- Department of Pediatric Cardiology, Heart Center, The First Hospital of Tsinghua University (Beijing Huaxin Hospital), Beijing, China
Early repolarization syndrome is rare in children. Mutation of genes encoding ion channels could display mixed electrophysiological phenotype of Kv4.3 including both cardiac phenotype (early repolarization syndrome, atrial fibrillation) and cerebral phenotype (epilepsy, intellectual disability). This situation is rare and was named as cardiocerebral channelopathy. Here, we report a case of an 11-year-old-girl with cardiocerebral channelopathy caused by KCND3 mutation, who was successfully treated with oral quinidine, metoprolol and implantable cardioverter-defibrillator. Clinicians should be vigilant on the risk of cardiogenic syncope and sudden cardiac death in a patient with epilepsy, intellectual disability and early repolarization pattern.
Introduction
Early repolarization pattern (ERP) is common in the general population and regarded as a benign electrocardiogram (ECG) phenomenon with an uneventful prognosis. Early repolarization syndrome (ERS) is diagnosed in a patient with an ERP on the ECG in addition to syncope or symptomatic arrhythmias, which significantly increase the risk of sudden cardiac death (SCD) (1, 2). However, to the best of our knowledge, ERS-related cardiocerebral channelopathy is extremely rare. Although the initial symptoms are epilepsy or intellectual disability, there is a possible SCD risk. Here, we report the case of an 11-year-old girl with ERS, atrial fibrillation (AF), epilepsy and intellectual disability caused by KCND3 mutation and describe the patient's treatment and follow-up.
Case presentation
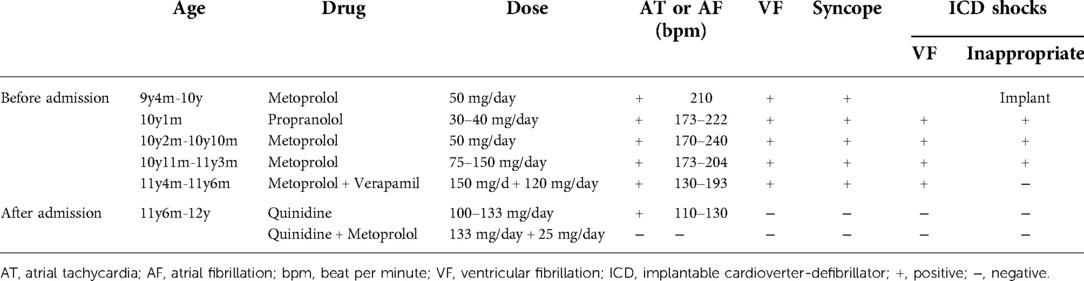
An 11-year-old girl with a weight of 45.3 kg (P 75–90th) and a height of 148 cm (P 50–75th) was admitted to the Pediatric Cardiologic Department due to “nine-year recurrent loss of consciousness”. She was diagnosed with febrile seizures at the age of 2 and had regular febrile seizures from age four (seven years ago); the seizures occurred with loss of consciousness and eye gazing. The seizure would usually last for several seconds and then suddenly disappear. Electroencephalogram (EEG) showed bilateral temporal spikes, central spikes, slow spikes, multiple slow spikes, sharp waves and slow sharp waves, which were predominant in the left temporal area. Brain magnetic resonance imaging (MRI) results was normal. She was diagnosed with epilepsy and treated with oral sodium valproate. The patient's seizures were successfully controlled with antiepileptic medications. EEGs re-examined at six months showed sharp waves and slow waves in each brain area, especially in the anterior head lead (Figure 1A). Presently, she continues treatment with oral sodium valproate. Intellectual disability was diagnosed at six years old (Wechsler scale score < 75). ECG showed ERP (Figure 2A), and a 24 h Holter monitor showed paroxysmal AF, premature atrial complexes (PACs), paired PACs and paroxysmal atrial tachycardia (AT). The result of the echocardiogram was normal. She was admitted to the emergency room 2 years ago, at age 9, for 8 episodes of Adams-Stokes attack that occurred in one day. Cardiac monitoring showed ventricular fibrillation (VF). ERS was diagnosed, and oral metoprolol 25 mg bid (twice per day) was prescribed.
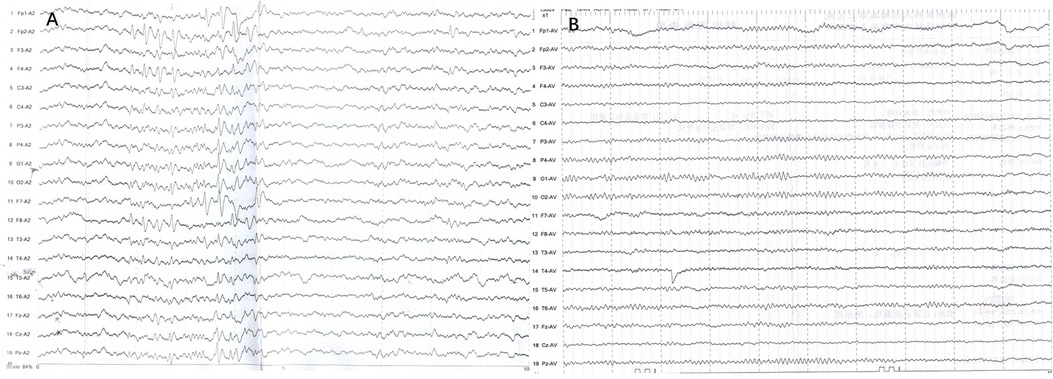
Figure 1. EEGs for this patients. (A) sharp waves and slow waves could be detected in each brain area which are predominant in anterior head lead. (B) EEG became normal during follow up.
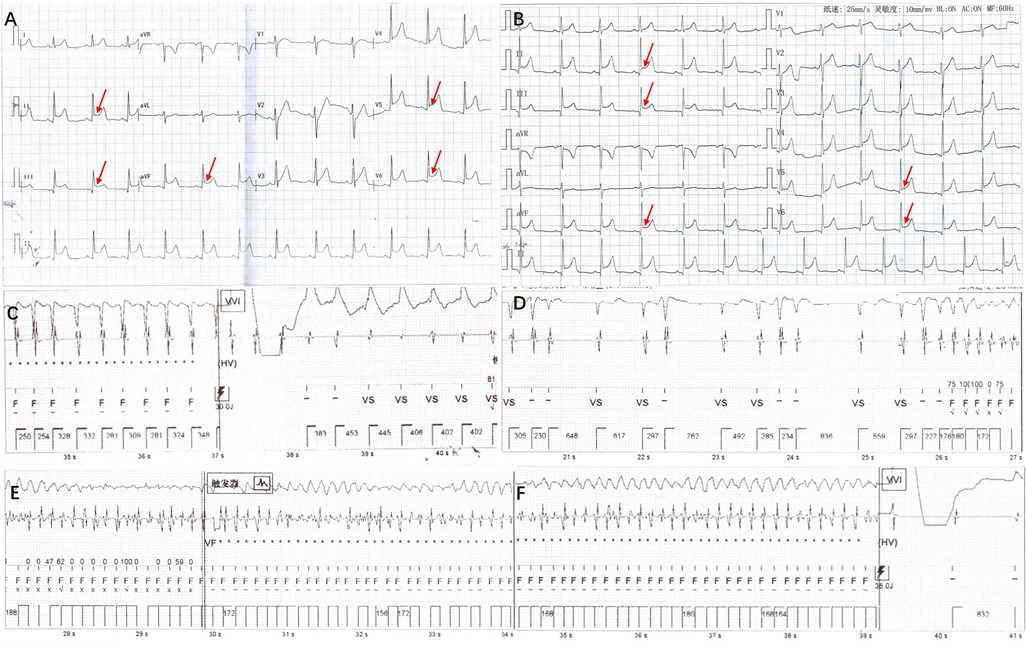
Figure 2. ECG and point diagram of ICD cavity for this patient. (A) ERP could be detected on ECG: elevation of J-point in inferior leads (II, III, AVF) and lateral leads (V5, V6). (B) J wave amplitudes were decreased slightly after administration of oral quinidine. (C) Diagram of ICD cavity shows atrial arrhythmias with electrical shock and ventricular pacing administered by ICD. (D–F) Diagram of ICD cavity shows VF occurred and was terminated by electrical shock of ICD.
One year ago, at age 10, she woke and cried during sleep, presenting with recurrent Adams-Stokes attack, but was rescued successfully by cardiopulmonary resuscitation and defibrillation. A single chamber implantable cardioverter-defibrillator (ICD, CD1231-40Q, St. Jude Medical and lead, 7122Q/65, St. Jude Medical) was implanted. In addition to implantation of the ICD, she was treated successively by oral propranolol 10 mg tid (three times per day)→15 mg (morning) 15 mg (noon) 10 mg (evening), metoprolol 25 mg bid→50 mg tid and combined therapy of metoprolol 50 mg tid with verapamil 40 mg tid but the treatment was poorly efficacious. Repeated ECG and Holter monitor showed paroxysmal AT, paroxysmal AF and R-on-T premature ventricular complexes (PVCs). ICD recorded multiple episodes of inappropriate shocks during AT (ventricular rate 254–288 bpm) without syncope recurrence (Figure 2C). Three months before admission, she had a total of 10 episodes of Adams-Stokes attack over an 8-day period. A diagram of the ICD cavity showed VF during Adams-Stokes syndrome and successful defibrillation by ICD (Figures 2D–F). She was admitted for further treatment.
There was no family history of hereditary disease, syncope or sudden death. Whole-exome sequencing showed KCND3 chr 1-112524433 and G306S (c.916G > A) (Figure 3, Beijing Maijinuo Medical Laboratory). The Sanger sequence was verified as a de novo mutation. According to ACMG guidelines, this variation evidenced PS2, PM2, PM5 [G306A had been reported (3)], PP3 (Bioinformatics protein function prediction software included SIFT, PolyPhen-2 and REVEL: harmful) and PP4 as a pathogenic mutation.
Figure 3. Results of KCND3 gene DNA sequencing patient has KCND3 chr 1-112524433 G306S (c.916G > A). Her parents and brother did not carry the same variant.
Diagnosis and treatment: After admission, oral metoprolol and verapamil were tapered gradually and ultimately withdrawn. Cardiac monitoring showed sinus rhythm with frequent short paroxysmal AT and occasional AF. Frequent R-on-T PVCs were detected. The diagnosis of ERS was clear, and the patient had a definite indication for ICD implantation. Because of atrial and ventricular arrhythmia, the patient suffered inappropriate shocks and electrical storms, for which antiarrhythmic drug therapy is appropriate. Because of the definitive role of the pathogenic gene and ERS diagnosis, propranolol, metoprolol and verapamil were ineffective, and we decided to administer quinidine, which was administered orally at 50 mg bid and gradually increased to 66.7 mg bid. Repeated ECGs showed no QT interval prolongation. PVCs disappeared after the administration of oral quinidine. Lasting several minutes up to one hour, paroxysmal short AT and paroxysmal AF with a ventricular rate of 110–130 bpm could still be detected 10 days after adding oral quinidine and usually occurred when the girl became angry. Atrial arrhythmias disappeared after a low dose of oral metoprolol 12.5 mg bid was added. Holter monitoring showed sinus rhythm with a total of 117,185 beats per 24 h. During 12 months of follow-up, no ICD shocks were detected. Holter monitor tests on three occasions showed no atrial or ventricular arrhythmias. ECG showed that the J wave amplitude decreased slightly (Figure 2B). There were no epileptic seizures, and her EEG result was normal (Figure 1B). Under the guidance of a pediatric neurologist, the sodium valproate dose is tapered. Antiarrhythmic drug therapy and evaluation of therapeutic effect are summarised in Table 1.
Discussion
KCND3 encodes Kv4.3 protein, ɑ subunit of the fast transient outward potassium (Ito) channel, which expressed on both cardiac and cerebral tissues. Clinical manifestations include both cardiac phenotype (ERS, AF) and cerebral phenotype (epilepsy, intellectual disability), collectively named cardiocerebral channelopathy (4). Gain of function of the potassium ion channel caused by the KCND3 mutation can lead to Brugada syndrome, early-onset AF and ERS (3), while loss of function of the potassium ion channel from the KCND3 mutation can lead to spinocerebellar ataxia (5). Recently, four cases(all of them over 12 years of age) were reported with KCND3 G306A or V392I mutations displaying a mixed electrophysiological phenotype of Kv4.3, including both cardiac and cerebral phenotypes (3, 6). The pathogenesis of the cardiac phenotype can be explained by the increased fast transient outward potassium current (3). Genetic analysis of this patient identified a KCND3 G306S mutation similar to the KCND3 G306A mutation reported in the literature. Both mutations affect the amino acid at position 306, which is a transmembrane region, and have similar phenotypes, suggesting that this mutation led to cardiocerebral channelopathy in our patient.
ERP has been regarded as a benign ECG phenomenon with an excellent prognosis (7). However, many recent studies have shown that ERP significantly increases the risk of SCD, cardiac death and death from any cause (2). There are no convincing studies that identify the epidemiological features of ERP in children and ERS is rare in children. Inferolateral with anterior ERP has been a key predictor for poor prognosis (8). The initial symptoms of our case were seizures, and ERP was discovered accidentally: inferior and lateral ERP on her ECG, which are concordant with the predictors of a poor prognosis as reported in the literature. Multiple occurrences of VF and surviving cardiac arrest after cardiopulmonary resuscitation indicate a high risk of SCD. Clinicians should be vigilant for cardiocerebral channelopathy and the risk of cardiogenic syncope and SCD in patients with epilepsy, intellectual disability and ERP.
It has been reported that at least 40% of ERS patients with a history of SCD or VF had VF recurrence during follow-up; therefore, implantation of ICD is recommended for patients who show ERP on their ECGs and also have a history of cardiac arrest or persistent ventricular arrhythmias (1, 9). A study showed that in patients with idiopathic VF and ICD implantation, inappropriate shock caused by AF was common (40%) (9). Our patient is a survivor of cardiac arrest with definite indication of ICD implantation and had atrial arrhythmias (paroxysmal AF and AT) in addition to ERS. Since a fast ventricular rate of atrial arrhythmias may lead to inappropriate shock by ICD, antiarrhythmic medication is indicated to control the atrial arrhythmias. Previous studies have shown that isoproterenol can effectively suppress VF electrical storms in patients with ERS and that the long-term use of quinidine can prevent the recurrence of ventricular arrhythmias (10, 11). Other antiarrhythmic medications have very limited effects in the prevention of ventricular arrhythmia recurrence (10). In our case, ventricular arrhythmias disappeared after quinidine treatment. The mechanism might quinidine could normalize the gain-of-function changes of mutant Kv4.3 (3). This was a case of ion channel disease caused by gene mutation, and the patient might present with ventricular arrhythmia and suffer the risk of syncope and SCD. Oral quinidine is safe and effective for ventricular arrhythmia, but long-term or lifelong quinidine therapy is often required. It should be emphasised that the Kv4.3 current increases during bradycardia because more Kv4.3 channels can recover from inactivation. Bradycardia might, possibly, worsen symptoms (12). In this case, low-dose oral metoprolol was added to control the atrial arrhythmias without causing bradycardia, and these anti-arrhythmic drugs did not increase the risk of ventricular arrhythmia. We still need follow-up to focus on bradycardia and ventricular arrhythmias.
Conclusions
Cardiocerebral channelopathy should be suspected in a patient with epilepsy and intellectual disability whose ECG shows ERP and AF. Quinidine is safe and effective for children with ERS.
Strengths and limitations
This was a very rare case. Since the KCND3 mutation present as a mixed electrophysiological phenotype of Kv4.3, including both cardiac and cerebral phenotype, doctors should be vigilant on the risk of cardiogenic syncope and SCD. Limitations: This case did not permit further functional study, but would be considered if future conditions permit.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the First Hospital of Tsinghua University. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
YZ and HJ treated the patient and drafted the manuscript. X-mL designed and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The Capital Clinical Characteristic Application Research (Z181100001718207) and Qinghua Yuyuan Medical Research Funding (20240000591).
Acknowledgments
The authors appreciate Zhang Yan for revising English expression.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart rhythm society. J Am Coll Cardiol. (2018) 72(14):e91–e220. doi: 10.1016/j.jacc.2017.10.054
2. Cheng Y, Lin X, Ji C, Chen X, Liu L, Tang K, et al. Role of early repolarization pattern in increasing risk of death. J Am Heart Assoc. (2016) 5(9):e003375. doi: 10.1161/jaha.116.003375
3. Takayama K, Ohno S, Ding WG, Ashihara T, Fukumoto D, Wada Y, et al. A de novo gain-of-function KCND3 mutation in early repolarization syndrome. Heart Rhythm. (2019) 16(11):1698–706. doi: 10.1016/j.hrthm.2019.05.033
4. Pollini L, Galosi S, Tolve M, Caputi C, Carducci C, Angeloni A, et al. KCND3-related neurological disorders: from old to emerging clinical phenotypes. Int J Mol Sci. (2020) 21(16):1–15. doi: 10.3390/ijms21165802
5. Wang J, Wen Y, Zhang Q, Yu S, Chen Y, Wu X, et al. Gene mutational analysis in a cohort of Chinese children with unexplained epilepsy: identification of a new KCND3 phenotype and novel genes causing dravet syndrome. Seizure. (2019) 66:26–30. doi: 10.1016/j.seizure.2019.01.025
6. Nakajima T, Kawabata-Iwakawa R, Kaneko Y, Hamano S, Sano R, Tamura S, et al. Novel cardiocerebral channelopathy associated with a KCND3 V392I mutation. Int Heart J. (2020) 61(5):1049–55. doi: 10.1536/ihj.20-203
7. Klatsky AL, Oehm R, Cooper RA, Udaltsova N, Armstrong MA. The early repolarization normal variant electrocardiogram: correlates and consequences. Am J Med. (2003) 115(3):171–7. doi: 10.1016/s0002-9343(03)00355-3
8. Kamakura T, Kawata H, Nakajima I, Yamada Y, Miyamoto K, Okamura H, et al. Significance of non-type 1 anterior early repolarization in patients with inferolateral early repolarization syndrome. J Am Coll Cardiol. (2013) 62(17):1610–8. doi: 10.1016/j.jacc.2013.05.081
9. Siebermair J, Sinner M, Beckmann B, Laubender R, Martens E, Sattler S, et al. Early repolarization pattern is the strongest predictor of arrhythmia recurrence in patients with idiopathic ventricular fibrillation: results from a single centre long-term follow-up over 20 years. Europace. (2016) 18(5):718–25. doi: 10.1093/europace/euv301
10. Haïssaguerre M, Sacher F, Nogami A, Komiya N, Bernard A, Probst V, et al. Characteristics of recurrent ventricular fibrillation associated with inferolateral early repolarization role of drug therapy. J Am Coll Cardiol. (2009) 53(7):612–9. doi: 10.1016/j.jacc.2008.10.044
11. Ergül Y, Kafalı HC, Gülgün M. Successful treatment of electrical storm in a child with early repolarization syndrome with orciprenaline and radiofrequency ablation. Anatol J Cardiol. (2019) 22(2):94–6. doi: 10.14744/AnatolJCardiol.2019.37085
Keywords: early repolarization syndrome, epilepsy, cardiocerebral channelopathy, atrial fibrillation, quinidine
Citation: Zhang Y, Jiang H and Li X (2022) Cardiocerebral channelopathy caused by KCND3 mutation in a child: A case report. Front. Pediatr. 10:1019122. doi: 10.3389/fped.2022.1019122
Received: 14 August 2022; Accepted: 11 November 2022;
Published: 28 November 2022.
Edited by:
Anastacia M Garcia, University of Colorado Denver, United StatesReviewed by:
Cheng Wang, Central South University, ChinaMinoru Horie, Shiga University of Medical Science, Japan
© 2022 Zhang, Jiang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao-mei Li bGl4aWFvbWVpMTM2OEBzaW5hLmNvbQ==
Specialty Section: This article was submitted to Genetics of Common and Rare Diseases, a section of the journal Frontiers in Pediatrics
 Yi Zhang
Yi Zhang He Jiang
He Jiang Xiao-mei Li
Xiao-mei Li