
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 05 December 2022
Sec. Neonatology
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.1032044
This article is part of the Research Topic Advances in Newborn Resuscitation View all 10 articles
 Chuanfeng Li1,†
Chuanfeng Li1,† Yuxuan Du2,3,4,5,6,†
Yuxuan Du2,3,4,5,6,† Kaiting Yang1
Kaiting Yang1 Huiling Cao2,3,4,5,6
Huiling Cao2,3,4,5,6 Hong Yang1
Hong Yang1 ChunXiu Zhang1
ChunXiu Zhang1 Xiongbin Li1
Xiongbin Li1 Xingmei Deng1,‡*
Xingmei Deng1,‡* Yuan Shi2,3,4,5,6,‡*
Yuan Shi2,3,4,5,6,‡*
Background: The purpose of this study was to assess the safety and efficacy of a new double-lumen tracheal tube for neonates, with a conventional tracheal tube as a control.
Method: Newborns with respiratory distress syndrome (RDS) requiring endotracheal intubation admitted to the tertiary neonatal intensive care unit (NICU) of Qujing Maternal and Child Healthcare Hospital in Yunnan Province between March 2021 and May 2022 were enrolled in this prospective cohort study. Outcome indicators related to effectiveness included mainly the number of intubations, duration of ventilation, duration of oxygenation, and length of stay; safety indicators included any clinical adverse effects during and after intubation. Appropriate stratified and subgroup analyses were performed according to the purpose of intubation, gestational age, and whether the drug was administered via endotracheal tube.
Result: A total of 101 neonates were included and divided into two groups based on the choice of tracheal tube: the conventional (n = 50) and new (n = 51) tracheal tube groups. There was no statistical difference between the two groups in terms of adverse effects during and after intubation (p > 0.05). In neonates who were mechanically ventilated without endotracheal surfactant therapy or newborns receiving InSurE technique followed by non-invasive ventilation, no significant differences were found between the two groups regarding any of the efficacy indicators (p > 0.05). However, for neonates on invasive mechanical ventilation, the new tracheal tube allowed for a significant reduction in the duration of mechanical ventilation (96.50[74.00, 144.00] vs. 121.00[96.00, 196.50] hours, p = 0.037) and total ventilation (205.71 ± 80.24 vs. 277.56 ± 117.84 h, p = 0.027), when used as a route for endotracheal drug delivery. Further analysis was performed according to gestational age for newborns requiring intratracheal surfactant administration during mechanical ventilation, and the data showed that for preterm infants, the new tracheal tube not only shortened the duration of mechanical ventilation (101.75 ± 39.72 vs. 155.50 ± 51.49 h, p = 0.026) and total ventilation (216.00 ± 81.60 vs. 351.50 ± 113.79 h, p = 0.010), but also demonstrated significant advantages in reducing the duration of oxygen therapy (9.75 ± 6.02 vs. 17.33 ± 8.43 days, p = 0.042); however, there was no statistical difference in efficacy outcomes between the two groups in full-term infants (p > 0.05).
Conclusion: The efficacy and safety of this new tracheal tube are promising in neonates with RDS, especially those requiring surfactant administration via a tracheal tube during mechanical ventilation. Given the limitations of this study, however, the clinical feasibility of this catheter needs to be further confirmed in prospective randomized trials with larger sample sizes.
Clinical Trial Registration: http://www.chictr.org.cn/showproj.aspx?proj=122073
The significance of surfactant replacement therapy, as a cornerstone in the treatment of respiratory distress syndrome (RDS), has been well documented (1–3). Conventional administration of exogenous surfactants requires coordination with an endotracheal tube, meaning that the newborn may face temporary interruptions in ventilation while receiving therapeutic surfactants, which may lead to regurgitation of expiratory-phase surfactants and prolonged duration of ventilation.
The effects of prolonged ventilation may be particularly pronounced in neonates receiving invasive mechanical ventilation (IMV). After all, while significantly improving neonatal survival, invasive mechanical ventilation may cause a variety of adverse effects, including ventilator-related lung injury, pulmonary infections, bronchopulmonary dysplasia (BPD), and even distant neurological abnormalities (4, 5). In response to these potential risks, several emerging technologies featuring non-invasiveness or minimally-invasiveness have emerged, represented by Less Invasive Surfactant Administration (LISA), Minimally Invasive Surfactant Therapy (MIST), aerosolisation administration, and laryngeal mask administration (6–10). However, these modes of administration are not fully applicable in neonates with inadequate initial respiratory drive or failure of non-invasive respiratory support (11), and there are substantial gaps in the clinical evidence regarding these techniques except for LISA (12). Therefore, traditional administration via endotracheal tube during mechanical ventilation remains an irreplaceable option. Unfortunately, clinical information on modified endotracheal tubes for neonates has been rather limited to date.
Considering the limitations of the existing traditional tracheal tube used in neonates and the lack of relevant clinical information, we developed a new dual-lumen tracheal tube. By designing an additional, thinner drug delivery tube within the wall of the dominant tube, we have artificially separated the inlet and most of the route of drug and airflow delivery, avoiding temporary interruptions in mechanical ventilation during drug administration. Furthermore, the drug delivery tube does not meet the dominant tube until it extends to the superior end of the Murphy Eye. In theory, such a design can not only avoid drug residue but also reduce the risk of the Murphy Eye becoming clogged with sputum or other secretions. However, these ideas are just speculation without the support of clinical evidence. To investigate the clinical safety and efficacy of the new double-lumen tracheal tube, we conducted a prospective cohort study in newborns with RDS, using a conventional tracheal tube as a control.
This is a single-center prospective cohort study conducted from March 2021 to May 2022 in the tertiary neonatal intensive care unit (NICU) of Qujing Maternal and Child Healthcare Hospital, Yunnan Province, China. The study complied with the principles of the Declaration of Helsinki and was approved by the Medical Ethics Committee (Approval serial number: QJFYLL2021-KY001), with a protocol registered at www.chictr.org.cn (ChiCTR2100043565).
All newborns diagnosed with RDS and prepared for initial postnatal tracheal intubation were eligible for enrollment, including those ready for invasive mechanical ventilation or the InSurE (intubation, surfactant, extubation) technique. The choice of tracheal tube type would be determined by the operating physician. Physicians trained in the clinical operation of the new tracheal tube would choose a double-lumen tube, while those without relevant training would apply a conventional tracheal tube. The physician-patient pairing is not influenced by personal preference, but by pre-defined scheduling rules within the ward. The diagnosis of RDS would be based on clinical manifestations and chest imaging features. Typical clinical manifestations include respiratory distress, tachypnea, nasal flaring, groan and cyanosis presenting within the first 24 h of life, while chest imaging features are represented by granular shadows, air bronchograms and white lungs (13). However, patients meeting any of the following criteria would be excluded: (1) endotracheal intubation was required for reasons other than RDS, (2) received surfactant administration prior to enrollment, (3) received both mechanical ventilation and InSurE technique during hospitalization, (4) severe congenital malformations, known complex congenital heart disease or chromosomal abnormalities, (5) intraventricular hemorrhage (IVH) of grade 3 or 4, (6) transferred to another medical facility during hospitalization. Written informed consent was given by the guardians of all infants.
In this study, two types of tracheal catheters were available, a conventional tube and a new non-capsular double-lumen tracheal tube (Manufacturer: Henan Tuoren Medical Device Co., Ltd.), for which a national patent (Application number: CN201821495880.4) was granted (Figure 1). To date, this double-lumen tracheal tube has not been used in any neonatal center other than our NICU. The dual-lumen tracheal tube consists of a dominant tube and a smaller diameter drug delivery tube. The main body of the drug delivery tube is located inside the wall of the dominant tube, with the distal end opening directly into the Murphy Eye in the distal wall of the main tube. Due to the small diameter of the drug delivery tube and its design within the wall of the dominant tube, the dual-lumen setup did not thicken our catheter or place additional strain on the fragile airway of the neonate. To facilitate drug administration, the proximal end of the drug delivery tube extends outside the wall of the dominant tube, and the injection port at its tip is closed with a plastic cap. There is no difference in the intubation operation between the conventional and new tracheal tubes. The diameter of the tracheal tube would be selected according to the weight of the newborn (Table 1). Additional information on the properties of the double-lumen tracheal tube can be found in Supplementary Tables S1, S2.
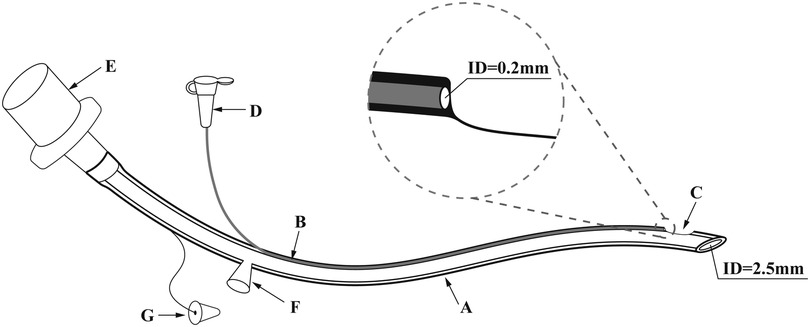
Figure 1. The novel doublt-lumen tracheal tube (take model 1 as an example.) ID: inner diameter. (A) The dominant tube, whose diameter is chosen according to the weight of the newborn. (Model 1: ID 2.5 mm; Model 2: ID 3.0 mm; Model 3: ID 3.5 mm; Model 4: ID 4.0 mm). (B) The drug delivery tube located inside the wall of the dominant tube, with an ID of 0.2 mm. (C) The Murphy Eye in direct communication with distal end of the drug delivery tube. (D) The plastic cap for closing the drug injection port. (E) The tube connector. (F) The aspiration cannula for the insertion of the suction tube. (G) The hole plug for closing the aspiration cannula when sputum aspiration is not required. In order to optimize the new double-lumen tracheal tube, the design of the aspiration cannula with its accompanying hole plug was eliminated in the actual production process.
In our NICU, eligible newborns would be selected for different modes of respiratory support based on individualization and weaned from ventilation when appropriate. Regardless of the mode used, the respiratory parameters would be adjusted according to the clinical presentation of the neonate to maintain oxygen saturation (SpO2) at 90%–94% (14). The specific criteria are shown below.
Criteria for the application of non-invasive ventilation: (1) extremely premature infants with spontaneous breathing, (2) infants at high risk of RDS, (3) arterial oxygen tension (PaO₂) < 50 mmHg (1 mmHg = 0. 133 kPa) or transcutaneous oxygen saturation (TcSO2) < 90%, when the fraction of inspired oxygen (FiO2) was set above 0.3 for oxygen via nasal cannula, mask or hood, (4) apnea of prematurity (15).
Using the short binasal prongs as an interface, we provided two modes of non-invasive ventilation with the support of the Infant Flow® SiPAP™ (Viasys Healthcare, Yorba Linda, CA, USA): nasal continuous positive airway pressure (NCPAP) and biphasic NCPAP (BP-NCPAP). The initial pressure of the NCPAP would be set at 5 cmH2O and could be adjusted between 4 and 8 cmH2O depending on the situation, while the initial breathing parameters for BIPAP would be set at a frequency of 10–30 breaths/min, a peak inspiratory pressure (PIP) of 15–25 cmH2O and a positive end-expiratory pressure (PEEP) of 4–6 cmH2O.
When the infant was clinically stable with a PEEP < 4 cmH2O and FiO2 < 25% for 24 h, weaning from non-invasive ventilation would be considered (16). If an infant presented with PaO2 < 50 mmHg or TcSO2 < 85% on air inhalation, oxygen would be supplied by appropriate means, including High-flow nasal cannula (HFNC), oxygen environment, and nasal cannula.
Infants meeting any of the following criteria would be supported with mechanical ventilation: (1) severe respiratory acidosis lasting 2 h, (2) recurrent apnoea (Frequency >3 times/h, requiring ventilation by airbag and mask) with bradycardia (Heart rate <100 beats/min), (3) hypoxemia (FiO2 > 0.5, PaO2 < 50 mmHg, or SpO 2 < 85%) for at least 2 h (17, 18). In addition, emergency tracheal intubation would be initiated when the neonate was faced with (1) severe respiratory distress, (2) neonatal pulmonary hemorrhage, (3) cardiopulmonary arrest without effective resuscitation requiring continued ventilation and rescue, or (4) other clinical emergencies (13).
In our NICU, conventional mechanical ventilation (CMV) would be the first choice, with high-frequency ventilation (HFV) as a remedial option after the failure of CMV. With the SLE 5000 ventilator as the ventilation device, mechanical ventilation and open lung strategies were used in combination. For infants receiving CMV, respiratory parameters were set as follows: respiratory rate of 30–40 breaths/min, inspiration time of 0.4 s, PEEP of 4–8 cmH2O, and PIP adjusted to maintain a target tidal volume (TV) of 4–6 ml/kg. For infants on HFV, mean airway pressure (MAP) ranged from 10 to 20 cmH2O (1 cmH20 = 0.098 kPa), oscillation amplitude from 25 to 45 cmH2O, and frequency from 10 to 15 Hz (1 Hz = 60 times/min), while inspiratory time was set at 33%.
The following are pre-defined criteria used to guide extraction and withdrawal: (1) weaning from CMV: RR < 30 beats/min, PIP < 16 cmH2O, PEEP < 5 cmH2O, and FiO2 < 30%, (2) weaning from HFV: MAP < 8 cm H2O, and FiO2 < 0.30 (19). In brief, extubation may be attempted when the infant on mechanical ventilation remains clinically stable with adequate spontaneous respiratory effort and FiO2 ≤ 0.30 for 6 h, followed by appropriate non-invasive ventilation or oxygen therapy support, with the specific criteria as described above (20).
When FiO2 was adjusted above 0.4 to maintain target SpO2, surfactant would be administered through an endotracheal tube. No more than four doses would be administered (16, 21).
The appropriate amount of surfactant (Curosurf, 200 mg/kg) with approximately 0.5 ml of air was dripped into the trachea of newborns in the supine position, via the catheter port (of a conventional tracheal tube) or the drug injection port (of a new tracheal tube), within 1 min under the push of a syringe. After administration, the infant's head was slightly elevated to reduce the risk of drug reflux (22). Avoid endotracheal suctioning for 1 h after administration unless there were obvious signs of endotracheal tube obstruction (22). After the endotracheal administration, the respiratory parameters would be flexibly adjusted in real-time to ensure the target oxygen saturation (23).
The InSurE technique would be used in infants receiving non-invasive ventilation, a group where pausing ventilation and tracheal intubation are mandatory steps in the medication administration, regardless of the type of tracheal tube chosen. However, temporary interruption of ventilation could be avoided for infants who were on invasive mechanical ventilation prior to drug administration, provided a new tracheal tube was chosen. In this specific population, endotracheal surfactant therapy through the injection port was administered while maintaining normal operation of the invasive ventilator, during which the neonate's clinical manifestations and oxygen saturation were monitored, and respiratory parameters could be adjusted accordingly if necessary. However, if a conventional catheter was chosen for a newborn on mechanical ventilation, a temporary interruption of ventilation was still unavoidable. After all, in this case, the ventilator must be withdrawn to make way for the drug drip until the end of the administration.
All premature newborns with a gestational age of fewer than 32 weeks and newborns presenting with apnea would be given caffeine (Caffeine Citrate Injection, Chiesi Pharmaceuticals, Parma, Italy; loading dose of 20 mg/kg with a maintenance dose of 5 mg/kg/day) (14, 24).
Observation of an enrolled infant would end if one of the following conditions was met: (1) death; (2) parental decision not to continue participation; (3) 1 month after discharge from the hospital.
Prior to the study, nurses in our NICU were given uniform instructions on the clinical care of conventional and new tracheal tubes, and willing operating physicians were given uniform training on the selection protocol for the new catheter type and the clinical manipulation of endotracheal surfactant administration through the new catheter. Physicians completing the training would use the new dual-lumen tracheal tube in the study, whereas physicians without the training were only qualified to apply the conventional tracheal tube. In addition, prior to the study, all medical staff agreed on criteria for the selection of ventilation mode, the regulation of respiratory parameters, the administration of endotracheal surfactant, and the assessment of outcome indicators.
Baseline data on the newborns, including gestational age (GA), birth weight, sex, Apgar score, history of antenatal steroids, mode of delivery, as well as maternal risk factors during pregnancy and delivery, would be collected through the electronic medical record system. Gestational age estimates were based on obstetric information, and the rest of the demographic information was primarily obtained through guardian dictation. Furthermore, data on the operation of the first endotracheal intubation (tube diameter, depth of tube insertion through the mouth, the experience of the performing physician, and whether an endotracheal medication was performed) were collected prospectively.
The safety of the tracheal tube was thoroughly evaluated based on the adverse reactions of neonates during and after intubation. Adverse reactions during intubation included drug reflux, oral mucosal injury, bleeding airway injury, respiratory depression, bradycardia (Heart rate <100 beats/min for at least 10 s), tachycardia (Heart rate >160 beats/min for at least 10 s), arterial hypotension, laryngospasm, blockage of the drug delivery tube, and distortion of the dominant tube. Adverse reactions after intubation were defined as coughing and wheezing, recurrent choking on milk, pulmonary hemorrhage, pneumothorax, emphysema, intraventricular hemorrhage grade 3 or 4, secondary infection, and concurrent laryngospasm. In this study, respiratory depression was used to describe weak and irregular breathing during intubation, as evidenced by a respiratory rate of <10 breaths/min and an oxygen saturation of <80%. Arterial hypotension was defined as a mean arterial pressure in mm Hg less than the gestational age measured in weeks (25). Blockage of the drug delivery tube was represented by laborious and poor drug injection. The diagnosis of pneumothorax and emphysema was determined by chest radiograph findings, while the grading of intraventricular hemorrhage relied on intracranial ultrasound and imaging.
The following outcomes were recorded as indicators to assess the effectiveness of the tracheal tube: the total number of intubations, duration of ventilation, duration of oxygen therapy, length of hospital stay, whether discharged with oxygen, and whether presenting with recurrent dyspnea one month after discharge. For infants receiving mechanical ventilation, the duration of invasive ventilation and non-invasive ventilation after withdrawal were calculated separately, and the duration of total ventilation was defined as the sum of both. The record of oxygen supply ended at the discharge, and home oxygen therapy (HOT) was not included. All infants would be assessed for recurrent respiratory distress one month after discharge by telephone follow-up.
Based on previous studies, the incidence of intraventricular hemorrhage (grade 3 or 4) after intubation with a conventional tracheal tube was approximately 17% (26). Assuming that the application of the new dual-lumen catheter would result in a 16% reduction in this risk, a minimum of 47 neonates per group was calculated by PASS software (version 15.0) at a 0.05 two-sided significance and an 80% power. Data analysis would be performed using SPSS statistical software (version 26.0). Normally distributed continuous variables would be presented as mean ± standard deviation (SD) (Range) and compared between groups using the independent-samples T test, while non-normally distributed continuous variables would be expressed as median [interquartile range (IQR)] and analyzed using the Mann–Whitney U test. For dichotomous variables represented as number (%), the χ2 test or Fisher's exact test would be chosen for the analysis. When the P-value was less than 0.05, statistical significance could be considered. Stratified analysis according to the purpose of intubation (Invasive mechanical ventilation and the InSurE technique) and gestational age (Full-term and preterm) would be conducted using the Cochran-Mantel-Haenszel test. When necessary, subgroup analysis of the primary outcomes would also be performed based on the reason for intubation (InSurE technique or invasive mechanical ventilation), gestational age, and whether the drug was administered endotracheally.
A total of 101 neonates diagnosed with RDS and endotracheally intubated were eligible, of whom 63 received invasive mechanical ventilation (HFOV, SIMV, or A/C) and the remaining 38 received the InSurE technique followed by non-invasive ventilation (NCPAP, or BP-NCPAP) (Figure 2). In our study, there was no crossover between mechanical ventilation and the InSurE technique. For infants with the InSurE technique, endotracheal intubation was performed to provide access to surfactant administration, but for mechanically ventilated neonates, surfactant therapy was not mandatory. Ultimately, 78 neonates, 40 of whom were from the group with invasive mechanical ventilation, received endotracheal surfactant administration due to respiratory immaturity; for these neonates, no doses of surfactant were received by routes other than the endotracheal tube. All neonates were followed up by telephone one month after discharge. No patients dropped out or missed visits, and there were no deaths. Based on the choice of the tracheal tube, these newborns were divided into two groups: the conventional tracheal tube group (n = 50), and the new tracheal tube group (n = 51).
Analysis of basic information on neonates and the operation of the first endotracheal intubation showed no statistical differences between the two groups, except for a significantly higher rate of surfactant administration in the new tracheal tube group than in the control group (44/51 vs. 34/50, p = 0.029; Table 2). Notably, the gestational age of the newborns in the new catheter group fluctuated between 28 and 41.57 weeks, with 32 of them being preterm, while the gestational age of the infants in the old catheter group ranged between 28 and 42.29 weeks, with only 23 being preterm. Stratified by gestational age (Supplementary Table S3) and the purpose of intubation (Supplementary Table S4), the data showed that for mechanically ventilated neonates (n = 63), the risk of surfactant application was 3.429 times higher in the new catheter group than in the old catheter group (OR = 3.429, 95% CI, 1.152–10.202, p = 0.024; Supplementary Table S4). Subsequently, a subgroup analysis was performed according to the purpose of endotracheal intubation (InSurE technique or invasive mechanical ventilation) (Table 3), and the data show a significantly higher percentage of preterm infants (13/31 vs. 6/32, p = 0.045; Table 3) and rate of surfactant administration (24/31 vs. 16/32, p = 0.024; Table 3) among neonates mechanically ventilated with the new tracheal tube; otherwise, there were no statistically significant differences (p > 0.05; Table 3). Separate stratified analyses according to gestational age (Full-term and preterm) were also performed for neonates receiving mechanical ventilation (Supplementary Table S5) and those receiving InSurE (Supplementary Table S6), and no significant association was found. Given the specificity of intratracheal administration and the fact that the response to surfactant may vary among neonates of different gestational ages (27), further subgroup analyses were performed and revealed no statistical differences in relevant indicators (p > 0.05; Tables 4–6).
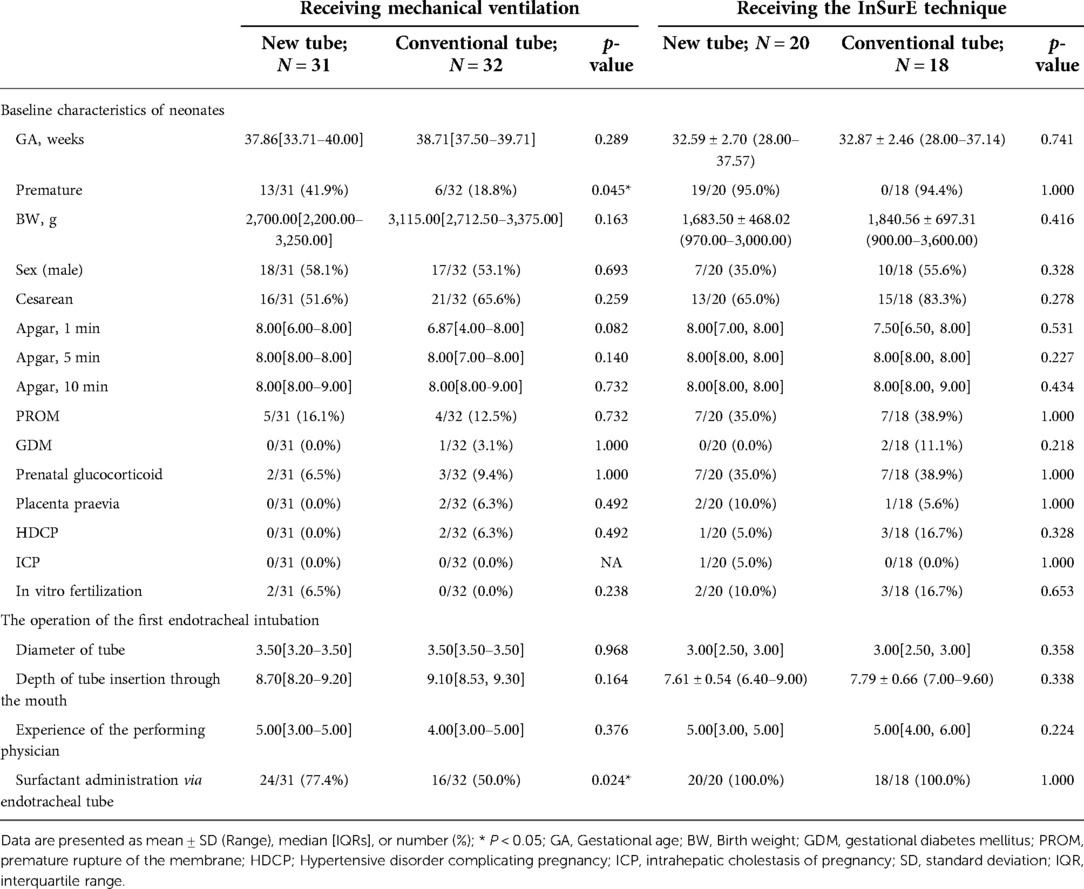
Table 3. Basic information on newborns receiving invasive mechanical ventilation and the InSurE technique.
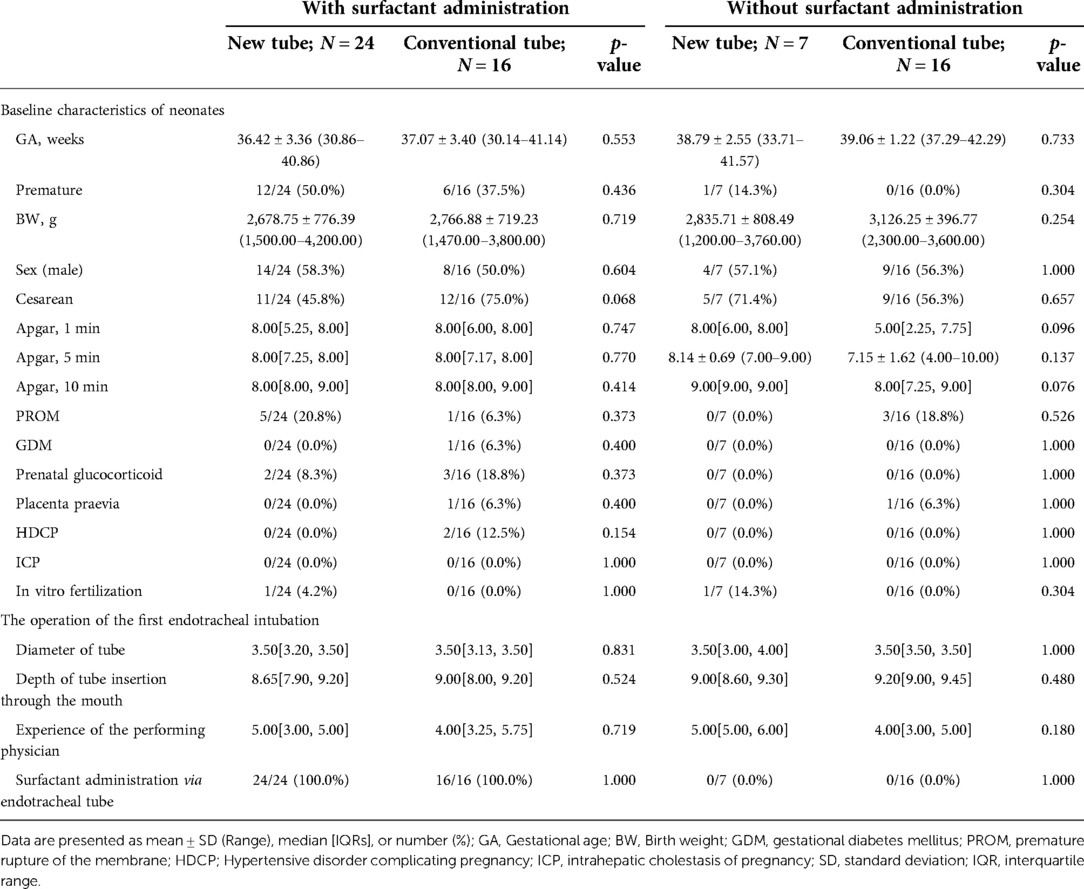
Table 4. Basic information on mechanically ventilated neonates with and without surfactant administration via endotracheal intubation.
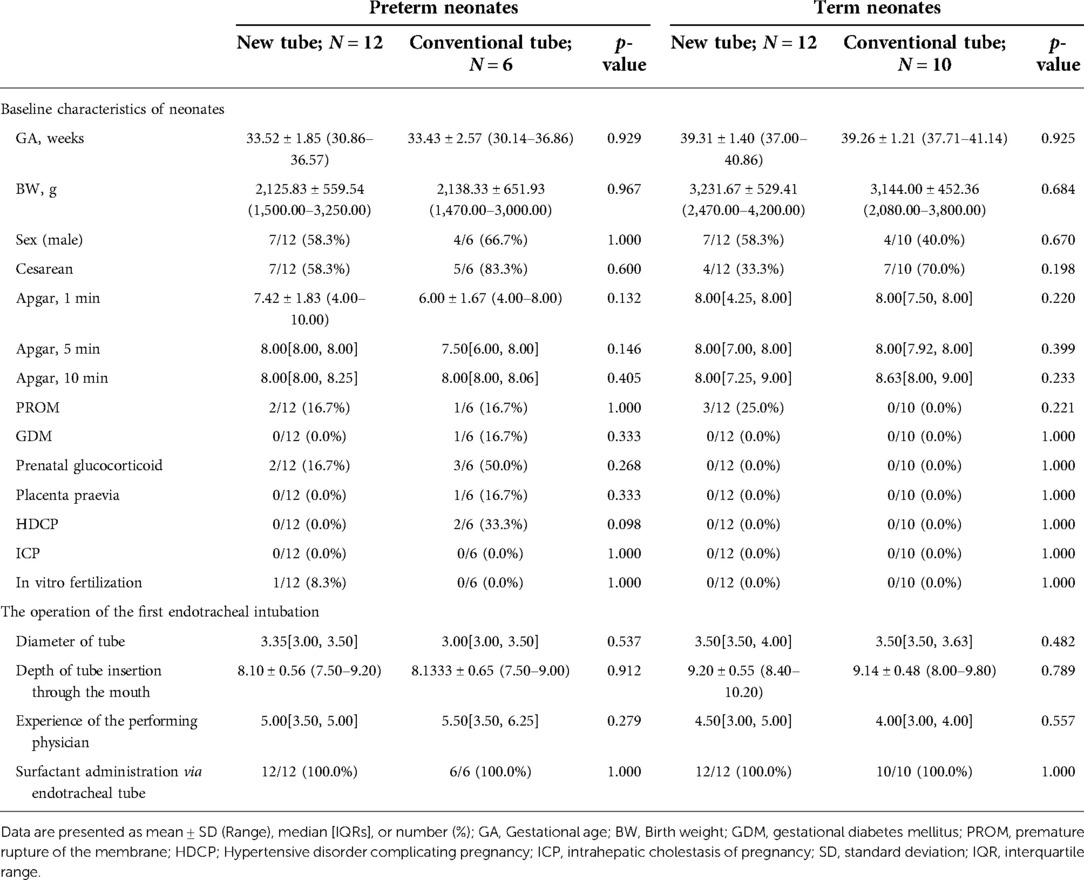
Table 5. Basic information on preterm and term neonates requiring surfactant administration via endotracheal tube during mechanical ventilation.
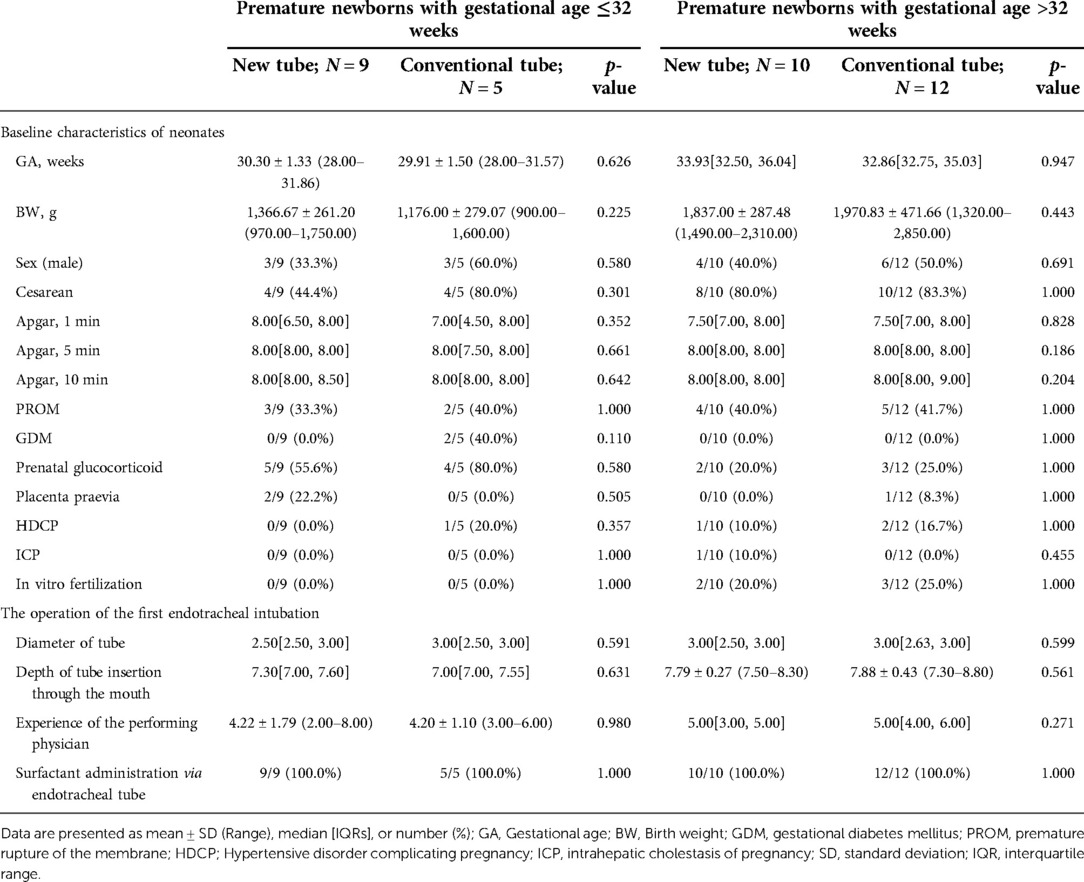
Table 6. Basic information on premature newborns with gestational age ≤32 weeks and >32 weeks, receiving the inSurE technique.
No statistical differences were found between the two groups in terms of available safety outcomes, either during (0/51 vs. 0/50, p = 1.000; Table 7) or after (2/51 vs. 3/50, p = 0.678; Table 7) intubation, even after subgroup analyses based on the reason for intubation (p > 0.05; Table 8), whether the drug was administered endotracheally (p > 0.05; Table 9), and gestational age (p > 0.05; Tables 10, 11). Similarly, no significant association was found between the application of the novel double-lumen tracheal tube and safety outcome indicators after controlling for the confounding factor of gestational age or the purpose of intubation. (p > 0.05; Supplementary Tables S7–10).
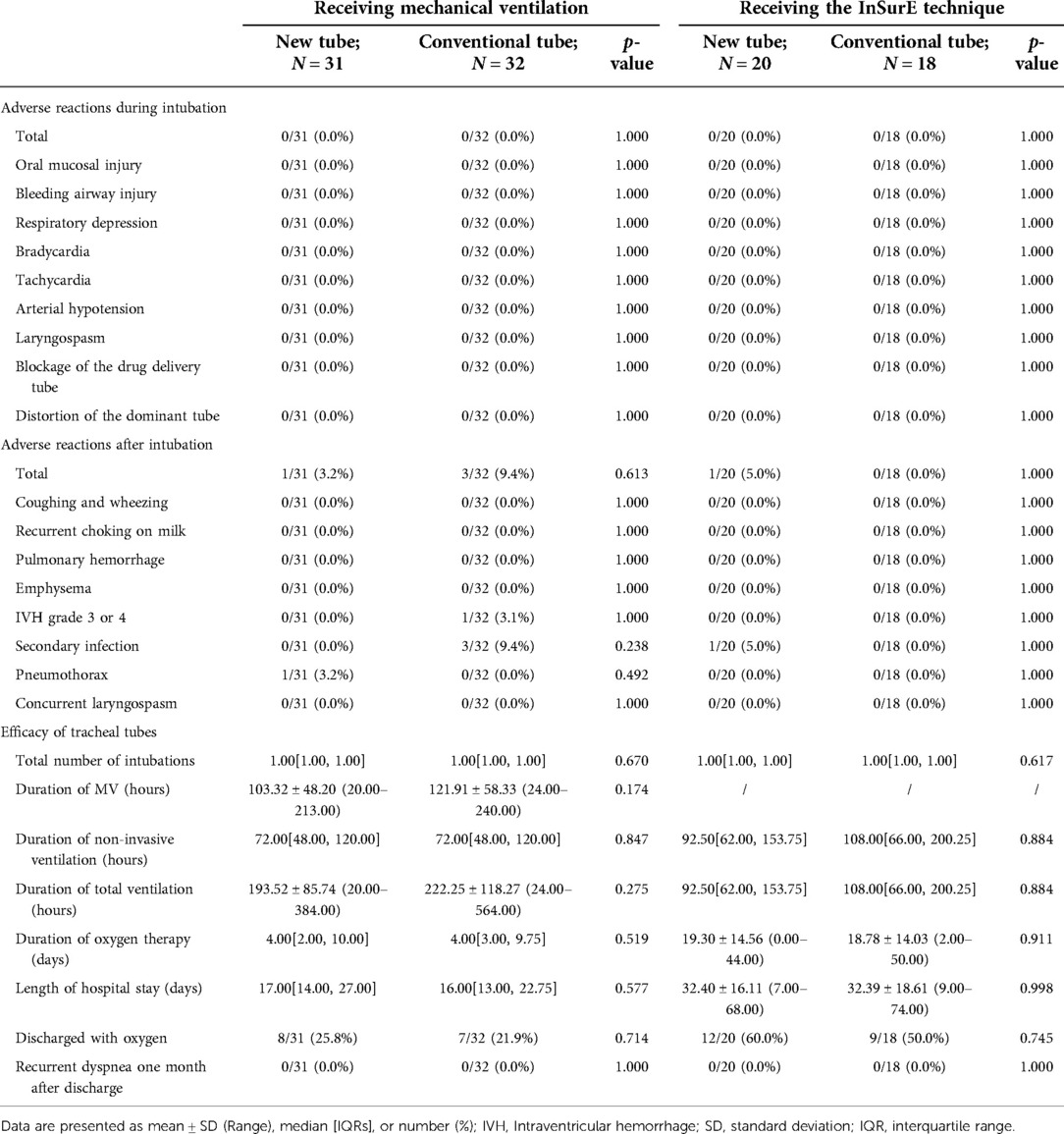
Table 8. Clinical outcomes of neonates receiving invasive mechanical ventilation and the inSurE technique.
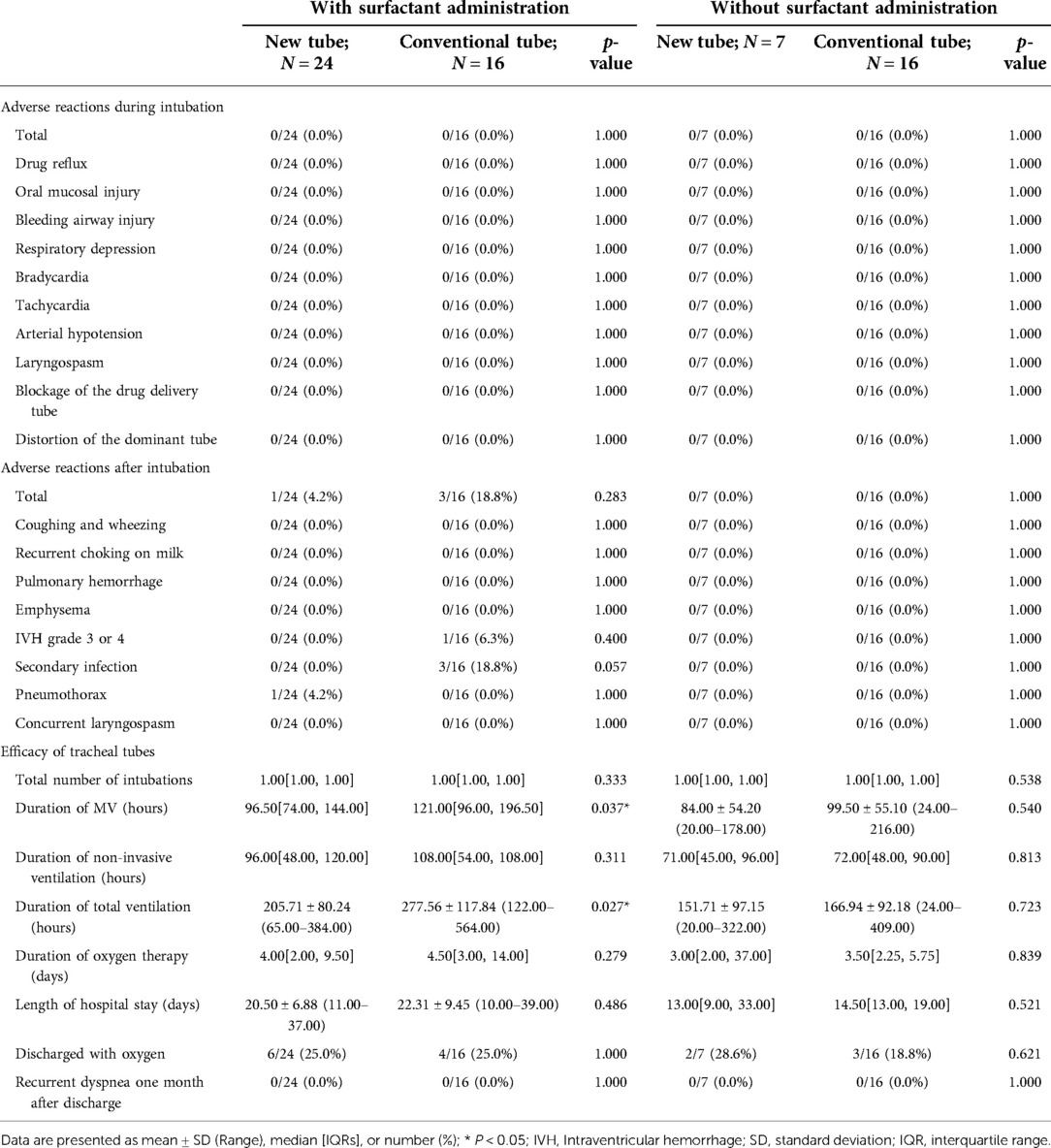
Table 9. Clinical outcomes of mechanically ventilated neonates with and without surfactant administration via endotracheal intubation.
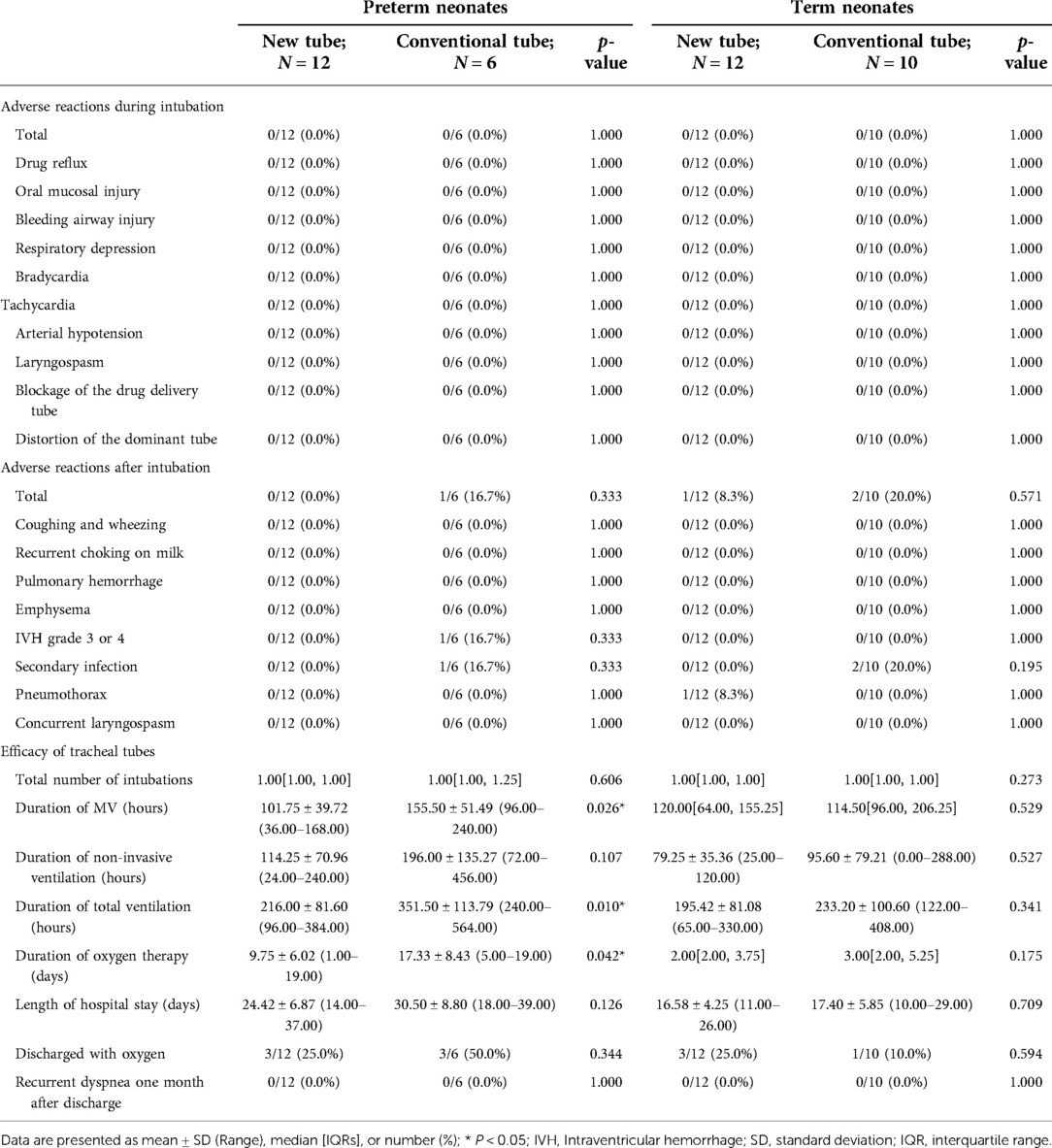
Table 10. Clinical outcomes of preterm and term neonates requiring surfactant administration via endotracheal tube during mechanical ventilation.
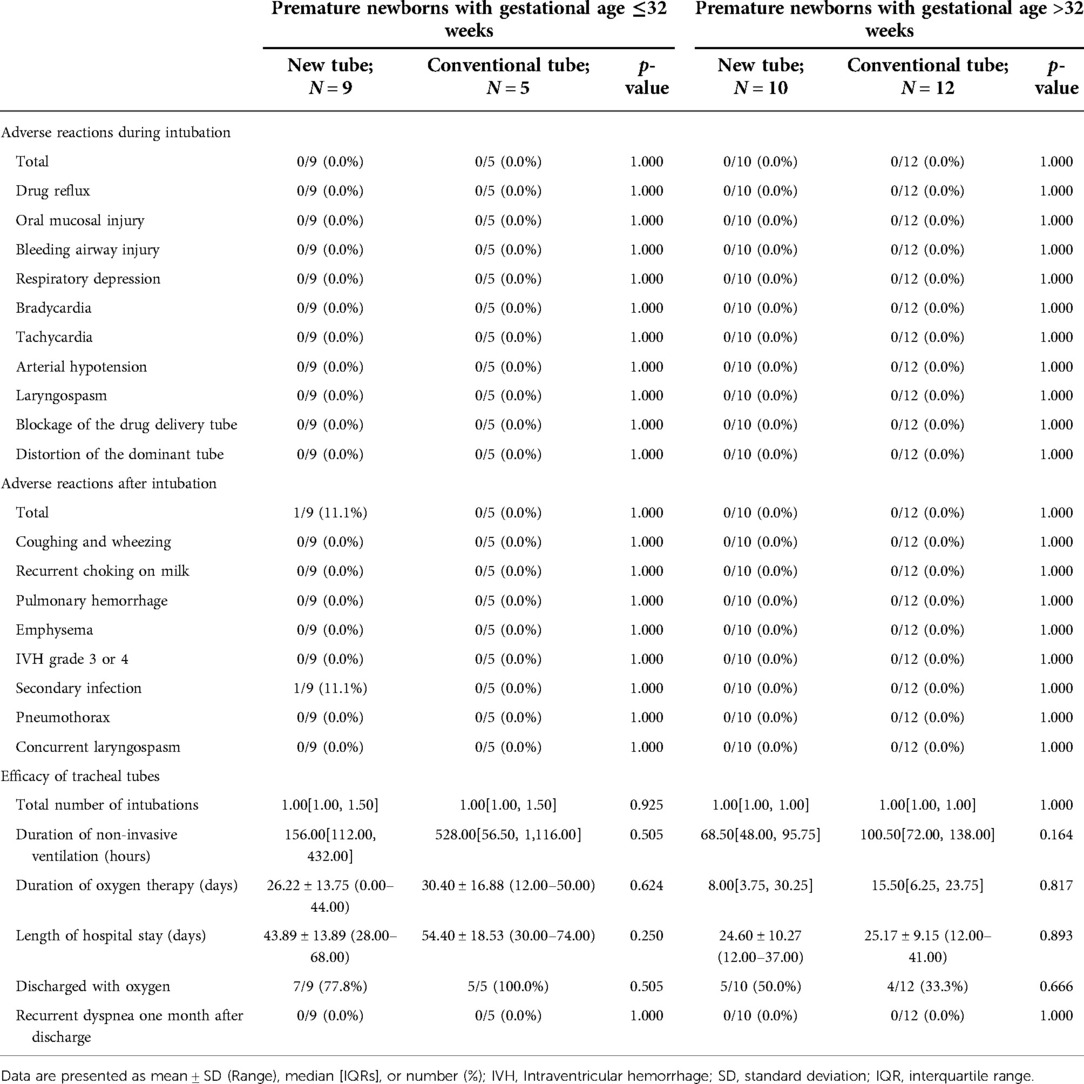
Table 11. Clinical outcomes of premature newborns receiving the inSurE technique with gestational age ≤32 weeks and >32 weeks.
The effectiveness of the new tracheal tube was evaluated using a conventional tracheal tube as a control, and the data showed no significant differences in all relevant indicators between the two groups of infants (p > 0.05; Table 7). A stratified analysis of the indicator “Discharged with oxygen” was performed according to gestational age (p = 0.856; Supplementary Table 11) and purpose of intubation (p = 0.493; Supplementary Table 11), and the data showed no significant association between the application of the new tracheal tube and the clinical outcome; for other outcome indicators targeting effectiveness, the stratified analysis was not applicable. Considering the limitations of the stratified analysis and the heterogeneity among study subjects, we performed some detailed subgroup analyses based on the above criteria (Tables 8–11).
For mechanically ventilated infants requiring surfactant administration (n = 40; GA fluctuating between 30.14 and 41.14 weeks), the application of the new tracheal tube resulted in a significant reduction in the duration of mechanical ventilation (96.50[74.00, 144.00] vs. 121.00[96.00, 196.50] hours, p = 0.037; Table 9) and total ventilation (205.71 ± 80.24 vs. 277.56 ± 117.84 h, p = 0.027; Table 9). However, there were no statistical differences between the two groups in terms of the number of intubations (1.00[1.00, 1.00] vs. 1.00[1.00, 1.00], p = 0.333; Table 9), duration of non-invasive ventilation (96.00[48.00, 120.00] vs. 108.00[54.00, 108.00] hours, p = 0.311; Table 9), duration of oxygen therapy (4.00[2.00, 9.50] vs. 4.50[3.00, 14.00] days, p = 0.279; Table 9), length of stay (20.50 ± 6.88 vs. 22.31 ± 9.45 days, p = 0.486; Table 9), rate of discharge with oxygen (6/24 vs. 4/16, p = 1.000; Table 9), and rate of recurrent dyspnea one month after discharge (0/24 vs. 0/16, p = 1.000; Table 9). Further analysis was performed according to gestational age (Table 10), and the data showed that for preterm infants (n = 18; GA fluctuating between 30.14 and 36.86 weeks), the new tracheal tube not only shortened the duration of mechanical ventilation (101.75 ± 39.72 vs. 155.50 ± 51.49 h, p = 0.026; Table 10) and total ventilation (216.00 ± 81.60 vs. 351.50 ± 113.79 h, p = 0.010; Table 10), but also demonstrated significant advantages in reducing the duration of oxygen therapy (9.75 ± 6.02 vs. 17.33 ± 8.43 days, p = 0.042; Table 10); interestingly, however, there was no statistical difference in efficacy outcomes between the two groups in full-term infants (n = 22; GA fluctuating between 37.00 and 41.14 weeks) (p > 0.05; Table 10).
For mechanically ventilated infants without endotracheal surfactant administration (n = 23; GA fluctuating between 33.71 and 42.29 weeks), there were no significant differences between the two groups regarding any effectiveness indicators (p > 0.05; Table 9). Notably, all patients in this subgroup were full-term (n = 22; GA fluctuating between 37.29 and 42.29 weeks), except for one preterm infant with a GA of 33.71 weeks in the novel catheter group. Therefore, no further subgroup analysis was performed based on gestational age.
In the detailed analysis of the effectiveness of the new catheter in infants with the InSurE technique (n = 38; GA fluctuating between 28.00 and 37.57 weeks), no statistically significant differences were found (p > 0.05; Table 8). Notably, the duration of non-invasive ventilation appeared to be shorter in neonates who opted for the new tracheal tube (n = 20; GA fluctuating between 28.00 and 37.57 weeks) than in those with the conventional tube (n = 18; GA fluctuating between 28.00 and 37.14 weeks), although the difference was not statistically significant (92.50[62.00, 153.75] vs. 108.00[66.00, 200.25] hours, p = 0.884; Table 8). Similarly, subgroup analysis was performed based on the gestational age, and no statistical differences were found in either subgroup (p > 0.05; Table 11).
In general, based on the available data, it can be tentatively determined that such a design is effective and safe for neonates with RDS, both preterm and term, regardless of whether the newborn receives invasive mechanical ventilation or the InSurE technique and whether the tracheal tube serves as a bridge for ventilation or a route for drug delivery. Given the design philosophy of this dual-lumen catheter, the investigation of its safety and effectiveness focused on newborns requiring surfactant administration via the tracheal tube during mechanical ventilation. In this target population, the application of the new catheter significantly reduced the duration of mechanical ventilation, total ventilation, and oxygen therapy in preterm infants; however, such an advantage was not evident in term neonates. Moreover, no significant differences were found between the new and conventional tracheal tubes regarding safety outcomes, either in the full-term or preterm infants subgroup. Furthermore, in neonates mechanically ventilated without surfactant administration and in neonates receiving the InSurE technique, the dual-lumen tracheal tube showed similar safety and efficacy to conventional tracheal tubes. Given the design rationale for this catheter, such data are actually consistent with our expectations and suggest that the application of this new catheter may not be limited to neonates requiring endotracheal surfactant administration during mechanical ventilation, although it was designed primarily for this group.
Notably, the rate of intratracheal surfactant administration was significantly higher in the new tracheal tube group compared to the conventional tracheal tube group. Considering that the primary purpose of endotracheal intubation is different for neonates receiving mechanical ventilation and those with the InSurE technique, we performed a subgroup analysis accordingly. The data showed that for mechanically ventilated neonates, the proportion of preterm infants and the rate of surfactant administration were significantly greater in the new tracheal tube group than in the conventional tracheal tube group; while for neonates receiving the InSurE technique, no significant differences were found between the two groups. Similarly, a stratified analysis based on the purpose of intubation suggested that mechanically ventilated neonates were more likely to face endotracheal surfactant administration when a double-lumen tracheal tube was selected. Such data suggest that the double-lumen tracheal tube may lead to worse ventilation in mechanically ventilated neonates, which casts a shadow on the effectiveness and safety of the new catheter. However, it is unreasonable to attribute a higher surfactant administration rate to the use of new tracheal tubes without eliminating the effect of prematurity on the outcome. In this context, a stratified analysis according to gestational age (Premature and full-term) for mechanically ventilated neonates was born, and the data showed no significant association between the choice of the new tracheal tube and the need for endotracheal surfactant therapy after excluding the confounding factor of prematurity. Therefore, it is reasonable to suspect that the higher rate of endotracheal surfactant administration is related to the characteristics of the participants themselves. Nevertheless, given the statistical validity limited by the small sample size in this study, we still cannot completely exclude the risk of the new tracheal tube leading to worse ventilation or other poor outcomes. In further randomized controlled trials with large sample sizes, a targeted assessment of the various risks that may be associated with a dual-lumen tracheal tube is necessary.
Even with the popularity of minimally invasive and noninvasive surfactant administration technologies, mechanical ventilation combined with drug administration via endotracheal tube remains an irreplaceable treatment protocol. However, due to the limitations of the conventional tracheal tube, temporary interruptions of mechanical ventilation are unavoidable, which may lead to an increased risk of various adverse effects (28, 29). Our study confirmed that for neonates requiring endotracheal surfactant during mechanical ventilation, this dual-lumen tube could avoid interruption of ventilation and facilitate ventilator withdrawal while ensuring safety. Furthermore, the applicability of such a design was also demonstrated in other groups, including mechanically ventilated neonates without endotracheal surfactant therapy and neonates receiving the InSurE technique. If the clinical feasibility can be confirmed in further studies, such a design may have the potential to replace conventional catheters for volume use in various centers.
To our knowledge, this is the second prospective report on a double-lumen tracheal tube for newborns, while the first prospective study was provided by Valls-i-Soler et al. from Spain in 1998 (26). In the design of Valls-i-Soler et al., the drug delivery tube and the dominant tube are always separate and unconnected, with the end of the drug delivery tube opening directly into the trachea to minimize the possibility of drug wastage. However, such a design may present other drawbacks or risks, such as uneven drug distribution, direct irritation of the trachea due to the greater pressure generated by rapid drug injection, and the risk of blockage of the Murphy Eye. Notable, the Murphy Eye can provide an alternative route to maintain ventilation when the main opening of the catheter is blocked for various reasons. On balance, in our design, the opening of the drug delivery tube was designed to be at the upper end of the Murphy Eye, allowing the drug to enter the trachea in as precise a dose as possible while diluting the sputum around the Murphy Eye to relieve the blockage. Furthermore, with the buffering space provided by the Murphy eye, the direct irritation to the fragile airways of the newborn due to the greater pressure generated by rapid drug injection can be reduced. Moreover, the combined effect of the buffer space and the airflow in the main tube may allow for a relatively even distribution of the drug, preventing it from always entering the trachea along the same side of the inner wall. In addition, a plastic cap was designed to replace the valve as a closure for the drug injection port in order to reduce costs.
In the trial by Valls-i-Soler et al. (26), no statistical differences were found between the effectiveness of the two tracheal tubes, as measured by the setting of respiratory parameters, duration of mechanical ventilation, and the number of surfactant doses required. In contrast, the effectiveness demonstrated by our new tracheal tube is more impressive in a population with the same attributes, i.e., neonates requiring intratracheal surfactant administration during mechanical ventilation, especially premature neonates. The shortened duration of mechanical ventilation may be closely related to the design advantages of the dual-lumen catheter, although accurate and in-depth validation is not yet available. In addition, such a significant advantage may also be related to the success of the new catheter in avoiding temporary interruption of mechanical ventilation. In our study, neonates who opted for mechanical ventilation were usually in a more unstable state compared to those on non-invasive ventilation. When ventilation was suspended followed by surfactant administration in this situation, fluctuations in oxygen saturation cannot be completely avoided, even if the respiratory parameters were appropriately adjusted immediately after the operation. Therefore, although the duration of the endotracheal surfactant administration was limited to a few minutes, neonates with conventional catheters might still face an unstable internal environment and hemodynamics for a short period of time, which would increase the need for subsequent mechanical ventilation in this vulnerable population (30).
However, such an essential advantage of the new catheter failed to be demonstrated in full-term infants, which may be related to the fact that the drug administered intratracheally in our study was limited to surfactant. Unlike preterm neonates, the mechanism underlying RDS in term newborns is not dominated by a deficiency or dysfunction of pulmonary surfactant; therefore, term newborns are generally less responsive to surfactant therapy (31, 32). Nevertheless, it is unreasonable to interpret our results as an amplification of the efficacy of surfactant in preterm newborns by the new tracheal tube based on these speculations alone. Studies with larger sample sizes are needed to rule out the possibility that the effectiveness of the new tracheal tube itself varies by gestational age.
There is no precise conclusion on the optimal mode of intratracheal surfactant administration (33). However, a meta-analysis of preterm infants with respiratory distress syndrome showed that surfactant administration via a thin catheter reduced the risk of death and BPD compared with that via a tracheal tube (34). Besides, a net meta-analysis showed that among the various popular methods of endotracheal surfactant administration, surfactant administration via a fine catheter could show a more substantial advantage in reducing mortality, the need for mechanical ventilation, and the risk of BPD (35). Therefore, after validating the clinical applicability of the dual-lumen tracheal catheter, it is necessary to shift the focus of the study to a comparison between the dual-lumen tracheal catheter and the thin catheter to update the assessment of the optimal surfactant delivery method. However, it is necessary to emphasize differences in the target populations for tracheal tubes and thin catheters. While the thin catheter is primarily used in neonates with spontaneous breathing and stable hemodynamics (36), the advantages of the new dual-lumen catheter focus on neonates receiving mechanical ventilation, although still suitable for neonates requiring tracheal intubation in other situations. It is this difference that establishes the unassailable position of surfactant administration via the tracheal tube. Therefore, even though the surfactant administration via a thin catheter demonstrates a stronger advantage in comparison with the InSurE technique with a double-lumen tracheal tube, the effectiveness of the double-lumen tracheal tube for mechanically ventilated neonates remains irreplaceable.
Overall, there are still some limitations in our study. First of all, this is a single-center study from China, and its statistical validity was limited by the small sample size. Moreover, the study population was limited to neonates with RDS, and all drugs administered intratracheally were surfactants. Furthermore, no subgroup analysis was performed based on the severity of RDS. In our study, repeated administration of surfactant was determined flexibly by the clinician based on the condition of the newborn, so that neonates with more severe symptoms may receive more frequent surfactant treatment. From a theoretical point of view, the advantages of the dual-lumen catheter should have been maximized in this particular group, especially those on mechanical ventilation, but unfortunately, this idea was not reflected in our study. In addition, there was no long-term follow-up of respiratory and neurological function.
To address these limitations, further multicenter prospective randomized trials with large sample sizes and long-term follow-up of multisystem functions are being planned. Some detailed subgroup analysis can be performed on the basis of large sample sizes, and assessments for a specific population, such as those at high risk for repeat dosing (37, 38), can also be considered, which will help us to assess the target population and the applicable scope of this catheter. Besides, the expanded sample size should not be limited to neonates with RDS requiring surfactant therapy; other therapies administered via the tracheal tube or surfactant therapies for other diseases deserve to be further explored. What's more, neonates receiving both the InSurE technique and invasive mechanical ventilation will be included in further clinical trials to assess the impact of a dual-lumen tracheal tube on the success rate of the InSurE technique. In addition, the double-lumen tracheal tube can be compared with other new tracheal tubes, and the method of surfactant administration via the new tracheal tube can also be compared with other techniques of endotracheal surfactant administration, to allow for a tailored program for newborns when conditions permit.
In general, preliminary evidence suggests that our new dual-lumen tracheal tube is safe and effective in neonates with RDS, either as an intermediary for ventilation or as a conduit for surfactant administration, but especially in preterm infants requiring intratracheal surfactant therapy during mechanical ventilation. Nevertheless, these results should be viewed with caution, given the limitations. Multicenter prospective randomized trials with large sample sizes are urgently needed for a more detailed assessment.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by Research Ethics Committee in the Qujing Maternal and Child Healthcare Hospital. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
CL and YD conceived and designed the study. KY and HY contributed substantially to enrolment, measurements and data collection. HC, CZ and XL performed the data analysis and prepared the figures and the tables. YD and CL wrote the manuscript. YS and XD revised it critically for important intellectual content. All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work. CL and YD contributed equally to this work and share first authorship. XD and YS contributed equally to this work and share corresponding authorship. All authors contributed to the article and approved the submitted version.
This research was funded by the Special Key Project for Technology Innovation and Application Development of Chongqing Science and Technology Bureau (CSTC2021jscx-gksb-N0015).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.1032044/full#supplementary-material.
1. Banerjee S, Fernandez R, Fox GF, Goss K, Mactier H, Reynolds P, et al. Surfactant replacement therapy for respiratory distress syndrome in preterm infants: united Kingdom national consensus. Pediatr Res. (2019) 86(1):12–4. doi: 10.1038/s41390-019-0344-5
2. Bahadue FL, Soll R. Early versus delayed selective surfactant treatment for neonatal respiratory distress syndrome. Cochrane Database Syst Rev. (2012) 11(11):Cd001456. doi: 10.1002/14651858.CD001456.pub2
3. SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network, Finer NN, Carlo WA, Walsh MC, Rich W, Gantz MG, Laptook AR, et al. Early CPAP versus surfactant in extremely preterm infants. N Engl J Med. (2010) 362(21):1970–9. doi: 10.1056/NEJMoa0911783
4. Terragni P, Ranieri VM, Brazzi L. Novel approaches to minimize ventilator-induced lung injury. Curr Opin Crit Care. (2015) 21(1):20–5. doi: 10.1097/MCC.0000000000000172
5. Natarajan G, Pappas A, Shankaran S, Kendrick DE, Das A, Higgins RD, et al. Outcomes of extremely low birth weight infants with bronchopulmonary dysplasia: impact of the physiologic definition. Early Hum Dev. (2012) 88(7):509–15. doi: 10.1016/j.earlhumdev.2011.12.013
6. Barkhuff WD, Soll RF. Novel surfactant administration techniques: will they change outcome? Neonatology. (2019) 115(4):411–22. doi: 10.1159/000497328
7. Choubey R, Pillai A, Kabra N. Aerosolized surfactant: what is the cost of avoiding intubation? Pediatrics. (2021) 147(3):e2020047985A. doi: 10.1542/peds.2020-047985A
8. Gupta BK, Saha AK, Mukherjee S, Saha B. Minimally invasive surfactant therapy versus InSurE in preterm neonates of 28 to 34 weeks with respiratory distress syndrome on non-invasive positive pressure ventilation-a randomized controlled trial. Eur J Pediatr. (2020) 179(8):1287–93. doi: 10.1007/s00431-020-03682-9
9. Mehler K, Broer A, Roll C, Göpel W, Wieg C, Jahn P, et al. Developmental outcome of extremely preterm infants is improved after less invasive surfactant application: developmental outcome after LISA. Acta Paediatr. (2021) 110(3):818–25. doi: 10.1111/apa.15565
10. Devi U, Roberts KD, Pandita A. A systematic review of surfactant delivery via laryngeal mask airway, pharyngeal instillation, and aerosolization: methods, limitations, and outcomes. Pediatr Pulmonol. (2022) 57(1):9–19. doi: 10.1002/ppul.25698
11. Pfister RH, Soll RF. Initial respiratory support of preterm infants: the role of CPAP, the INSURE method, and noninvasive ventilation. Clin Perinatol. (2012) 39(3):459–81. doi: 10.1016/j.clp.2012.06.015
12. Kakkilaya V, Gautham KS. Should less invasive surfactant administration (LISA) become routine practice in US neonatal units? Pediatr Res. (2022) 1–11. doi: 10.1038/s41390-022-02265-8
13. Chen L, Wang L, Li J, Wang N, Shi Y. Noninvasive ventilation for preterm twin neonates with respiratory distress syndrome: a randomized controlled trial. Sci Rep. (2015) 5:14483. doi: 10.1038/srep14483
14. Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, Te Pas A, et al. European consensus guidelines on the management of respiratory distress syndrome—2019 update. Neonatology. (2019) 115(4):432–50. doi: 10.1159/000499361
15. Cao H, Li H, Zhu X, Wang L, Yi M, Li C, et al. Three non-invasive ventilation strategies for preterm infants with respiratory distress syndrome: a propensity score analysis. Arch Med Sci. (2020) 16(6):1319–26. doi: 10.5114/aoms.2020.93541
16. Chen L, Wang L, Ma J, Feng Z, Li J, Shi Y. Nasal high-frequency oscillatory ventilation in preterm infants with respiratory distress syndrome and ARDS after extubation: a randomized controlled trial. Chest. (2019) 155(4):740–8. doi: 10.1016/j.chest.2019.01.014
17. Liu K, Chen L, Xiong J, Xie S, Hu Y, Shi Y. HFOV Vs CMV for neonates with moderate-to-severe perinatal onset acute respiratory distress syndrome (NARDS): a propensity score analysis. Eur J Pediatr. (2021) 180(7):2155–64. doi: 10.1007/s00431-021-03953-z
18. Chawla S, Natarajan G, Gelmini M, Kazzi SN. Role of spontaneous breathing trial in predicting successful extubation in premature infants. Pediatr Pulmonol. (2013) 48(5):443–8. doi: 10.1002/ppul.22623
19. Zhu J, Bao Y, Du L, Huang H, Lv Q, Jiang Y, et al. Less invasive surfactant administration versus endotracheal surfactant instillation followed by limited peak pressure ventilation in preterm infants with respiratory distress syndrome in China: study protocol for a randomized controlled trial. Trials. (2020) 21(1):516. doi: 10.1186/s13063-020-04390-3
20. Dunn MS, Kaempf J, de Klerk A, de Klerk R, Reilly M, Howard D, et al. Randomized trial comparing 3 approaches to the initial respiratory management of preterm neonates. Pediatrics. (2011) 128(5):e1069–76. doi: 10.1542/peds.2010-3848
21. Singh N, Halliday HL, Stevens TP, Suresh G, Soll R, Rojas-Reyes MX. Comparison of animal-derived surfactants for the prevention and treatment of respiratory distress syndrome in preterm infants. Cochrane Database Syst Rev. (2015) 2015(12):CD010249. doi: 10.1002/14651858.CD010249.pub2
22. Laughon M, Bose C, Moya F, Aschner J, Donn SM, Morabito C, et al. A pilot randomized, controlled trial of later treatment with a peptide-containing, synthetic surfactant for the prevention of bronchopulmonary dysplasia. Pediatrics. (2009) 123(1):89–96. doi: 10.1542/peds.2007-2680
23. Polin RA, Carlo WA, Committee on Fetus and Newborn, American Academy of Pediatrics. Surfactant replacement therapy for preterm and term neonates with respiratory distress. Pediatrics. (2014) 133(1):156–63. doi: 10.1542/peds.2013-3443
24. Roberts CT, Halibullah I, Bhatia R, Green EA, Kamlin C, Davis PG, et al. Outcomes after introduction of minimally invasive surfactant therapy in two Australian tertiary neonatal units. J Pediatr. (2021) 229:141–6. doi: 10.1016/j.jpeds.2020.10.025
25. Dempsey EM. What should we do about low blood pressure in preterm infants. Neonatology. (2017) 111(4):402–7. doi: 10.1159/000460603
26. Valls-i-Soler A, Fernández-Ruanova B, López-Heredia y Goya J, Román Etxebarría L, Rodriguez-Soriano J, Carretero V. A randomized comparison of surfactant dosing via a dual-lumen endotracheal tube in respiratory distress syndrome. The Spanish Surfactant Collaborative Group. Pediatrics. (1998) 101(4):E4. doi: 10.1542/peds.101.4.e4
27. Moya F, Sinha S, D’Agostino RB Sr. Surfactant-replacement therapy for respiratory distress syndrome in the preterm and term neonate: congratulations and corrections. Pediatrics. (2008) 121(6):1290–2. doi: 10.1542/peds.2008-0710
28. Danan C, Durrmeyer X, Brochard L, Decobert F, Benani M, Dassieu G. A randomized trial of delayed extubation for the reduction of reintubation in extremely preterm infants. Pediatr Pulmonol. (2008) 43(2):117–24. doi: 10.1002/ppul.20726
29. Lee BK, Shin SH, Jung YH, Kim EK, Kim HS. Comparison of NIV-NAVA and NCPAP in facilitating extubation for very preterm infants. BMC Pediatr. (2019) 19(1):298. doi: 10.1186/s12887-019-1683-4
30. Lal M, Tin W, Sinha S. Automated control of inspired oxygen in ventilated preterm infants: crossover physiological study. Acta Paediatr. (2015) 104(11):1084–9. doi: 10.1111/apa.13137
31. Sun H, Xu F, Xiong H, Kang W, Bai Q, Zhang Y, et al. Characteristics of respiratory distress syndrome in infants of different gestational ages. Lung. (2013) 191(4):425–33. doi: 10.1007/s00408-013-9475-3
32. Jain L, Eaton DC. Physiology of fetal lung fluid clearance and the effect of labor. Semin Perinatol. (2006) 30(1):34–43. doi: 10.1053/j.semperi.2006.01.006
33. Fortas F, Loi B, Centorrino R, Regiroli G, Ben-Ammar R, Shankar-Aguilera S, et al. Enhanced INSURE (ENSURE): an updated and standardised reference for surfactant administration. Eur J Pediatr. (2022) 181(3):1269–75. doi: 10.1007/s00431-021-04301-x
34. Abdel-Latif ME, Davis PG, Wheeler KI, De Paoli AG, Dargaville PA. Surfactant therapy via thin catheter in preterm infants with or at risk of respiratory distress syndrome. Cochrane Database Syst Rev. (2021) 5(5):Cd011672. doi: 10.1002/14651858.CD011672.pub2
35. Bellos I, Fitrou G, Panza R, Pandita A. Comparative efficacy of methods for surfactant administration: a network meta-analysis. Arch Dis Child Fetal Neonatal Ed. (2021) 106(5):474–87. doi: 10.1136/archdischild-2020-319763
36. Devi U, Pandita A. Surfactant delivery via thin catheters: methods, limitations, and outcomes. Pediatr Pulmonol. (2021) 56(10):3126–41. doi: 10.1002/ppul.25599
37. Greiner E, Wittwer A, Albuisson E, Hascoët JM. Outcome of very premature newborn receiving an early second dose of surfactant for persistent respiratory distress syndrome. Front Pediatr. (2021) 9:663697. doi: 10.3389/fped.2021.663697
Keywords: newborn resuscitation, endotracheal intubation, invasive mechanical ventilation, respiratory distress syndrome, surfactant replacement therapy
Citation: Li C, Du Y, Yang K, Cao H, Yang H, Zhang C, Li X, Deng X and Shi Y (2022) Safety and efficacy of a novel double-lumen tracheal tube in neonates with RDS: A prospective cohort study. Front. Pediatr. 10:1032044. doi: 10.3389/fped.2022.1032044
Received: 30 August 2022; Accepted: 16 November 2022;
Published: 5 December 2022.
Edited by:
Laila Springer, University Children’s Hospital Tübingen, GermanyReviewed by:
Kari Roberts, University of Minnesota Children’s Hospital, United States© 2022 Li, Du, Yang, Cao, Yang, Zhang, Li, Deng and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xingmei Deng ZHhtMTM4ODcxOTg4NjZAMTI2LmNvbQ== Yuan Shi c2hpeXVhbkBob3NwaXRhbC5jcW11LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
‡These authors contributed equally to this work
Specialty Section: This article was submitted to Neonatology, a section of the journal Frontiers in Pediatrics
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.