- Department of Pediatric Allergy and Immunology, The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, Wenzhou, China
Introduction: Allergen immunotherapy (AIT) is considered to be the only treatment that may change the natural process of allergic diseases. Subcutaneous immunotherapy (SCIT) is a type of allergen immunotherapy that is commonly used in clinical practice. However, SCIT has inconsistent effects on individuals, and it is yet unclear what factors affect therapeutic efficacy. In recent years, vitamin D levels have been speculated as a potential factor influencing SCIT efficacy.
Objective: To investigate the effect of serum vitamin D level on the SCIT efficacy in children with allergic rhinitis and/or asthma caused by dust mite allergy.
Methods: According to the panel consensus, children with asthma and/or allergic rhinitis who received SCIT were divided into the vitamin D deficiency group (<12 ng/ml), vitamin D insufficiency group (12–20 ng/ml), and vitamin D sufficiency group (>20 ng/ml). Serum 1–25(OH) D3, blood eosinophil, total IgE, dermatophagoides pteronyssinus (Dp), and dermatophagoides farina (Df) specific IgE (sIgE) were detected, and questionnaires of symptom and medication scores were collected before and after one year of treatment.
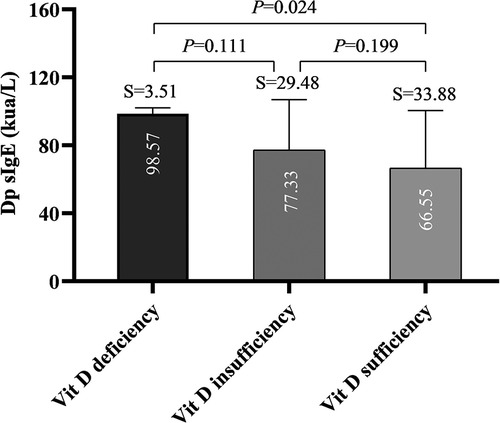
Results: After one year of SCIT treatment, the symptom and medication score significantly decreased (P < 0.05), but there was no difference between the efficacy in different groups (P > 0.05). Our study found a statistical difference in Dp sIgE level between the vitamin D deficiency and the sufficiency groups (P = 0.024), and vitamin D levels become lower with children's growth (Y = −0.8981*X + 34.26, P = 0.0025).
Conclusions: No difference was found between the efficacy of one-year SCIT and serum vitamin D levels based on symptom and medication scores. Nevertheless, higher vitamin D levels may be associated with a decreased indicator of Dp allergy.
Introduction
In recent years, the prevalence of allergic rhinitis and asthma has remained high and caused a heavy social burden (1, 2). However, allergen immunotherapy (AIT) is considered the only disease-modifying treatment for type I allergy, including allergic rhinitis and asthma (3). Through high-dose allergen exposure, AIT induces dendritic cells to produce interleukin (IL)-10, IL-12, and IL-27, meanwhile stimulating regulatory T cells (Treg) and regulatory B (Breg) cells to produce immunoglobulin A (IgA), IgG and blocking IgG/IgG4 antibodies, thus to correct immune dysfunction caused by allergy, leading to the tolerance of allergens and improving clinical symptoms (4). Subcutaneous immunotherapy (SCIT) is a type of allergen immunotherapy that is commonly used in clinical practice. A large number of animal and clinical studies and meta-analyses have confirmed the efficacy and safety of SCIT (5–8). Nevertheless, there is a lack of ideal and effective immunological indicators for predicting the efficacy of subcutaneous immunotherapy.
Vitamin D is one of the immunomodulators. 1–25 (OH) D3 regulates immune function by inhibiting the differentiation and maturation of dendritic cells and enhances the frequency of two distinct populations of Treg cells: IL-10 secreting and Foxp3+ Treg cell (9, 10). Noticeably, dendritic cells, Treg cells, and IL-10 also play a significant role in the mechanism of allergen immunotherapy. Moreover, some studies have found that subcutaneous immunotherapy combined with vitamin D can enhance the therapeutic effect (11, 12). It has been reported that there is a significant correlation between vitamin D deficiency and the risk of asthma attack development in children (13). Accordingly, we further speculate if vitamin D levels in the body could affect the efficacy of immunotherapy. An epidemiological study showed that sufficient baseline serum vitamin D levels might enhance the efficacy of SCIT in adults with hay pollen allergic rhinitis (14). But for dust mite allergic children, the effect of vitamin D levels on SCIT has not been reported. Therefore, this study aimed to investigate the relationship between baseline serum vitamin D levels and the clinical efficacy of subcutaneous immunotherapy in children with dust mite allergic rhinitis and/or asthma.
Materials and methods
Study population
In the study, a total of 76 children with bronchial asthma and/or allergic rhinitis who received allergen specific immunotherapy were set as the observation group, and their serum vitamin D levels were detected. According to the global panel recommendations (15), the population was divided into three groups based on serum vitamin D levels: vitamin D deficiency group (<30 nmol/L, i.e., <12 ng/ml), vitamin D insufficiency group (30–50 nmol/L, i.e., 12–20 ng/ml) and vitamin D sufficiency group (>50 nmol/L, i.e., >20 ng/ml) (16). All the children were given the symptom scores and medication scores by filling in the questionnaire at two time points: before they were given specific immunotherapy and after one year of AIT treatment.
Inclusion criteria: (1) diagnosis of mild to moderate asthma and/or allergic rhinitis; (2) age 4–14 years old; (3) course of rhinitis or asthma >1 year; (4) determination of serum specific IgE antibody level >grade 3, dust mite was the main source of allergen; (5) treatment time >1 year, injection times >20 times.
Exclusion criteria: (1) severe asthma; (2) Use of beta-blockers; (3) cardiovascular dysfunction; (4) immune deficiency and/or chronic infectious diseases.
The study was approved by the medical ethical committee of The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, and written informed consent was obtained from the parent or legal guardian of the children.
Subcutaneous immunotherapy administration regimens
According to different administration methods, allergen specific immunotherapy commonly used in clinical practice includes subcutaneous immunotherapy (SCIT) and sublingual immunotherapy. All participants included in this study received SCIT. House dust mite allergen preparation (Alutard sq Der p, ALK Abello company, Denmark) was used for subcutaneous injection. The course of injection was divided into initial treatment (dose increase) and maintenance treatment (dose maintenance). In the initial treatment stage, the allergen dose gradually increased from 100 SQ-U/ml to 100,000 SQ-U/ml. Children with bronchial asthma used conventional therapy. In the initial treatment stage, the injection is usually once a week, which takes 15 weeks. Cluster therapy (Rush immunotherapy, RIT) is used for children with simple allergic rhinitis, and the initial treatment stage is 7 weeks. After reaching the maintenance dose, the first injection was injected every 2 weeks, and the second injection was injected every 4 weeks. After that, according to the changes in the children’s condition, the interval of injection was 4–8 weeks. It is generally recommended that the children should be injected once every 6 weeks. The efficacy was evaluated after 1 year of treatment (after 20 injections).
Asthma and allergic rhinitis definition
According to the Global Initiative for Asthma guideline, asthma was diagnosed by professional pediatric physicians based on the history of respiratory symptoms such as wheeze, cough, chest tightness, and shortness of breath that change over time and in intensity, as well as variable expiratory airflow limitation (17). Severe asthma was defined using the European Respiratory Society and the American Thoracic Society’s severe asthma definition (2014) (18).
Allergic rhinitis was defined as an inflammation of the nasal lining induced by inhaling something allergic, such as animal dander or pollen, with symptoms such as sneezing, nasal congestion, runny nose, post-nasal drip, and nasal itching (19).
Allergenic index
Allergenic indexes were detected before entering the group.
(1) Peripheral blood eosinophil level: the count of eosinophils in peripheral blood was detected by an automatic hematology analyzer (produced by Shenzhen Mindray Pharmaceutical Co., Ltd.), and its value <0.45 * 109/L was considered normal.
(2) Serum total IgE: peripheral venous blood was collected, and serum total IgE was determined by fluorescence enzyme-linked immunosorbent assay. The detection ranged from 0.00 IU/ml to 2,500 IU/ml.
(3) Serum specific IgE: serum sIgE was measured by fluoroimmunoassay technique (UniCAP, Pharmcia Diagnostics, Uppsala, Sweden). The detection range was 0.1–100 kul/L. The value was divided into 7 grades: <0.35 kua/L is grade 0, 0.35−<0.7 kua/L is grade 1, 0.7−<3.5 kua/L is grade 2, 3.5−<17.5 kua/L is grade 3, 17,5−<50 kua/L is grade 4, 50−<100 kua/L is grade 5, ≥100 is grade 6.
Efficacy evaluation of subcutaneous immunotherapy
Before each allergen injection, patients were instructed to grade their symptoms retrospectively in the past week. Visual analog scales and Drug scores were evaluated for both rhinitis and asthma at the start of treatment and then every 2 or 3 months. SCIT is considered effective if the medication score of concomitant drugs decreases and the symptoms are well controlled after treatment (5, 6).
(1) Symptom scores assessment (20): the following symptoms were assessed and scored on a scale of 0 to 10: chest (cough, wheeze, breathlessness, chest tightness), nose (sneeze, blockage, and running), eye (itching, streaming, redness, and swelling), and mouth and throat (dryness and itching) symptoms.
(2) Medication scores assessment (21): it was totaled for the allowed rescue treatments: each oral antihistamines tablet (5 mg) was scored 1. Inhaled corticosteroids (200 µg budesonide or equivalent) were scored 1. Short-acting β-agonist (one application) was scored 1. Nasal corticosteroids (50 µg budesonide or equivalent) were scored 0.75. Nasal antihistamines (one application) were scored 0.25.
Statistical analysis
Considering the response rate of allergic rhinitis to subcutaneous allergen immunotherapy (80%) using the following formula:
if d = 0.15p and P = 0.8, the minimum sample size to detect a response was estimated at 44 patients (14).
Statistical analysis was carried out by statistics software (SPSS 21). The data of normal distribution are described by arithmetic mean and 95% confidence interval. Nonnormal data were expressed as a 95% confidence interval or median and interquartile distance (IQR) after geometric mean and logarithmic transformation. Multiple comparisons of ANOVA were used for pairwise comparisons among the three groups. A covariance test was used to compare the symptom scores before and after treatment. Linear regression analysis was applied to analyze the association between vitamin D levels and age. Graphical representations were made by using the GraphPad Prism statistical package. For all tests, P < 0.05 was considered statistically significant.
Results
Characteristics of the study population
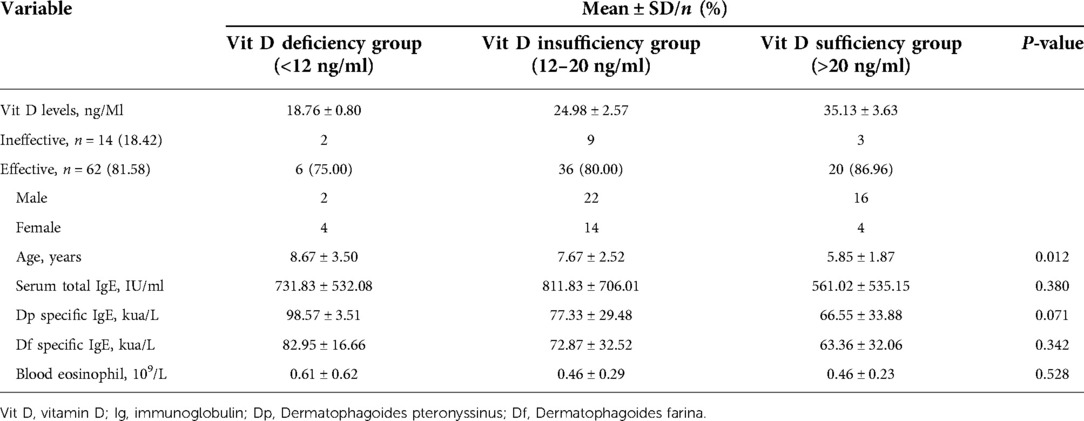
A total of 76 children participated in the study. And symptom and medication scores of 62 patients improved with an effective rate of 81.58% (62/76). Eventually, the 62 effective patients were selected for subsequent analysis. There were 6 participants in the vitamin D deficiency group, 36 participants in the vitamin D insufficiency group, and 20 participants in the vitamin D sufficiency group, with the effective rate of 75.00% (6/8), 80.00% (36/45), and 86.96% (20/23), respectively. The eosinophil count of the vitamin D deficiency group, vitamin D insufficiency group and vitamin D sufficiency group were 0.61*109/L, 0.46*109/L, and 0.46*109/L, respectively (Table 1).
Vitamin D levels and age
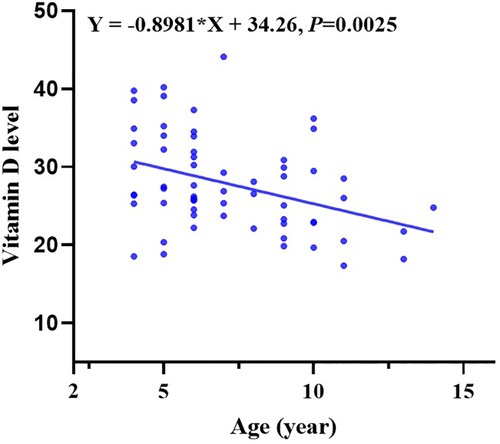
Moreover, multiple comparisons showed that the age in the vitamin D deficiency group, vitamin D insufficiency group, and vitamin D sufficiency group were statistically different with a P value <0.05 (Table 1). Vitamin D levels become lower with children’s growth. We further conducted a linear regression analysis and found a negative relationship between vitamin D levels and age with a regression coefficient (−0.8981) <0 and P-value <0.05 (Figure 1).
Symptom score and medication score before and after AIT
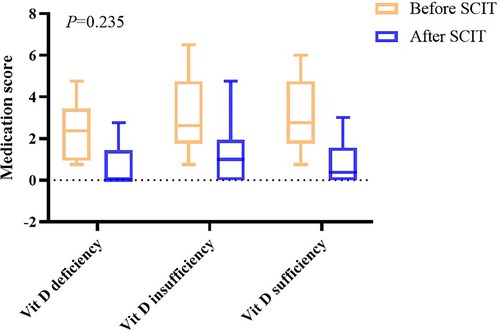
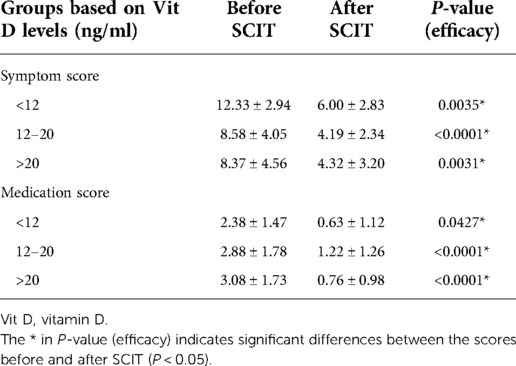
After one year of AIT treatment, the symptom and medication scores statistically decreased (P < 0.05) (Table 2), but analysis of covariance showed there was no difference between the effectiveness in different groups (P = 0.409 in symptom scores and P = 0.235 in medication scores) (Figures 2, 3). Moreover, no difference was found between the symptom score of conventional therapy and cluster therapy (Supplementary Table S1).
Dp sIgE level and df sIgE level between groups
Multiple comparisons among groups showed there was a significant difference in Dp SIgE level between the vitamin deficiency group and vitamin D sufficient group (P = 0.024), but no significant difference between the other two groups comparison (Figure 4). Nevertheless, we found no difference between Df sIgE levels in the vitamin D deficiency group, vitamin D insufficiency group, and vitamin D sufficiency group (P > 0.05) (Figure S1).
Discussion
Our study suggested that subcutaneous immunotherapy could provide adequate relief of allergic rhinitis and asthma symptoms and improve medication scores. However, serum vitamin D levels were weakly associated with the efficacy of one-year subcutaneous immunotherapy. Still, vitamin D levels may be related to the decreased Dp sIgE levels in children receiving SCIT, which is one of the vital indicators for evaluating allergy.
The growing body of evidence indicated that vitamin D as an adjuvant combined with subcutaneous immunotherapy could enhance the efficacy of subcutaneous immunotherapy. Meanwhile, a recent epidemiological study suggested that baseline serum vitamin D levels were associated with subcutaneous immunotherapy in adults with hay pollen allergic rhinitis (14). Nevertheless, the effect of serum vitamin D levels on the subcutaneous immunotherapy of dust mite allergy in children has not been reported. According to the meta-analysis (at least 19 studies), lower vitamin D levels were correlated with a higher allergic rhinitis prevalence in children (22), and vitamin D affects not only the occurrence but also the course and treatment of asthma (23–25). Additionally, previous clinical studies found that in children with dust mite-induced asthma, vitamin D as an adjuvant participating in subcutaneous immunotherapy could achieve a better clinical effect compared to single subcutaneous immunotherapy (26). Experimental studies in mice have also demonstrated that adjuvant use of vitamin D may enhance the efficacy of subcutaneous immunotherapy in grass pollen-induced allergic asthma (11, 12). Vitamin D is an essential immune regulatory factor and may influence the development of asthma and allergy susceptibility through different mechanisms (27). Vitamin D affects innate immunity by inhibiting toll-like receptors and regulates acquired immunity by inhibiting T cell proliferation. Especially in the allergic state, vitamin D acts on the human immune system by inhibiting the growth cycle of human dendritic cells and regulating the function of T cells by increasing the secretion of IL-10 (27, 28). In addition, research has demonstrated that vitamin D is essential for maintaining the integrity of nasal mucosa epithelium, which makes it a potent defense against environmental allergens (27). From this, we further speculate that vitamin D levels in the body may affect the efficacy of immunotherapy through immune regulation.
Intriguingly, our study observed a substantial difference in the Dp SIgE level between the vitamin deficiency group and the vitamin D sufficiency group. Similarly, serum vitamin D levels are closely related to asthma and allergic diseases and were inversely correlated with serum total IgE levels (29). A prior study showed that long-term immune tolerance to allergens might be influenced by a decline in allergen-specific IgE concentrations (4). Therefore, this might mean that high vitamin D levels may not impair AIT’s effectiveness based on symptom and medication scores in our study, but they might make it easier for the body to develop long-term immunological tolerance.
In addition, this study also found that vitamin D deficiency becomes more severe with children’s growth, which is consistent with the previous research (30). Vitamin D levels are frequently influenced by diet and sun exposure. However, as information about diet and outdoor activities is lacking from our investigation, further epidemiological studies are needed to confirm the finding. Meanwhile, a meta-analysis reported that vitamin D levels had significant age-specific relations to the risk of aeroallergen sensitization and allergic rhinitis. Children with serum 25(OH)D ≥ 75 nmol/L were associated with decreased risk of aeroallergen sensitization, but vitamin D supplementation in infancy was not related to the risk of allergic rhinitis (31). Overall, our study suggests that although no correlation was found between serum vitamin D levels and the efficacy of specific immunotherapy, we should pay more attention to the vitamin D levels in school-age children and adolescents, especially in the allergy population.
Furthermore, this study has some limitations. The participants are all from Wenzhou, which does not accurately represent the country’s population. Further study is required to corroborate this finding because of the relatively small sample size and limited representation of participants.
Conclusions
There were no differences between the efficacy of one-year allergen immunotherapy and serum vitamin D levels based on symptom scores and medication scores. We nevertheless discovered that lower vitamin D levels are linked to higher IgE levels of the dermatophagoides pteronyssinus allergy.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
XJ and HZ contributed equally to this work and shared the first authorship. XJ designed the study and coordinated data collection. HZ was responsible for the statistical analysis of data and revised the manuscript. XY and HD assist with data collection. QX designed the study and drafted the initial manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Wenzhou Municipal Science and Technology Bureau (Y20170618).
Acknowledgments
We are indebted to The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University for providing the data.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.1018549/full#supplementary-material.
References
1. Ozdoganoglu T, Songu M. The burden of allergic rhinitis and asthma. Ther Adv Respir Dis (2012) 6(1):11–23. doi: 10.1177/1753465811431975
2. Stern J, Pier J, Litonjua AA. Asthma epidemiology and risk factors. Semin Immunopathol (2020) 42(1):5–15. doi: 10.1007/s00281-020-00785-1
3. Jutel M, Agache I, Bonini S, Burks AW, Calderon M, Canonica W, et al. International consensus on allergy immunotherapy. J Allergy Clin Immunol (2015) 136(3):556–68. doi: 10.1016/j.jaci.2015.04.047
4. Shamji MH, Durham SR. Mechanisms of allergen immunotherapy for inhaled allergens and predictive biomarkers. J Allergy Clin Immunol. (2017) 140(6):1485–98. doi: 10.1016/j.jaci.2017.10.010
5. Dhami S, Kakourou A, Asamoah F, Agache I, Lau S, Jutel M, et al. Allergen immunotherapy for allergic asthma: a systematic review and meta-analysis. Allergy (2017) 72(12):1825–48. doi: 10.1111/all.13208
6. Dhami S, Nurmatov U, Arasi S, Khan T, Asaria M, Zaman H, et al. Allergen immunotherapy for allergic rhinoconjunctivitis: a systematic review and meta-analysis. Allergy. (2017) 72(11):1597–631. doi: 10.1111/all.13201
7. Mueller RS, Jensen-Jarolim E, Roth-Walter F, Marti E, Janda J, Seida AA, et al. Allergen immunotherapy in people, dogs, cats and horses—differences, similarities and research needs. Allergy. (2018) 73(10):1989–99. doi: 10.1111/all.13464
8. Hesse L, van Ieperen N, Habraken C, Petersen AH, Korn S, Smilda T, et al. Subcutaneous immunotherapy with purified Der p1 and 2 suppresses type 2 immunity in a murine asthma model. Allergy. (2018) 73(4):862–74. doi: 10.1111/all.13382
9. Jeffery LE, Wood AM, Qureshi OS, Hou TZ, Gardner D, Briggs Z, et al. Availability of 25-hydroxyvitamin D(3) to APCs controls the balance between regulatory and inflammatory T cell responses. J Immunol. (2012) 189(11):5155–64. doi: 10.4049/jimmunol.1200786
10. van der Aar AMG, Sibiryak DS, Bakdash G, van Capel TMM, van der Kleij HPM, Opstelten D-JE, et al. Vitamin D3 targets epidermal and dermal dendritic cells for induction of distinct regulatory T cells. J Allergy Clin Immunol. (2011) 127(6):1532–40.e7. doi: 10.1016/j.jaci.2011.01.068.
11. Hesse L, Petersen AH, Oude Elberink JNG, van Oosterhout AJM, Nawijn MC. 1,25(OH)VitD3 Supplementation enhances suppression of grass pollen-induced allergic asthma by subcutaneous and sublingual immunotherapy in a mouse model. Sci Rep. (2020) 10(1):8960. doi: 10.1038/s41598-020-65946-6
12. Hesse L, van Ieperen N, Petersen AH, Elberink JNGO, van Oosterhout AJM, Nawijn MC. High dose vitamin D empowers effects of subcutaneous immunotherapy in a grass pollen-driven mouse model of asthma. Sci Rep. (2020) 10(1):20876. doi: 10.1038/s41598-020-77947-6
13. Arikoglu T, Kuyucu S, Karaismailoglu E, Batmaz SB, Balci S. The association of vitamin D, cathelicidin, and vitamin D binding protein with acute asthma attacks in children. Allergy Asthma Proc. (2015) 36(4):51–8. doi: 10.2500/aap.2015.36.3848
14. Joudi M, Farid Hosseini R, Khoshkhui M, Salehi M, Kouzegaran S, Ahoon M, et al. Effects of serum vitamin D and efficacy of subcutaneous immunotherapy in adult patients with allergic rhinitis. Allergy Asthma Immunol Res. (2019) 11(6):885–93. doi: 10.4168/aair.2019.11.6.885
15. Munns CF, Shaw N, Kiely M, Specker BL, Thacher TD, Ozono K, et al. Global consensus recommendations on prevention and management of nutritional rickets. J Clin Endocrinol Metab. (2016) 101(2):394–415. doi: 10.1210/jc.2015-2175
16. Pludowski P, Holick MF, Grant WB, Konstantynowicz J, Mascarenhas MR, Haq A, et al. Vitamin D supplementation guidelines. J Steroid Biochem Mol Biol. (2018) 175:125–35. doi: 10.1016/j.jsbmb.2017.01.021
17. Reddel HK, Bateman ED, Becker A, Boulet L-P, Cruz AA, Drazen JM, et al. A summary of the new GINA strategy: a roadmap to asthma control. Eur Respir J. (2015) 46(3):622–39. doi: 10.1183/13993003.00853-2015
18. Lommatzsch M, Virchow JC. Severe asthma: definition, diagnosis and treatment. Dtsch Arztebl Int. (2014) 111(50):847–55. doi: 10.3238/arztebl.2014.0847
19. Seidman MD, Gurgel RK, Lin SY, Schwartz SR, Baroody FM, Bonner JR, et al. Clinical practice guideline: allergic rhinitis. Otolaryngol Head Neck Surg. (2015) 152(1 Suppl):S1–43. doi: 10.1177/0194599814561600
20. Walker SM, Pajno GB, Lima MT, Wilson DR, Durham SR. Grass pollen immunotherapy for seasonal rhinitis and asthma: a randomized, controlled trial. J Allergy Clin Immunol. (2001) 107(1):87–93. doi: 10.1067/mai.2001.112027
21. Kuna P, Kaczmarek J, Kupczyk M. Efficacy and safety of immunotherapy for allergies to Alternaria alternata in children. J Allergy Clin Immunol. (2011) 127(2):502–8 e1-6. doi: 10.1016/j.jaci.2010.11.036
22. Kim YH, Kim KW, Kim MJ, Sol IS, Yoon SH, Ahn HS, et al. Vitamin D levels in allergic rhinitis: a systematic review and meta-analysis. Pediatr Allergy Immunol. (2016) 27(6):580–90. doi: 10.1111/pai.12599
23. Wang M, Liu M, Wang C, Xiao Y, An T, Zou M, et al. Association between vitamin D status and asthma control: a meta-analysis of randomized trials. Respir Med. (2019) 150:85–94. doi: 10.1016/j.rmed.2019.02.016
24. Kaaviyaa AT, Krishna V, Arunprasath TS, Ramanan PV. Vitamin D deficiency as a factor influencing asthma control in children. Indian Pediatr. (2018) 55(11):969–71. doi: 10.1007/s13312-018-1420-3
25. Liu M, Wang J, Sun X. A meta-analysis on vitamin D supplementation and asthma treatment. Front Nutr. (2022) 9:860628. doi: 10.3389/fnut.2022.860628
26. Baris S, Kiykim A, Ozen A, Tulunay A, Karakoc-Aydiner E, Barlan IB. Vitamin D as an adjunct to subcutaneous allergen immunotherapy in asthmatic children sensitized to house dust mite. Allergy. (2014) 69(2):246–53. doi: 10.1111/all.12278
27. Sikorska-Szaflik H, Sozańska B. The role of vitamin D in respiratory allergies prevention. Why the effect is so difficult to disentangle? Nutrients. (2020) 12(6):1801. doi: 10.3390/nu12061801
28. Penna G, Adorini L. 1 Alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol. (2000) 164(5):2405–11. doi: 10.4049/jimmunol.164.5.2405
29. Mohammadzadeh I, Darvish S, Qujeq D, Hajiahmadi M, Vaghari-Tabari M. Association of serum 25-OH vitamin D₃ with serum IgE and the Pediatric Asthma Severity Score in patients with pediatric asthma. Allergy Asthma Proc. (2020) 41(2):126–33. doi: 10.2500/aap.2020.41.190025
30. Zhu Z, Zhan J, Shao J, Chen W, Chen L, Li W, et al. High prevalence of vitamin D deficiency among children aged 1 month to 16 years in Hangzhou, China. BMC Public Health. (2012) 12:126. doi: 10.1186/1471-2458-12-126
Keywords: 1-25 (OH) D3, dermatophagoides pteronyssinus, dermatophagoides farina, allergy, subcutaneous immunotherapy
Citation: Jia X, Zheng H, Yan X, Dai H and Xiang Q (2022) Effect of baseline serum vitamin D level on symptom and medication scores of subcutaneous immunotherapy in children with mite allergy. Front. Pediatr. 10:1018549. doi: 10.3389/fped.2022.1018549
Received: 13 August 2022; Accepted: 11 October 2022;
Published: 1 November 2022.
Edited by:
Rongfei Zhu, Huazhong University of Science and Technology, ChinaReviewed by:
Yubao Cui, Wuxi People’s Hospital Affiliated to Nanjing Medical University, ChinaHaiyu Hong, Sun Yat-sen University, China
Lisha Li, Peking Union Medical College Hospital (CAMS), China
© 2022 Jia, Zheng, Yan, Dai and Xiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiangwei Xiang NzA4ODI2OUBxcS5jb20=
†These authors have contributed equally to this work and share first authorship
Specialty Section: This article was submitted to Pediatric Immunology, a section of the journal Frontiers in Pediatrics
 Xiaoxiao Jia†
Xiaoxiao Jia† Hang Zheng
Hang Zheng Xiumei Yan
Xiumei Yan Qiangwei Xiang
Qiangwei Xiang