoas muscle surface area is now considered a feasible method that provides objective data to assess the psoas or skeletal muscle surface area in children (6, 9, 11).
Moreover, in adults with cirrhosis, there is substantial evidence that sarcopenia is associated with increased morbidity and mortality before and after liver transplantation (LT); thus, some authors have advocated the inclusion of sarcopenia in the criteria of organ allocation for LT (12, 13). Poor studies have investigated the prevalence of sarcopenia in children with CLD undergoing LT and its correlation with transplant outcomes (6, 14–19).
The aim of our study was to assess the prevalence of sarcopenia among children with CLD who are candidates for LT.
The secondary aim was to evaluate the impact of sarcopenia on selected transplant outcomes and identify the distinctive features of sarcopenic subjects.
This was a retrospective, single-center study conducted at Bambino Gesù Children's Hospital (Rome, Italy), evaluating all children (0–16 years old) with CLD listed for LT from July 2016 to July 2021, with an abdominal CT scan performed and available up to 3 months before LT.
Abdominal CT was performed in all subjects as part of the LT assessment protocol adopted at our center.
Patients candidates to LT without a chronic liver disease (acute liver failure) or with conditions that may impact on sarcopenia beyond the role of liver disease (i.e., chronic kidney disease, tumors, neuromuscular or endocrine disorders) were excluded (Figure 1).
Demographic (age, sex, main diagnosis, days on waiting list), anthropometric (weight, height, body surface area [BSA], pediatric end-stage liver disease (PELD)/model for end-stage liver disease (MELD) score), clinical (portal hypertension), and laboratory (full blood count, alanine transaminase, aspartate transaminase, gamma-glutamyl transferase, total bilirubin, international normalized ratio, serum albumin, vitamin D, alpha-fetoprotein, creatinine, and serum sodium) data were collected from patient charts. Laboratory results were registered only if they were performed within 1 month of CT imaging. Data on nutritional supplementation via a nasogastric tube (NGT), parenteral nutrition (PN), or both before LT were collected. The decision to start artificial nutrition was based on the clinical assessment of nutritional status (20). Early and late morbidities after LT represent the study outcomes.
The following outcomes were recorded during LT hospitalization: overall hospital and intensive care unit (ICU) length of stay (LOS); need for inotropes; need for re-intubation; Pediatric Index of Mortality 3 and Pediatric Organ Logistic Dysfunction 2 scores; and episodes of ICU infections and multiple organ dysfunction syndrome (MODS). MODS was defined as the presence of failure in at least two organs. ICU infections were defined as the presence of a positive bacterial culture in blood, urine, peritoneal fluid, or bronchoalveolar lavage detected during the ICU stay.
The incidence of sepsis, respiratory (acute respiratory distress syndrome, pneumothorax, pleural effusions, pneumonia), surgical (need for re-laparotomy, biliary or vascular complications), and graft-related (primary non-function, hepatic artery, or portal vein thrombosis) complications were recorded.
Finally, mortality, number of acute rejections based on the BANFF criteria (21), and catch-up growth in the first 12 months after LT were also considered.
Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin. The study was carried out according to the rules of Helsinki declaration.
CT scans were recovered using the Picture Archiving and Communication System and reviewed by an experienced radiologist (LM) with experience of over 20 years in abdominal radiology. The total psoas muscle surface area (t-PMSA) was defined as the sum of the left and right psoas muscle surface areas measured at L4-L5 on axial images. The t-PMSA z-score was calculated according to reference data, and sarcopenia was defined as a t-PMSA z-score of ≤−2 (Figure 2) (11).
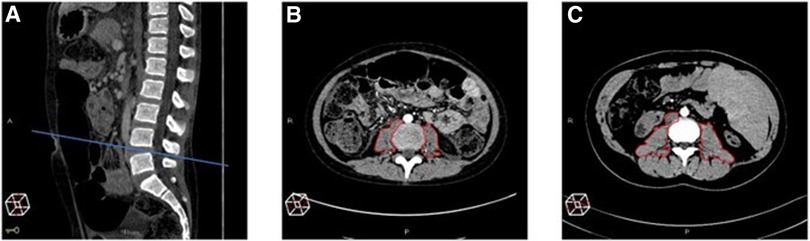
Figure 2. Measurement of the total psoas muscle surface area (t-PMSA). For the measurement of the total psoas muscle surface area (t-PMSA), the L4–L5 level in the sagittal plane is identified (A) then the left and right psoas muscle areas are measured in the axial plane (B,C). (B) Shows the psoas muscles of a subject with sarcopenia t-PMSA: 981.6 mm2, z-score: −2.88, while (C) those of a non-sarcopenic subject (t-PMSA: 2,390 mm2, z-score: 0.1).
To date, there is no standardized definition of sarcopenia for children aged <12 months as there are no reference values for t-PMSA and t-PMSA z-score available.
In this group, we calculated the psoas muscle index (PMI) as follows: PMI = (t-PMSA/(100 × BSA)) (mm2/(kg/m2)) and decided to stratify the population into two halves according to the median PMI. Infants with a PMI <50th percentile were conventionally considered to be sarcopenic.
Continuous variables are expressed as median and 25th–75th centile or mean ± standard deviation, as appropriate. Categorical data are expressed as frequencies and percentages. Comparisons between continuous variables were performed by Mann–Whitney test or t-test. The chi-square test was used for proportions. Linear or binary logistic regression analyses were performed to study the t-PMSA z-scores or PMI (independent variables) and outcomes (dependent variables).
Statistical significance was set at p < 0.05. Statistical analysis was performed using SPSS v.22.0 (IBM Corp., Armonk, New York, United States).
Eighty-four children with CLD (41 males, 48.8%) who were listed for LT were evaluated. Thirty-six (16 males, 44%) were infants (<12 months old) and 48 (25 males, 52%) were aged 1–16 years old (Figure 1). Eighty-two of 84 (97.6%) patients underwent LT, while two were still on the waiting list at the end of the study. The most common indication for LT was biliary atresia (n = 60, 71%), followed by genetic disorders (n = 14, 16%) (Supplementary Table S1). Table 1 shows the demographic characteristics of the study population.
Because of the lack of a validated definition of sarcopenia for children aged <12 months, we analyzed data from the CLD populations separately according to age.
The prevalence of sarcopenia in this group was 56.3% (27 out 48). In patients with sarcopenia, the median t-PMSA z-score and mean PMI were −3.26 (±1.06) and 7.62 (6.12–8.67) compared to −0.737 (±0.989) and 10.73 (9.85–12.1), respectively (p < 0.001) (Table 2).
Patients with and without sarcopenia and CLD had similar weight and height z-scores at LT listing. In addition, the median growth increase one year after LT was comparable between the two groups.
There were no differences in the etiology of liver diseases, liver function tests, PELD/MELD score calculated at listing or LT, and median time spent on the waiting list. Patients with sarcopenia had a higher incidence of portal hypertension (n = 18, 66.7% vs. n = 8, 38.1%) and moderate-to-severe ascites than those without sarcopenia (n = 16, 59.3% vs. n = 5, 23.8%) (p < 0.05).
Forty-six out of 48 (96%) patients with CLD aged 1–16 years old underwent LT during the study.
During ICU stay, the proportion of patients with inotropes after surgery was higher among those with sarcopenia (n = 11, 40.7% vs. n = 3, 10.8%) (Table 3) (Figure 3). The median duration of mechanical ventilation was similar between the two groups (12, 9.3–18.3 h vs. 13.1, 5.2–17.1 h), but children with sarcopenia more frequently failed to maintain noninvasive ventilatory support needing a second intubation (n = 8, 29.2% vs. n = 2, 10.5%).
No differences were observed in the incidence of MODS, ICU-related infections, or ICU mortality.
At the end of LT hospitalization, patients with sarcopenia showed an increased incidence of LT-related complications (n = 21, 77.8% vs. n = 8, 42.1%) (p < 0.05) (Table 4). In particular, a higher percentage of subjects had respiratory complications (n = 18, 66.7% vs. n = 8, 42.1%), while no differences were noted in surgical, infectious, and graft-related complications.
Patients with and without sarcopenia showed comparable ICU and overall LOS, as well as mortality and rate of acute rejections in the 12-months following LT.
Regression analyses revealed that the t-PMSA z-score was a significant predictor of the need for inotropes after surgery (odds ratio [OR] 0.487 [0.279–0.850], p < 0.05) and the risk of developing any complications [OR 0.535 (0.316–0.905), p < 0.05].
We reviewed data from 36 infants (16 males; 6, 5.2–8.4 months) with CLD listed for LT with a median PMI of 9.5 (7.8–11.2) mm2.
To estimate the impact of sarcopenia in this population, we conventionally considered patients with sarcopenia and PMI of <50th centile (<9.5 mm2) and patients without sarcopenia those and PMI of ≥50th percentile (≥9.5 mm2).
As for older patients, infants with and without sarcopenia had similar anthropometric, clinical, and laboratory features (Table 2).
The proportion of children receiving enteral nutrition was comparable between the two groups (n = 7, 38.9% vs. 8, 44.4%), as was the duration of support.
Infants with sarcopenia tended to remain on the waiting list for a longer period (60, 31–86 vs. 34, 18–51 days), even if PELD scores at both listing and transplantation were similar between the two groups.
Median ICU and overall hospitalization stays were longer in infants with sarcopenia (18, 5–26 and 44, and 23–56 days) than in those without (7, 5–10 and 24, and 19–43 days) (Tables 3, 4).
Moreover, patients with lower muscle mass presented a higher incidence of ICU-related infections (11 patients, 61.1% vs. 5 patients, 27.8%) and MODS both on the first (n = 8, 44.4% vs. n = 2, 11.1%) and second postoperative days (n = 7, 38.9% vs. n = 0, 0%) (Figure 3).
No differences in the rates of mortality and acute rejection in the first year after LT were found between the two groups.
Regression analysis revealed that PMI was a statistically significant predictor of length of hospitalization with a β coefficient of −4.303 [95% confidence interval (CI) −8.09 to −0.52] (p = 0.027) and MODS (OR: 0.513, 95% CI: 0.275–0.957) (p = 0.036) in the population of children aged 0–12 months.
Intervention on modifiable prognostic factors is part of the management of patient candidates for LT to improve their overall outcome. Recent literature has highlighted that sarcopenia is a common feature of adult patients with CLD, with a significant negative impact on both LT waitlist mortality and outcomes (22, 23).
Our study evaluated the prevalence and impact of sarcopenia in pediatric patients with CLD who were candidates for LT.
Various techniques are currently available for the assessment of skeletal muscle mass. Dual-energy x-ray absorptiometry and bioelectrical impedance analyses have been widely used in the past, but they may be affected by the patient's hydration status and lack reproducibility among different vendors or accuracy compared with other methods (24). Instead, abdominal CT scan and magnetic resonance evaluation of specific features of the psoas muscle (surface area measured at L3/L4/L5 or thickness) have proven to be reliable and reproducible with greater accuracy compared to other methods, especially in specific settings, such as patients with cirrhosis (24–27).
The main limitation of CT is the exposure to ionizing radiation, which is a major issue, mostly in pediatric patients. In our center, CT scan is performed in all patient candidates for LT as a part of the local listing protocol; therefore, we could retrospectively review the scans in our patients in order to assess skeletal muscle mass.
In our study population aged 1–16 years, we found a sarcopenia prevalence of 56%. This proportion is higher than those reported in previous studies, but comparable to that described in a recent Dutch study (55.6%) conducted on a population of 34 children with CLD (6, 14–19). Table 5 summarizes the results of the most recent reports on this topic.
Malnutrition is a known predictor of poor outcomes in children undergoing LT (19). However, currently available clinical and laboratory tools to accurately determine poor nutritional status in children are affected by organomegaly and ascites, among others. In our populations, we did not identify differences between subjects with and without sarcopenia regarding auxological parameters. Although muscle mass development and anthropometrics share some pathophysiological patterns, the lack of correlations between these variables may indicate that they are independently present in patients with CLD.
This finding is further supported by the same proportion of patients requiring artificial nutritional support regardless of the presence of sarcopenia. In 2021, Woolfson et al. highlighted that children with sarcopenia more frequently needed enteral or parenteral support (70%) than those without sarcopenia (15.6%); however, the population with sarcopenia also showed worse weight and height z-scores, which may explain the higher proportion of patients requiring nutritional support (18).
We suggest that t-PMA z-scores are a more sensitive and reliable marker for muscle mass and, therefore, are a more accurate method for assessing nutritional status in children with CLD.
The most recent guidelines for nutritional management of children with CLD are focused on advising an increased caloric intake to 130%–150% of dietary reference values but lack a clear indication on how to prevent or adjust nutritional support in case of sarcopenia (20). In this regard, the assessment of sarcopenia should be implemented during the evaluation of patients with CLD, which might become a novel indication for a dedicated nutritional regimen.
Our study highlighted that both patients with and without sarcopenia had similar PELD and MELD scores. In adults, the evidence that sarcopenia is associated with the onset of unfavorable outcomes, both before and after LT, led to the development of special MELD models corrected for including sarcopenia (“Sarco-MELD”) (12, 28, 29). The optimization of MELD with sarcopenia parameters remains controversial. Data from adult studies have shown that patients with sarcopenia are often burdened by prolonged and complicated hospitalizations, with increased mortality. This finding might ideally lead to the prioritization of allocation in this specific setting; however, the state of low functional reserve determined by sarcopenia may in itself impact pre- and post-transplant outcomes (30). Moreover, the presence of severe sarcopenia could burden the total score, and patients may be recommended LT at an early and compensated stage of liver disease. For these reasons, to date, Sarco-MELD is not widespread in routine clinical practice, and its transfer to the pediatric setting seems to have a narrow window.
By analyzing the outcomes after LT, we found that infants with lower muscle mass tended to be hospitalized for a longer period, especially in the ICU setting. This outcome was pointed out in previous studies; in particular, Jitwongwai et al. showed that the median hospital LOS after LT in patients with sarcopenia was longer than that in patients without (53 vs. 45 days) (14). Similarly, Verhagen et al. demonstrated a negative correlation between skeletal muscle index and overall (r = −0.3, p = 0.01) and ICU LOS (r = −0.3, p = 0.01) (16). In children, prolonged hospitalization is a typical feature of frailty and is associated with a significant increase in medical complications and economic costs, as has already been determined in adult studies (31, 32).
According to the European Working Group on Sarcopenia in Older People (EWGSOP), frailty is a multidimensional syndrome that increases vulnerability to poor health outcomes. It has been historically labeled as a “geriatric” condition as it is strictly related to aging, even if recent studies have begun to translate this concept in pediatric patients with chronic diseases (3, 33–35). Frailty encompasses both social and physical domains, with sarcopenia representing the main determinant.
In the analysis of LT outcomes, we found that patients with sarcopenia were more prone to developing complications (e.g., ICU infections, respiratory complications, and MODS) than patients without, while the presence of sarcopenia did not increase the risk of death or graft failure after LT. Similarly, previous pediatric studies demonstrated worse clinical evolution in sarcopenic children with various LT outcomes (e.g., risk of sepsis and vascular complications). Regarding mortality, only two works identified a correlation between t-PMSA and survival after LT (17, 19). In particular, Booster et al. described an increased risk of death by 60% per 100 mm2 decrease in PMSA in a population of 57 children (0–18 years old) listed for LT. Moreover, in our study, indices of sarcopenia (t-PMSA and PMI) were independent predictors of some LT outcomes; therefore, we may speculate that the presence of sarcopenia negatively impacts the evolution of LT, and thus is considered as a paradigm of frailty in CLD pediatric patients.
This study bears several limitations. First, it has a retrospective design. The timing of CT imaging was heterogeneous (<3 months before LT), and further variations in muscle mass may have occurred before LT. The secondary limitation is the lack of reference values for t-PMSA in children aged <12 months, as the only available data refer to a population aged 1–16 years old (11). Moreover, the current definition of sarcopenia also considers the functional impact of reduced muscle mass; however, to date, there are no standardized tests available to assess this aspect, especially in younger age groups (9).
In conclusion, reduced muscle mass is now recognized as a frequent feature of children and adolescents with CLD and is related to a more complicated post-transplant course. Early identification of sarcopenia is crucial to enable targeted treatment and prevention across pediatric clinical populations, especially for those requiring LT.
In the future, it will be necessary to identify new noninvasive, reliable, and reproducible techniques for diagnosing and monitoring muscle mass with widespread use in clinical practice.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
SV, AP and GM conceptualized and designed the study. SV, AP, TA, AM, DL, and MSB collected data. LM, GS, and LR retrieved and reviewed radiological data. SV, AP, DCC, MC, FT and FC analyzed and interpreted the data. SV, AP, MS, GM, LM, TA, AM, DL, and MSB wrote the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.1033570/full#supplementary-material.
1. Zhou WC, Zhang QB, Qiao L. Pathogenesis of liver cirrhosis. World J Gastroenterol. (2014) 20(23):7312–24. doi: 10.3748/wjg.v20.i23.7312
2. Dancygier H. Complications of liver cirrhosis. In Clinical hepatology: Principles and practice of hepatobiliary diseases. Berlin, Heidelberg: Springer Berlin Heidelberg (2010). p. 967–1031. doi: 10.1007/978-3-642-04519-6_26
3. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. (2019) 48(1):16–31. doi: 10.1093/ageing/afy169
4. Vandewoude MF, Alish CJ, Sauer AC, Hegazi RA. Malnutrition-sarcopenia syndrome: is this the future of nutrition screening and assessment for older adults? J Aging Res. (2012) 2012:651570. doi: 10.1155/2012/651570
5. Merli M. Pediatric sarcopenia: exploring a new concept in children with chronic liver disease. J Pediatr (Rio J). (2020) 96(4):406–8. doi: 10.1016/j.jped.2019.08.001
6. Lurz E, Patel H, Frimpong RG, Ricciuto A, Kehar M, Wales PW, et al. Sarcopenia in children with end-stage liver disease. J Pediatr Gastroenterol Nutr. (2018) 66(2):222–6. doi: 10.1097/MPG.0000000000001792
7. Mangus RS, Bush WJ, Miller C, Kubal CA. Severe sarcopenia and increased fat stores in pediatric patients with liver, kidney, or intestine failure. J Pediatr Gastroenterol Nutr. (2017) 65(5):579–83. doi: 10.1097/MPG.0000000000001651
8. Juby AG, Mager DR. A review of nutrition screening tools used to assess the malnutrition-sarcopenia syndrome (MSS) in the older adult. Clin Nutr ESPEN. (2019) 32:8–15. doi: 10.1016/j.clnesp.2019.04.003
9. Ooi PH, Thompson-Hodgetts S, Pritchard-Wiart L, Gilmour SM, Mager DR. Pediatric sarcopenia: a paradigm in the overall definition of malnutrition in children? JPEN J Parenter Enteral Nutr. (2020) 44(3):407–18. doi: 10.1002/jpen.1681
10. Carey EJ, Lai JC, Sonnenday C, Tapper EB, Tandon P, Duarte-Rojo A, et al. A North American expert opinion statement on sarcopenia in liver transplantation. Hepatology. (2019) 70(5):1816–29. doi: 10.1002/hep.30828
11. Montano-Loza AJ, Duarte-Rojo A, Meza-Junco J, Baracos VE, Sawyer MB, Pang JX, et al. Inclusion of sarcopenia within MELD (MELD-sarcopenia) and the prediction of mortality in patients with cirrhosis. Clin Transl Gastroenterol. (2015) 6:e102. doi: 10.1038/ctg.2015.31
12. Kim HY, Jang JW. Sarcopenia in the prognosis of cirrhosis: going beyond the MELD score. World J Gastroenterol. (2015) 21(25):7637–47. doi: 10.3748/wjg.v21.i25.7637
13. Jitwongwai S, Lertudomphonwanit C, Junhasavasdikul T, Fuangfa P, Tanpowpong P, Gesprasert G, et al. Low psoas muscle index as an unfavorable factor in children with end-stage liver disease undergoing liver transplantation. Pediatr Transplant. (2021) 25(5):e13996. doi: 10.1111/petr.13996
14. Takeda M, Sakamoto S, Uchida H, Shimizu S, Yanagi Y, Fukuda A, et al. Impact of sarcopenia in infants with liver transplantation for biliary atresia. Pediatr Transplant. (2021) 25(5):e13950. doi: 10.1111/petr.13950
15. Verhagen MV, Levolger S, Hulshoff JB, Werner MJM, van der Doef HPJ, Viddeleer AR, et al. Utility of preoperative computed tomography-based body metrics in relation to postoperative complications in pediatric liver transplantation recipients. Liver Transpl. (2021) 27(12):1779–87. doi: 10.1002/lt.26205
16. Boster JM, Browne LP, Pan Z, Zhou W, Ehrlich PF, Sundaram SS. Higher mortality in pediatric liver transplant candidates with sarcopenia. Liver Transpl. (2021) 27(6):808–17. doi: 10.1002/lt.26027
17. Woolfson JP, Perez M, Chavhan GB, Johara FT, Lurz E, Kamath BM, et al. Sarcopenia in children with end-stage liver disease on the transplant waiting list. Liver Transpl. (2021) 27(5):641–51. doi: 10.1002/lt.25985
18. Dag N, Karatoprak S, Ozturk M, Karatoprak NB, Sigirci A, Yilmaz S. Investigation of the prognostic value of psoas muscle area measurement in pediatric patients before liver transplantation: a single-center retrospective study. Clin Transplant. (2021) 35(10):e14416. doi: 10.1111/ctr.14416
19. Mouzaki M, Bronsky J, Gupte G, Hojsak I, Jahnel J, Pai N, et al. Nutrition support of children with chronic liver diseases: a joint position paper of the north American society for pediatric gastroenterology, hepatology, and nutrition and the European society for pediatric gastroenterology, hepatology, and nutrition. J Pediatr Gastroenterol Nutr. (2019) 69(4):498–511. doi: 10.1097/MPG.0000000000002443
20. An International Panel. Banff schema for grading liver allograft rejection: an international consensus document. Hepatology. (1997) 25(3):658–63. doi: 10.1002/hep.510250328
21. Lurz E, Patel H, Lebovic G, Quammie C, Woolfson JP, Perez M, et al. Paediatric reference values for total psoas muscle area. J Cachexia Sarcopenia Muscle. (2020) 11(2):405–14. doi: 10.1002/jcsm.12514
22. Meeks AC, Madill J. Sarcopenia in liver transplantation: a review. Clin Nutr ESPEN. (2017) 22:76–80. doi: 10.1016/j.clnesp.2017.08.005
23. Duong N, Sadowski B, Rangnekar AS. The impact of frailty, sarcopenia, and malnutrition on liver transplant outcomes. Clin Liver Dis (Hoboken). (2021) 17(4):271–6. doi: 10.1002/cld.1043
24. Gilligan LA, Towbin AJ, Dillman JR, Somasundaram E, Trout AT. Quantification of skeletal muscle mass: sarcopenia as a marker of overall health in children and adults. Pediatr Radiol. (2020) 50(4):455–64. doi: 10.1007/s00247-019-04562-7
25. Schweitzer L, Geisler C, Pourhassan M, Braun W, Glüer CC, Bosy-Westphal A, et al. What is the best reference site for a single MRI slice to assess whole-body skeletal muscle and adipose tissue volumes in healthy adults? Am J Clin Nutr. (2015) 102(1):58–65. doi: 10.3945/ajcn.115.111203
26. Giusto M, Lattanzi B, Albanese C, Galtieri A, Farcomeni A, Giannelli V, et al. Sarcopenia in liver cirrhosis: the role of computed tomography scan for the assessment of muscle mass compared with dual-energy x-ray absorptiometry and anthropometry. Eur J Gastroenterol Hepatol. (2015) 27(3):328–34. doi: 10.1097/MEG.000000000000027
27. Gu DH, Kim MY, Seo YS, Kim SG, Lee HA, Kim TH, et al. Clinical usefulness of psoas muscle thickness for the diagnosis of sarcopenia in patients with liver cirrhosis. Clin Mol Hepatol. (2018) 24(3):319–30. doi: 10.3350/cmh.2017.0077
28. van Vugt JLA, Alferink LJM, Buettner S, Gaspersz MP, Bot D, Darwish Murad S, et al. A model including sarcopenia surpasses the MELD score in predicting waiting list mortality in cirrhotic liver transplant candidates: a competing risk analysis in a national cohort. J Hepatol. (2018) 68(4):707–14. doi: 10.1016/j.jhep.2017.11.030
29. Lai Q, Magistri P, Lionetti R, Avolio AW, Lenci I, Giannelli V, et al. Sarco-Model: a score to predict the dropout risk in the perspective of organ allocation in patients awaiting liver transplantation. Liver Int. (2021) 41(7):1629–40. doi: 10.1111/liv.14889
30. Stirnimann G, Ebadi M, Tandon P, Montano-Loza AJ. Should sarcopenia increase priority for transplant or is it a contraindication? Curr Gastroenterol Rep. (2018) 20(11):50. doi: 10.1007/s11894-018-0656-3
31. Chang SF, Lin HC, Cheng CL. The relationship of frailty and hospitalization among older people: evidence from a meta-analysis. J Nurs Scholarsh. (2018) 50(4):383–91. doi: 10.1111/jnu.12397
32. Zhang X, Zhang W, Wang C, Tao W, Dou Q, Yang Y. Sarcopenia as a predictor of hospitalization among older people: a systematic review and meta-analysis. BMC Geriatr. (2018) 18(1):188. doi: 10.1186/s12877-018-0878-0
33. Panchangam C, White DA, Goudar S, Birnbaum B, Malloy-Walton L, Gross-Toalson J, et al. Translation of the frailty paradigm from older adults to children with cardiac disease. Pediatr Cardiol. (2020) 41(5):1031–41. doi: 10.1007/s00246-020-02354-7
34. Verwaaijen EJ, van Hulst A, Fiocco M, Hartman A, Grootenhuis M, Pluijm S, et al. Dexamethasone-Induced sarcopenia and physical frailty in children with acute lymphoblastic leukemia: protocol for a prospective cohort study. JMIR Res Protoc. (2022) 11(4):e33517. doi: 10.2196/33517
Keywords: chronic liver disease, liver transplantation, sarcopenia, frailty, pediatric patients
Citation: Veraldi S, Pietrobattista A, Soglia G, Monti L, Alterio T, Mosca A, Liccardo D, Basso MS, Della Corte C, Russo L, Candusso M, Chiusolo F, Tortora F, Spada M and Maggiore G (2022) Sarcopenia in children with chronic liver disease: Prevalence and impact on liver transplant outcomes. Front. Pediatr. 10:1033570. doi: 10.3389/fped.2022.1033570
Received: 31 August 2022; Accepted: 28 September 2022;
Published: 18 October 2022.
Edited by:
Pietro Vajro, University of Salerno, ItalyReviewed by:
Loreto Hierro, University Hospital La Paz Research Institute (IdiPAZ), Spain© 2022 Veraldi, Pietrobattista, Soglia, Monti, Alterio, Mosca, Liccardo, Basso, Della Corte, Russo, Candusso, Chiusolo, Tortora, Spada and Maggiore. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Silvio Veraldi c2lsdmlvLnZlcmFsZGlAb3BiZy5uZXQ=
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Pediatric Gastroenterology, Hepatology and Nutrition, a section of the journal Frontiers in Pediatrics
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.