- 1Department of Child Health, Dr. Cipto Mangunkusumo Hospital, Faculty of Medicine, University of Indonesia, Jakarta, Indonesia
- 2Clinical Epidemiology and Evidence-Based Medicine Unit, Dr. Cipto Mangunkusumo Hospital, Faculty of Medicine, University of Indonesia, Jakarta, Indonesia
Introduction: Several scoring systems are available to assess the severity of sepsis in pediatric patients in diverse settings worldwide. This study investigates the quality and applicability of predictive models for determining pediatric sepsis mortality, especially in acute care and limited-resource settings.
Data sources: Mortality prediction factors and models were searched in four databases using the following criteria: developed for pediatric health care, especially in acute settings, and with mortality as an outcome.
Study selection: Two or more reviewers performed the study selection to ensure no bias occurred. Any disagreements were solved by consensus or by the decision of a third reviewer.
Data extraction: The authors extracted the results and mapped the selected studies qualitatively to describe the prognostic properties of the risk factors and models proposed in the study.
Data synthesis: The final analysis included 28 mortality prediction models. Their characteristics, analysis, and performance measures were summarized. Performance was described in terms of calibration and discrimination, including assessing for risk of bias and applicability. A modified version of the PRISM-III score based on physiologic criteria (PRISM-III-APS) increased its predictive value to 0.85–0.95. The vasoactive-inotropic score at 12 h had a strong independent association with death. Albumin had an excellent predictive value when combined with other variables. Lactate, a biomarker widely measured in patients with sepsis, was highly associated with mortality. The bioimpedance phase angle was not considered applicable in our setting. Measurement using more straightforward methods, such as mid-upper arm circumference, was feasible in numerous health care facilities.
Conclusion: Leveraging prognostic models to predict mortality among pediatric patients with sepsis remains an important and well-recognized area of study. While much validation and development work remains to be done, available prognostic models could aid clinicians at the bedside of children with sepsis. Furthermore, mortality prediction models are essential and valuable tools for assessing the quality of care provided to critically ill pediatric patients.
Introduction
Sepsis is a systemic inflammatory response syndrome triggered by infections caused by various pathogens, resulting in severe sepsis and septic shock (1). Sepsis remains a significant cause of morbidity, mortality, and high health care costs in the pediatric population worldwide (2). In the United States, the incidence of severe sepsis was 5.16 per 1,000 infants (1). In children in pediatric intensive care units (PICU) in developing countries, the sepsis mortality rate is higher than 50% (2). The World Health Organization has estimated that sepsis causes 4 million deaths per year worldwide in children under five years old (3).
Several scoring systems are available to assess the severity of sepsis in pediatric patients (4–6). However, these systems were created across many different settings worldwide; they therefore might not be ideal for pediatric patients with sepsis in developing countries or in otherwise resource-limited settings. Thus, a scoring model that can assess pediatric sepsis in a stratified manner is needed to guide physicians in promptly treating these patients, particularly in acute care settings during the initial stages of sepsis (5, 7, 8). Although sepsis is one of the leading causes of mortality in hospitalized patients, information regarding predictive factors for mortality and morbidity is limited (2–4, 7, 9–13).
As a preliminary step, we searched for existing reviews of predictive factors and models to predict pediatric sepsis mortality in several databases and search platforms, such as PubMed, Cochrane Central, ProQuest, PROSPERO, the WHO Trial Registry, the Clinical Trial Registry, and Google Scholar, and did not find any similar studies. Therefore, we conducted this scoping review to provide a comprehensive, systematic overview of the various predictive models and scores available to guide clinicians in managing pediatric sepsis. This study investigates the quality and applicability of predictive models for assessing pediatric sepsis mortality, especially in acute care and resource-limited settings.
Methods
The main objective of the present review was to synthesize the evidence associated with broad research topics and to identify the forms of evidence available. The flexibility of this review method allowed us to broadly explore and incorporate different study designs. Although quality assessment is not included in this review, the methodology applied to synthesize this knowledge is systematic and thereby accessible for critical analysis.
This study explored the literature on the prognostic models available between 2010 and 2020 to predict pediatric sepsis mortality. In developing this study, we used the checklist of review processes from the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR). The framework of this study consists of several steps, including (1) identifying the research question, (2) identifying inclusion and exclusion criteria, (3) developing a search strategy and selecting evidence, (4) extracting and analyzing data, and (5) presenting the results.
Stage 1: Research question
The diversity among the available scoring systems for predicting sepsis mortality could lead to late diagnosis or misdiagnosis, thus potentially increasing the mortality rate. The research question was developed in consideration of this problem. The primary question was formulated by incorporating the Population, Concept, and Context (PCC) elements. Our primary question was “What prognostic models are available for predicting mortality in pediatric patients with sepsis?” and the sub-question was the application of those prognostic models in acute care, particularly in the crucial initial phases when decisions about further treatment must be made promptly, and in the context of resource-limited settings.
Stage 2: Inclusion and exclusion criteria
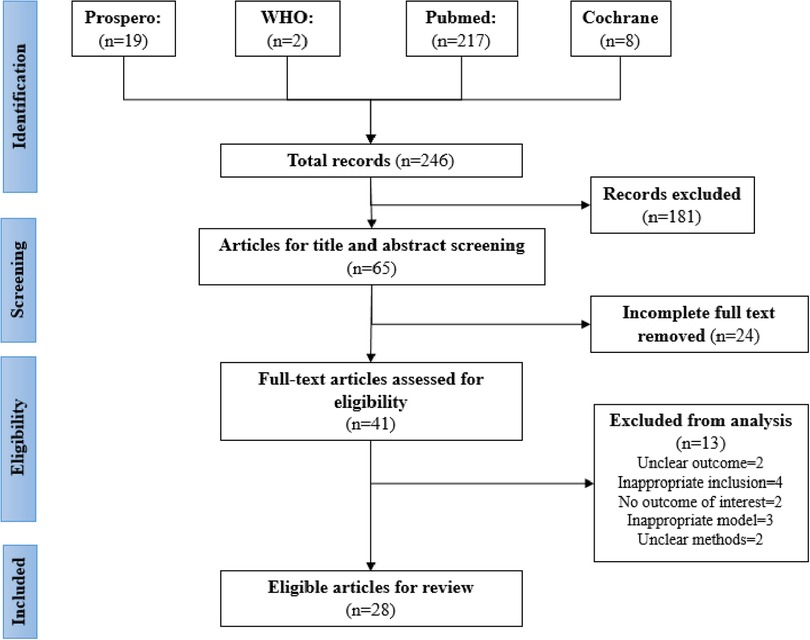
The inclusion and exclusion criteria for this study are shown in Figure 1. Evidence was selected based on the inclusion criteria, with the selection performed by two or more reviewers to ensure no bias occurred. Any disagreements were solved by consensus or by the decision of a third reviewer. The reviewers conducted their screening according to the PRISMA-ScR checklist. Ineligible papers were eliminated.
Stage 3: Search strategy and evidence selection
We searched MEDLINE (PubMed), PROSPERO, EMBASE, ProQuest, the WHO Registry, the Clinical Trial Registry, and the Cochrane Library using a tailored search strategy to identify all the relevant titles and abstracts of studies published in English between January 2010 and December 2020 that discussed predictive/prognostic scores or models that could be used in the management of sepsis. The main keywords in the search strategy were “prognostic” OR “predictive” OR “prognosis,” coupled with (AND) “model” OR “score,” coupled with (AND) “sepsis” OR “septic shock” OR “severe sepsis,” coupled with (AND) “pediatric” OR “paediatric” OR “child” OR “infant,” and excluding (NOT) “neonate” OR “neonates.” Gray literature was obtained by identifying similar articles in the references of eligible articles.
We excluded editorials, case studies, conference abstracts, unpublished studies, and expert commentaries. For studies with more than one publication of findings, we selected the most recent publication. We also excluded studies that contained models or scores aimed at diagnosing sepsis. We intended to limit the scope of the study to only those models that could be used to predict severity, mortality, or risk of complications. Three independent reviewers screened the titles and abstracts to ensure compliance with the inclusion and exclusion criteria mentioned above and settled any conflicts by mutual agreement.
Stage 4: Data extraction and analysis
The three independent reviewers used data extraction sheets that were prepared before screening to obtain the following details for inclusion in the final review: last name of the first author; date of publication; period of patient recruitment and follow-up; country of study; aims/purpose; sample size; age group; methodology; type of predictive model; the name of the model; and outcomes and how they were measured. The authors extracted the results and mapped the selected studies qualitatively to describe the prognostic properties of the models used to predict mortality as proposed in their respective studies.
Stage 5: Presentation of the results
We presented our results in a table to clarify which prognostic models are adequate to predict mortality rates in pediatric sepsis. This table also helped in identifying gaps where further studies are needed.
Results
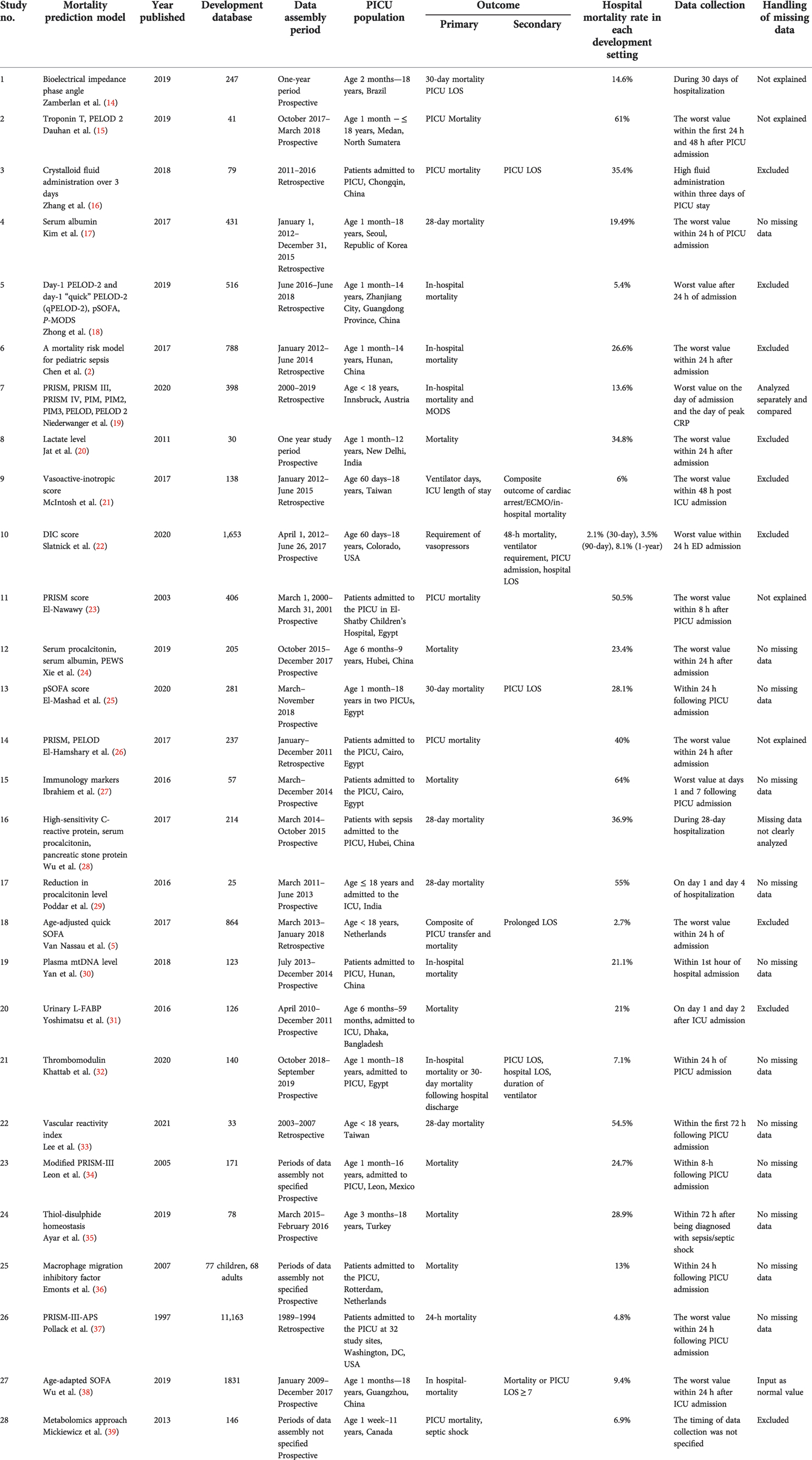
The selection of the source of evidence is described in Figure 1. Out of the 246 articles selected from four databases, 181 duplicate articles were excluded. Then, during screening, approximately 24 articles that had incomplete full text were removed. Articles that had unclear (two articles) or no (two articles) outcome of interest, inappropriate prediction models (three articles), inappropriate inclusion criteria (four articles), or unclear methods (two articles) were also excluded. The final review included 28 eligible articles, comprising nine studies with a single predictor, twelve studies with prognostic models [e.g., pediatric risk of mortality (PRISM), disseminated intravascular coagulation (DIC) score, vasoactive-inotropic score (VIS), pediatric logistic organ dysfunction (PELOD) score, or pediatric sequential organ failure assessment (pSOFA) scor], and seven studies that investigated the performance of a single predictor mixed with available predictor models. Tables 2–6 presents a comparison of the included studies.
Characteristics of the mortality prediction models
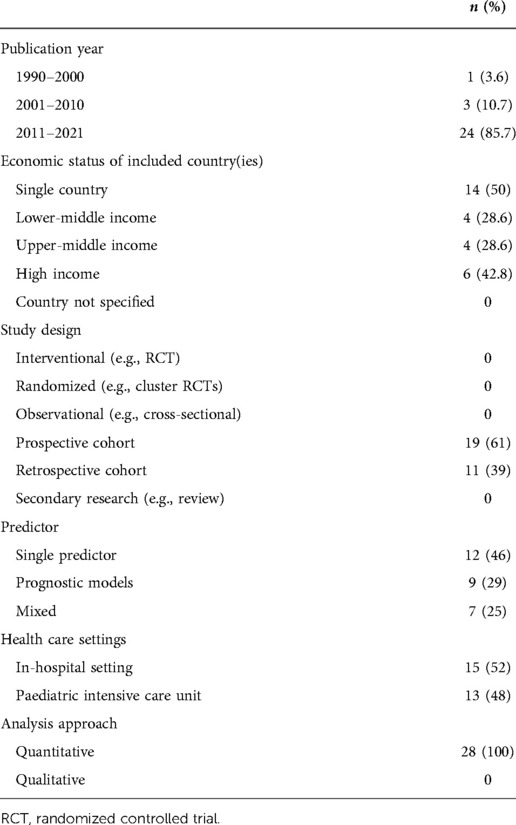
The characteristics of the mortality prediction models are presented in Tables 1, 5–7. Out of 28 prediction models, 19 were developed prospectively, while 11 used retrospectively collected data. The study durations varied from 4 months to 9 years. Three studies did not specify the study duration or data collection time (34, 36, 39). One study included both children and adult patients, and one study was multi-center (36). Eight studies did not specify the ages of their patients. Seven studies were done in developed countries (5, 19, 22, 35–37, 39). These studies included several parameters that are typically unavailable in developing countries, including pancreatic stone protein, macrophage migration inhibitory factors, plasma mitochondrial DNA, and metabolomic studies (28, 30, 36, 39). The number of included patients in each study ranged from 25 to 11,163. Several studies limited the participants to all patients admitted to the PICU (16, 23, 26–28, 30, 36, 37).
Outcomes measured
The timing of mortality outcomes varied between studies. Nevertheless, only eight studies specified the timing of mortality, and two had mortality as a secondary outcome (21, 22). Most studies did not specify the timing of mortality. Nine studies reported secondary outcomes such as length of hospital/PICU stay, ventilator requirement, or vasoactive agent usage (5, 16, 21, 22, 25, 32, 38). Mortality rates ranged between 4.8%and 64%. Missing data were excluded in seven studies (2, 5, 16, 18, 20–22, 31, 39). Only one study entered the missing data as normal values (38).
Discussion
The predictive value of each mortality model is shown in Table 2.
PELOD-2 had good predictive power. Estimating PELOD-2 at day 1 of admission had a high area under the curve (AUC) (0.916; 95% CI, 0.888–0.938). Even with some modification of PELOD-2, its AUC was still 0.802 (95% CI, 0.765–0.836) (18). Combining PELOD-2 with C-reactive protein (CRP) increased its predictive value to 0.84 (95% CI, 0.77–0.91) (19). These findings showed a better predictive value for PELOD-2 compared to PELOD. The SOFA score returned similar results. The predictive value of pSOFA was similar to that of PELOD. pSOFA measured at day 1 also had a high AUC (0.937; 95% CI, 0.913–0.957) (18). Its AUC showed good predictive value even with modifications such as qSOFA and age-adapted SOFA (with AUCs of 0.72 and 0.771, respectively) (5, 25). PRISM-III had lower predictive power than PELOD-2. Overall, the studies had a PRISM-III predictive power above 0.7 (19, 26, 34). A modification of PRISM-III based on physiologic criteria (PRISM-III-APS) increased its predictive value to 0.85–0.95 (38, 39).
The VIS had a strong independent association with death. For every unit increase of VIS at 12 h, there was a 14% increase in the odds of subsequently experiencing the composite outcome (p < 0.001). This finding was independent of the measured Pediatric Index of Mortality-3 (PIM3) score (21).
The Vascular Reactivity Index, defined as a systemic vascular resistance index subdivided by VIS (SVRI/VIS) measured at hour 0 in children with persistent refractory shock, had an AUC of 0.85—the highest measured (95% CI, 0.65–0.95; p = 0.001)—for predicting 28-day mortality when administered during the first 72 h. A VRI <18 at 0 h had 100% specificity for predicting mortality. The best cutoff values of the VRI increased from more than 30 at 0 h–12 h to more than 60 at 30 h–48 h. Most children with a cutoff VRI below 30 had a 100% likelihood of mortality, even after aggressive resuscitation, whereas most of those with a VRI >80 at 0 h–18 h and >100 at 24 h–48 h had the highest likelihood of survival (sensitivity 100%) (33).
Crystalloid fluid administration is associated with mortality in pediatric patients with severe sepsis or septic shock. The high crystalloid group (>193 ml/kg body weight) had a higher PICU mortality (46.2% vs. 25%; odds ratio [OR] 2.57; 95% CI, 0.99–6.67; p = 0.041) compared to the low crystalloid group (16).
Albumin had an excellent predictive value when combined with other variables. Albumin alone had a predictive power of 70.2%–76.1%. When combined with PIM3 and PRISM-III scores, the predictive power increased to 82% and 85.7%, respectively (17). When combined with other variables, such as B-type natriuretic peptide (BNP), total bilirubin, D-dimer, mechanical ventilation, and lactate, it had an increased predictive value, with an AUC in the range of 84.4%–85.4% (2). The highest recorded predictive power of albumin, 90.8%, was in combination with serum procalcitonin and the Pediatric Early Warning Score (PEWS).
Serum procalcitonin alone had a predictive value of 73%–83% (24, 28). When combined with serum albumin and PEWS, the predictive power increased to 90.8% (24). In addition, when serum procalcitonin was combined with high-sensitivity C-reactive protein (hsCRP) and pancreatic stone protein, the predictive power increased to 92% (28). Other studies investigated the predictive power of a reduction in procalcitonin levels for sepsis mortality. Nevertheless, an estimation could not be made because of the low number of outcomes (29). Troponin I had the highest predictive power (AUC 92.6%) compared with other biomarkers, but only a few centers were able to evaluate it; its highest predictive power was at 48 h (15).
Lactate had the greatest association with mortality. Lactate levels above 5 mmol/L had their highest predictive power (AUC 79.2%; 95% CI, 0.597–0.986) and association with mortality (OR 12.5; 95% CI, 1.85–84.442; p = 0.005) when measured at 12 h (20). The immediate measurement of the lactate level was more associated with mortality when using a higher cutoff (2 mmol/L [OR 1.556; 95% CI, 1.061–2.282; p < 0.024] vs. 5 mmol/L [OR 6.7; 95% CI, 1.047–42.431; p = 0.034]) (2, 20). Slatnick et al. found that a DIC score ≥3 predicted an increased mortality risk for up to 1 year, with a hazard ratio (HR) of 3.55 (95% CI, 1.46–8.64; p = 0.005). It was slightly higher than that of the lactate level measured within 24 h of admission (HR 3.03; 95% CI, 1.28–7.72; p = 0.012). Moreover, the DIC score had a predictive power with an AUC of 69% in predicting 1-year mortality (22).
Several biomarkers also had a predictive value for sepsis mortality, such as first urine liver-type fatty acid binding protein (L-FABP), natural killer (NK) cell concentration, and serum thrombomodulin. The diagnostic performance of the first urine L-FABP was analyzed using the receiver operating characteristic (ROC) curve, and it was found to have an AUC of 0.647 (95% CI, 0.500–0.795) (31). The relative concentration of NK cells (CD3–CD56/16+%) at day 1 had a significant predictive ability (p < 0.001) to detect mortality (AUC 0.950; 95% CI, 0.889–1.0) (27). In addition, the serum thrombomodulin level had an AUC of 0.711 for predicting mortality (31). Other biomarkers, such as plasma mtDNA, phase angle value, macrophage migration inhibitory factor, and plasma thiol-disulfide, also showed a significant association and positive correlation with mortality (14, 30, 35, 36).
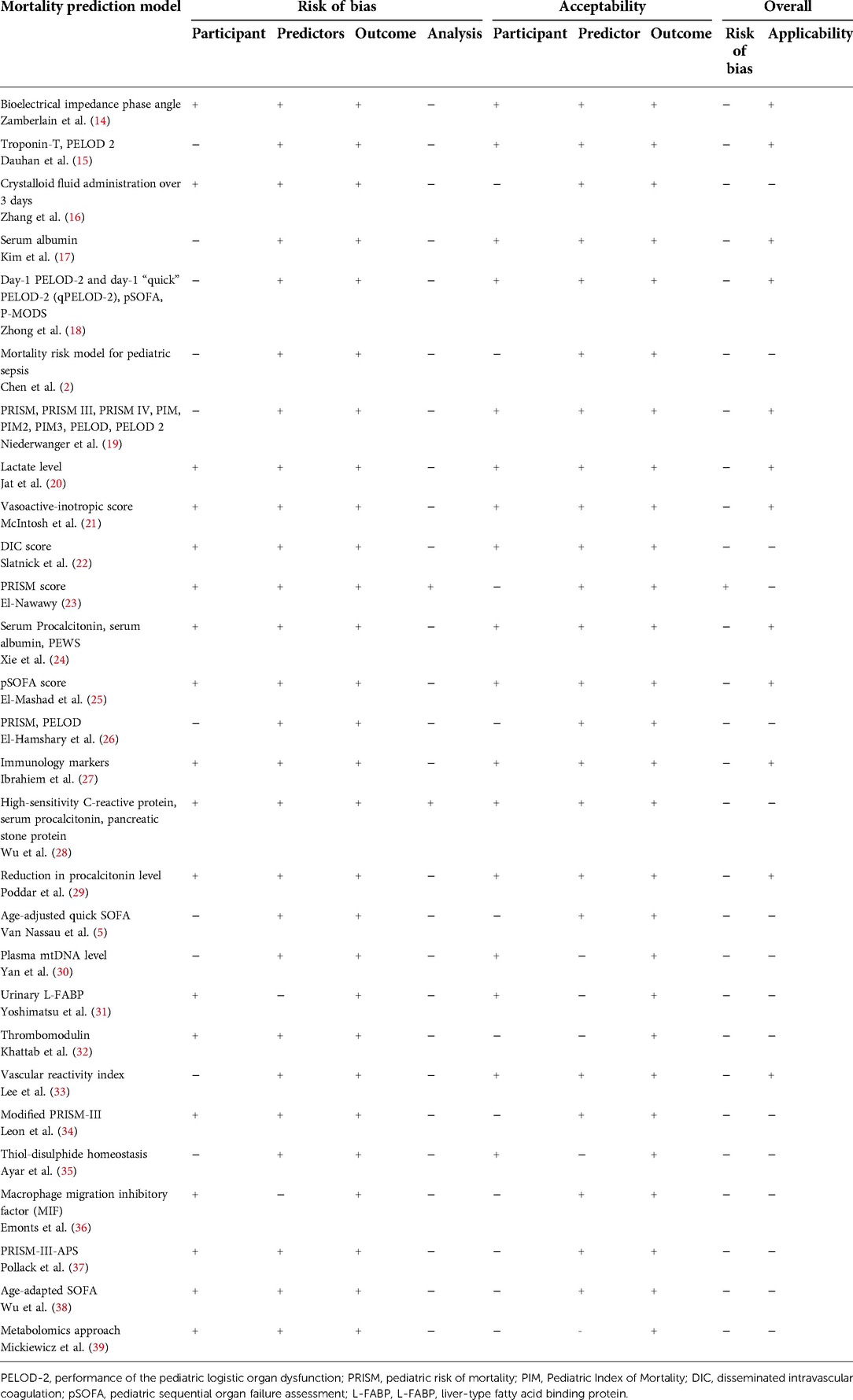
A tool with a discriminatory ability of 0.80 (AUC) or more was identified as good for discrimination. The closer the ROC curve area was to 1.0, the better the prediction model. Modified prediction models, i.e., the pSOFA (0.937), PELOD-2 at day 1 admission (0.916), and the Pediatric Risk of Mortality-III-Acute Physiology Score (PRISM-III-APS) (0.85–0.95), met these benchmarks, indicating that these three tools can discriminate between survival and non-survival in pediatric patients, primarily PRISM-III in combination with other predictors such as albumin (predictive power increased to 85.7%) (17).
All studies were assessed for risk of bias by evaluating the calibration and discrimination using the Hosmer–Lemeshow goodness-of-fit test and concordance index. However, one study showed a high risk of bias due to inappropriate analysis, no clear inclusion and exclusion criteria, and its handling of missing data (23). A total of 15 models had similar characteristics to their participants and matched predictors and outcomes with the research question.
Within the prediction models using biomarkers, serum albumin is highly applicable in acute care in resource-limited settings. Acute care refers to secondary healthcare, where a patient receives active but short-term treatment of sepsis in the emergency department or PICU. Furthermore, serum albumin evaluation was widely available and cost less than other biomarkers. Serum albumin had the best predictive power compared to other biomarkers, especially when combined with other predictors, such as serum procalcitonin and PEWS (AUC 90.8%, sensitivity 87.23%, specificity 85.11%) (24).
Serum procalcitonin had an even higher predictive power (AUC 92%) when combined with hsCRP and pancreatic stone protein. However, the study examining it showed a high risk of bias due to unclear participant selection and analysis (28). Rarer biomarkers, such as NK cell concentration, were still applicable in our setting, even though they are not widely used or available. The study evaluating NK cell concentration as a prediction model was considered to have a low risk of bias, even with a small sample size, because of its clear participant selection, predictor, and outcome. In addition, the discrimination value of NK cell evaluation was considered suitable due to the high AUC (95%; 95% CI 0.889–1.0) (27).
Lactate, a biomarker widely measured in patients with sepsis, was highly associated with mortality. It was applicable due to being widely available in numerous health care facilities. However, its predictive value is lower compared with other biomarkers; it therefore might be better to evaluate mortality with predictors with better discrimination, such as serum albumin. The bioimpedance phase angle was not considered applicable in our setting. Nevertheless, the study also included measurements using more straightforward methods, such as the mid-upper arm circumference, which was feasible in numerous health care facilities (14).
One of the limitations of this study was the study selection. Only studies in English were eligible for analysis. In addition, some prediction models did not have an AUROC analysis, so the predictive power was more challenging to determine. Despite its limitations, lactate is an easily measured laboratory parameter that can provide helpful information for the bedside clinician when incorporated into the appropriate clinical context. Thus, it is essential to interpret lactate cautiously, as its reported level can be due to tissue hypoperfusion, decreased lactate clearance, or use of epinephrine.
The strength of our study lies in the fact that it is the first scoping review to investigate the prognostic models and predictors that are available in developing countries.
Conclusion
Leveraging prognostic models to predict mortality among pediatric patients with sepsis remains an important and well-recognized area of study. While much validation and development work remains to be done, available prognostic models could aid clinicians at the bedside of children with sepsis. Furthermore, mortality prediction models are essential and valuable tools for assessing the quality of care provided to critically ill pediatric patients. In the future, these models should be prospectively validated and refined across diverse patient populations.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
IY, CNH, SFA, and ANS proposed the idea, performed the analysis, and drafted the manuscript. ANS, ACD, and KN interpreted the results and revised the manuscript. RWR and EDS contributed to framing the study's overall aims and helped analyze the data. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from the Universitas Indonesia (NKB-1342/UN2.RST/HKP.05.00/2020). The sponsor of this study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hu L, Zhu Y, Chen M, Li X, Lu X, Liang Y, et al. Development and validation of a disease severity scoring model for pediatric sepsis. Iran J Public Health. (2016) 45:875–84. MID: 27516993; PMCID: PMC4980341
2. Chen M, Lu X, Hu L, Liu P, Zhao W, Yan H, et al. Development and validation of a mortality risk model for pediatric sepsis. Medicine. (2017) 96:e6923. doi: 10.1097/MD.0000000000006923
3. Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the global burden of disease study. Lancet. (2020) 395:200–11. doi: 10.1016/S0140-6736(19)32989-7
4. Gonçalves JP, Severo M, Rocha C, Jardim J, Mota T, Ribeiro A. Performance of PRISM III and PELOD-2 scores in a pediatric intensive care unit. Eur J Pediatr. (2015) 174:1305–10. doi: 10.1007/s00431-015-2533-5
5. Nassau S, Beek R, Driessen G, Hazelzet J, Wering H, Boeddha N. Translating sepsis-3 criteria in children: prognostic accuracy of age-adjusted quick SOFA score in children visiting the emergency department with suspected bacterial infection. Front Pediatr. (2018) 6:266. doi: 10.3389/fped.2018.00266
6. Zhang L, Huang H, Cheng Y, Xu L, Huang X, Pei Y, et al. Predictive value of four pediatric scores of critical illness and mortality on evaluating mortality risk in pediatric critical patients. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. (2018) 30:51–6. doi: 10.3760/cma.j.issn.2095-4352.2018.01.010
7. Bilan N, Galehgolab BA, Emadaddin A, Shiva S. Risk of mortality in pediatric intensive care unit, assessed by PRISM-III. Pak J Biol Sci PJBS. (2009) 12:480–5. doi: 10.3923/pjbs.2009.480.485
8. Wong HR, Dalton HJ. The PICU perspective on monitoring hemodynamics and oxygen transport. Pediatr Crit Care Med. (2011) 12:S66–8. doi: 10.1097/PCC.0b013e3182211c60
9. Lacroix J, Cotting J. Severity of illness and organ dysfunction scoring in children. Pediatr Crit Care Med. (2005) 6:S126–134. doi: 10.1097/01.PCC.0000161287.61028.D4
10. El-Nawawy A, Mohsen AA, Abdel-Malik M, Taman SO. Performance of the pediatric logistic organ dysfunction (PELOD) and (PELOD-2) scores in a pediatric intensive care unit of a developing country. Eur J Pediatr. (2017) 176:849–55. doi: 10.1007/s00431-017-2916-x
11. Kaur G, Vinayak N, Mittal K, Kaushik JS, Aamir M. Clinical outcome and predictors of mortality in children with sepsis, severe sepsis, and septic shock from Rohtak, Haryana: a prospective observational study. Indian J Crit Care Med. (2014) 18:437–41. doi: 10.4103/0972-5229.136072
12. Weiss SL, Nicolson SC, Naim MY. Clinical update in pediatric sepsis: focus on children with pre-existing heart disease. J Cardiothorac Vasc Anesth. (2020) 34:1324–32. doi: 10.1053/j.jvca.2019.10.029
13. de Souza DC, Machado FR. Epidemiology of pediatric septic shock. J Pediatr Intensive Care. (2019) 8:3–10. doi: 10.1055/s-0038-1676634
14. Zamberlan P, Feferbaum R, Filho UD, de Carvalho WB, Delgado AF. Bioelectrical impedance phase angle and morbidity and mortality in critically ill children. Nutr Clin Pract. (2019) 34:163–71. doi: 10.1002/ncp.10201
15. Dauhan AC, Lubis AD, Mutiara E, Lubis M. Correlation of troponin level (troponin T, troponin I) with PELOD-2 score in sepsis as a predictive factor of mortality. Maced J Med Sci. (2019) 7:4072–7. doi: 10.3889/oamjms.2019.806
16. Zhang S, Dai X, Guo C. Crystalloid fluid administration was associated with outcomes in pediatric patients with severe sepsis or septic shock. Medicine. (2018) 97:e12663. doi: 10.1097/MD.0000000000012663
17. Kim YS, Sol IS, Kim MJ, Kim SY, Kim JD, Kim YH, et al. Serum albumin as a biomarker of poor prognosis in the pediatric patients in intensive care unit. Korean J Crit Care Med. (2017) 32:347–55. doi: 10.4266/kjccm.2017.00437
18. Zhong M, Huang Y, Tufeng L, Xiong L, Lin T, Li M, et al. Day-1 PELOD-2 and day-1 “quick” PELOD-2 scores in children with sepsis in the PICU. J Pediatr. (2020) 96:660–5. doi: 10.1016/j.jped.2019.07.007
19. Niederwanger C, Varga T, Hell T, Stuerzel D, Prem J, Gassner M, et al. Comparison of pediatric scoring systems fro mortality in septic patients and the impact of missing information on their predictive power: a retrospective analysis. PeerJ. (2020) 8:e9993. doi: 10.7717/peerj.9993
20. Jat KR, Jhamb U, Gupta VK. Serum lactate levels as the predictor of outcome in pediatric septic shock. Indian J Crit Care Med. (2011) 15(2):102–7. doi: 10.4103/0972-5229.83017
21. McIntosh AM, Tong S, Deakyne SJ, Davidson JA, Scott HF. Validation of the vasoactive-inotropic score in pediatric sepsis. Pediatr Crit Care Med. (2017) 18:750–7. doi: 10.1097/PCC.0000000000001191
22. Slatnick LR, Thornhill D, Davies SJD, Ford JB, Scott HF, Manco-Johnson MJ, et al. Disseminated intravascular coagulation is an independent predictor of adverse outcomes in children in the emergency department with suspected sepsis. J Pediatr. (2020) 225:198–206. doi: 10.1016/j.jpeds.2020.06.022
23. El-Nawawy A. Evaluation of the outcome of patients admitted to the pediatric intensive care unit in alexandria using the pediatric risk of mortality (PRISM) score. J Trop Pediatr. (2003) 49:109–14. doi: 10.1093/tropej/49.2.109
24. Xie X, Li M, Xiong TT, Wang R, Xiao L. Nested case-control study of multiple serological indexes and Brighton pediatric early warning score in predicting death of children with sepsis. World J Clin Cases. (2019) 7:431–40. doi: 10.12998/wjcc.v7.i4.431
25. El-Mashad GM, El-Mekkawy MS, Zayan MH. Pediatric sequential organ failure assessment (pSOFA) score: a new mortality prediction score in the paediatric intensive care unit. An Pediatr. (2020) 92:2777–85. doi: 10.1016/j.anpedi.2019.05.018
26. El-Hamshary AAE, El-Sherbini SA, Elgebaly HAF, Amin SA. Prevalence of multiple organ dysfunction in the pediatric intensive care unit: pediatric risk of mortality III versus pediatric logistic organ dysfunction scores for mortality prediction. Rev Bras Ter Intensiva. (2017) 29:206–12. doi: 10.5935/0103-507X.20170029
27. Ibrahiem SK, Galal YS, Youssef MRL, Sedrak AS, Khateeb EME, Abdel-Hameed ND, et al. Prognostic markes among Egyptian children with sepsis in the intensive care unit, Cairo university hospitals. Allergol Immunopathol. (2016) 44:46–53. doi: 10.1016/j.aller.2015.07.005
28. Wu Q, Nie J, Wu F, Zou X, Chen F. Prognostic value of high-sensitivity C-reactive protein, procalcitonin, and pancreatic stone protein in pediatric sepsis. Med Sci Monit. (2017) 23:1533–9. doi: 10.12659/MSM.900856
29. Poddar B, Gurjar M, Singh S, Aggarwal A, Baronia A. Reduction in procalcitonin level and outcome in critically ill children with severe sepsis/septic shock-a pilot study. J Crit Care. (2016) 36:230–3. doi: 10.1016/j.jcrc.2016.07.022
30. Yan H, Li M, Lu X, Zhu Y, Ou-yang W, Xiao Z, et al. Use of plasma mitochondrial DNA levels for determining disease severity and prognosis in pediatric sepsis: a case control study. BMC Pediatr. (2018) 18:267–74. doi: 10.1186/s12887-018-1239-z
31. Yoshimatsu S, Sugaya T, Hossain MI, Islam MM, Chisti MJ, Kamoda T, et al. Urinary L-FABP as a mortality predictor in <5-year-old children with sepsis in Bangladesh. Pediatr Int. (2016) 58:185–91. doi: 10.1111/ped.12765
32. Khattab AA, Dawood AAER, Saleh NY. Value of thrombomodulin as a marker for sepsis in critically ill children. Indian J Pediatr. (2021) 88:864–71. doi: 10.1007/s12098-020-03564-w
33. Lee EP, Zhao LL, Hsia SH, Chan OW, Lin CY, Su YT, et al. Vascular reactivity index as and effective predictor of mortality in children with refractory septic shock. J Intensive Care Med. (2021) 36:589–96. doi: 10.1177/0885066620914850
34. Leon ALP, Romero-Gutierrez GR, Valenzuela CA, Gonzales-Bravo FE. Simplified PRISM III score and outcome in the pediatric intensive care unit. Pediatr Int. (2005) 47:80–3. doi: 10.1111/j.1442-200x.2004.01997.x
35. Ayar G, Sahin S, Atmaca YM, Yazici MU, Neselioglu S, Erel O. Thiol-disulphide homeostasis is an oxidative stress indicator in critically ill children with sepsis. Arch Argent Pediatr. (2019) 117:143–8. doi: 10.5546/aap.2019.eng.143
36. Emonts M, Sweep FCG, Grebenchtchikov N, Geurts-Moespot A, Knaup M, Chanson AL, et al. Association between high levels of blood macrophage migration inhibitory factor, inappropriate adrenal response, and early death in patients with severe sepsis. Clin Infect Dis. (2007) 44:1321–8. doi: 10.1086/514344
37. Pollack MM, Patel KM, Ruttimann UE. The pediatreic risk of mortality III—acute physiology score (PRISM III-APS): a method of assessing physiologic instability for pediatric intensive care unit patients. J Pediatr. (1997) 131:575–81. doi: 10.1016/S0022-3476(97)70065-9
38. Wu Z, Liang Y, Li Z, Lio G, Zheng J, Zuo Y, et al. Accuracy comparison between age-adapted SOFA and SIRS in predicting in –hospital mortality of infected children at China's PICU. Shock. (2019) 52:347–52. doi: 10.1097/SHK.0000000000001261
Keywords: sepsis, scoring, PELOD, PRISM, mortality, prediction
Citation: Yuniar I, Hafifah CN, Adilla SF, Shadrina AN, Darmawan AC, Nasution K, Ranakusuma RW and Safitri Eka Dian (2023) Prognostic factors and models to predict pediatric sepsis mortality: A scoping review. Front. Pediatr. 10:1022110. doi: 10.3389/fped.2022.1022110
Received: 18 August 2022; Accepted: 28 October 2022;
Published: 23 February 2023.
Edited by:
Oliver Karam, Yale Medicine, United StatesReviewed by:
Jon Kaufman, Children's Hospital Colorado, United StatesChristopher Ray, Virginia Commonwealth University Health System, United States
© 2023 Yuniar, Hafifah, Adilla, Shadrina, Darmawan, Nasution, Ranakusuma and Safitri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Arifah Nur Shadrina YXJpZmFoLnNoYWRyaW5hQGdtYWlsLmNvbQ==
Specialty Section: This article was submitted to Pediatric Critical Care, a section of the journal Frontiers in Pediatrics
 Irene Yuniar1
Irene Yuniar1 Arifah Nur Shadrina
Arifah Nur Shadrina Respati W. Ranakusuma
Respati W. Ranakusuma