- 1Department of Pediatric Surgery, Wuhan Children's Hospital (Wuhan Maternal and Child Healthcare Hospital), Tongji Medical College, Huazhong University of Science & Technology, Wuhan, China
- 2Department of Ultrasound, Wuhan Children's Hospital (Wuhan Maternal and Child Healthcare Hospital), Tongji Medical College, Huazhong University of Science & Technology, Wuhan, China
This paper reports the diagnosis and treatment process of a case of fetal mesenchymal hamartoma of the liver (MHL), and reviews the previous literature reports. At 38+2 weeks of gestation, prenatal ultrasound found a well bound mixed solid and cystic mass, which was located at the lower edge of the right lobe of the liver and in front of the right kidney of the fetus, but the source and nature of the mass were not clear by ultrasound. Due to the approaching due date, the fetus showed no other abnormal symptoms, and no special treatment was given with the consent of the family members. A female fetus was delivered weighing 3,520 g at 39 weeks. An exploratory laparotomy was performed on the eighth day after delivery. During the operation, it was found that the tumor originated from the fifth, sixth and seventh hepatic segment and the corresponding hepatic segments were removed. Recovery was uneventful and the infant was discharged on the 6th day after surgery. Follow-up at 2 years showed a thriving young girl, and there was no tumor recurrence.
Introduction
MHL is the second most common benign liver tumor in children after infantile hemangioma, accounting for 6%–8% of liver tumors in children (1), and about 80% of them are found in infants under 2 years old (2). MHL can occur as early as the fetus, and there are often no obvious symptoms in the early stage. The tumor can grow rapidly in the abdominal cavity of the fetus, which may cause the death of the fetus in the intrauterine and neonatal period (3). At present, there are a few reports about fetal MHL, most of which are individual cases. The prenatal diagnosis rate is low, and there is no unified treatment plan. It is necessary to summarize the cases of fetal HML in order to understand and treat them comprehensively.
Materials and methods
Case report
A 38-year-old G3P2 female was admitted to our hospital for routine prenatal examination at 38+2 weeks of gestation. Past medical history was negative for medical illnesses, drug or medication exposure, smoking or alcohol ingestion. Diabetes screening was negative. Family history was likewise unremarkable. Maternal serum alpha-feto protein screening was not performed. Ultrasound examination at 34 weeks showed no significant abnormalities. The examination at 38 weeks, however, revealed an intraabdominal well-bounded mixed solid and cystic mass measuring 46 × 29 mm. The mass was located at the lower edge of the right lobe of the fetal liver and in front of the right kidney. It was polycystic with uneven internal echo (Figure 1). The Color Doppler Flow Imaging indicated that there was no obvious blood flow signal. Both kidneys, abdominal wall, stomach bubble, and bladder were normal. There was no evidence of fetal edema. Fetal development was consistent with fetal age. The amniotic fluid index was 7.2 cm. Amniocentesis was not performed. Due to the approaching due date, the fetus showed no other abnormal symptoms, and no special treatment was given with the consent of the family members.
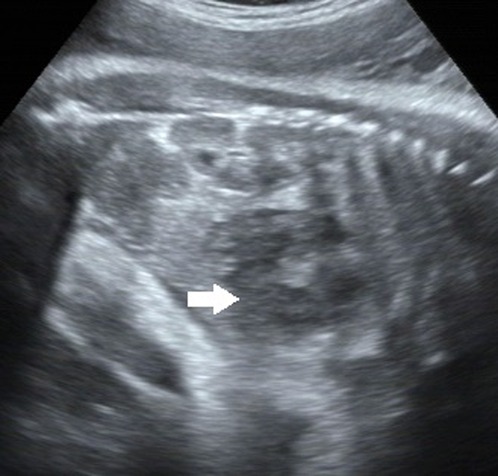
Figure 1. A well-defined 46 mm × 29 mm mixed mass at the lower margin of the right lobe and in front of the right kidney, which is polycystic with uneven internal echo. Arrows point to the mass.
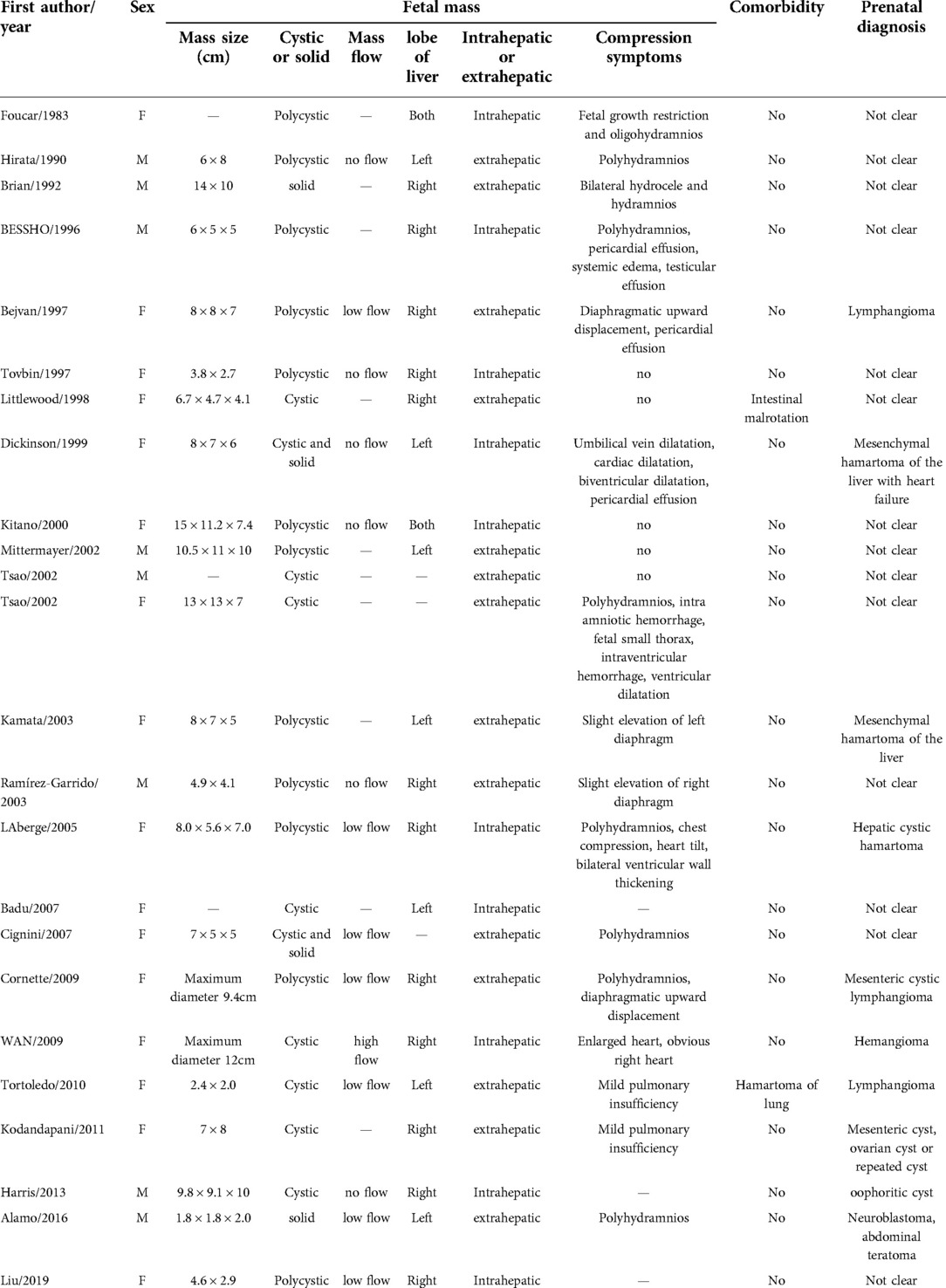
A female fetus weighing 3,520 g was delivered at 39 weeks. The newborn had no acute respiratory distress, the abdominal shape was normal, the abdominal muscles were soft, and no obvious mass was touched. The liver transaminase, direct and indirect bilirubin in the blood were normal, γ Glutalmy transpeptidase (346 u/l) increased. Abdominal computed tomography (CT) revealed a circular cystic mass in the right lobe of the liver, about 41 mm × 29 mm × 53 mm in size, with clear boundary, and multiple linear partitions were seen in it. Intrahepatic bile duct dilatation was also found. On contrast enhanced CT scans, the capsule and septum enhanced, but no enhancement was found inside the tumor. The medial side of the tumor was adjacent to the main portal vein, and the portal vein ran normally, with local compression of the right branch changing (Figure 2). Subcutaneous edema was found in bilateral iliolumbar, lower abdominal wall and proximal thigh. Spleen, kidney and pancreas were normal. The mass was diagnosed as MHL.

Figure 2. Computed tomography (CT) scans of the abdomen. CT scans showed a circular cystic mass in the right lobe of the liver, with multiple linear partitions and clear boundaries. On contrast enhanced CT scans, the capsule and septum enhanced, but no enhancement was found inside the tumor (arrow).
The operation was performed on the eighth day after birth. During the operation, it was found that the tumor originated from the fifth, sixth and seventh hepatic segment and the corresponding hepatic segment were removed. The tumor was grayish brown, measuring 70 × 55 × 40 mm. Multiple cysts with a diameter of 5–35 mm could be seen, and cysts were filled with yellow liquid. Part of the liver was gelatinous. Microscopically, nonmalignant proliferation of hepatocytes and fibroblasts could be seen in the edematous collagen matrix (Figure 3). The pathological findings were consistent with MHL.
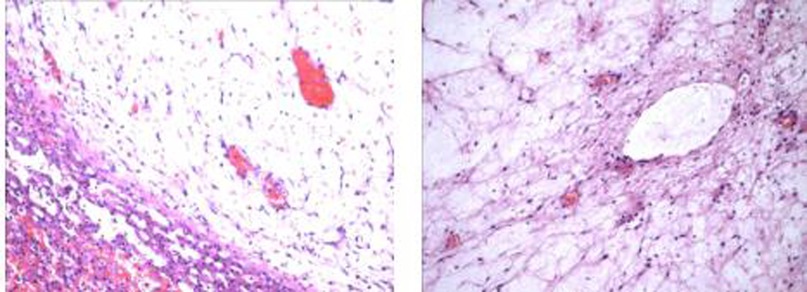
Figure 3. Nonmalignant proliferation of hepatocytes and fibroblasts could be seen in the edematous collagen matrix.
The infant recovered and was discharged on the 6th day after operation. Follow-up at 2 years showed a thriving young girl, and there was no tumor recurrence.
Literature review
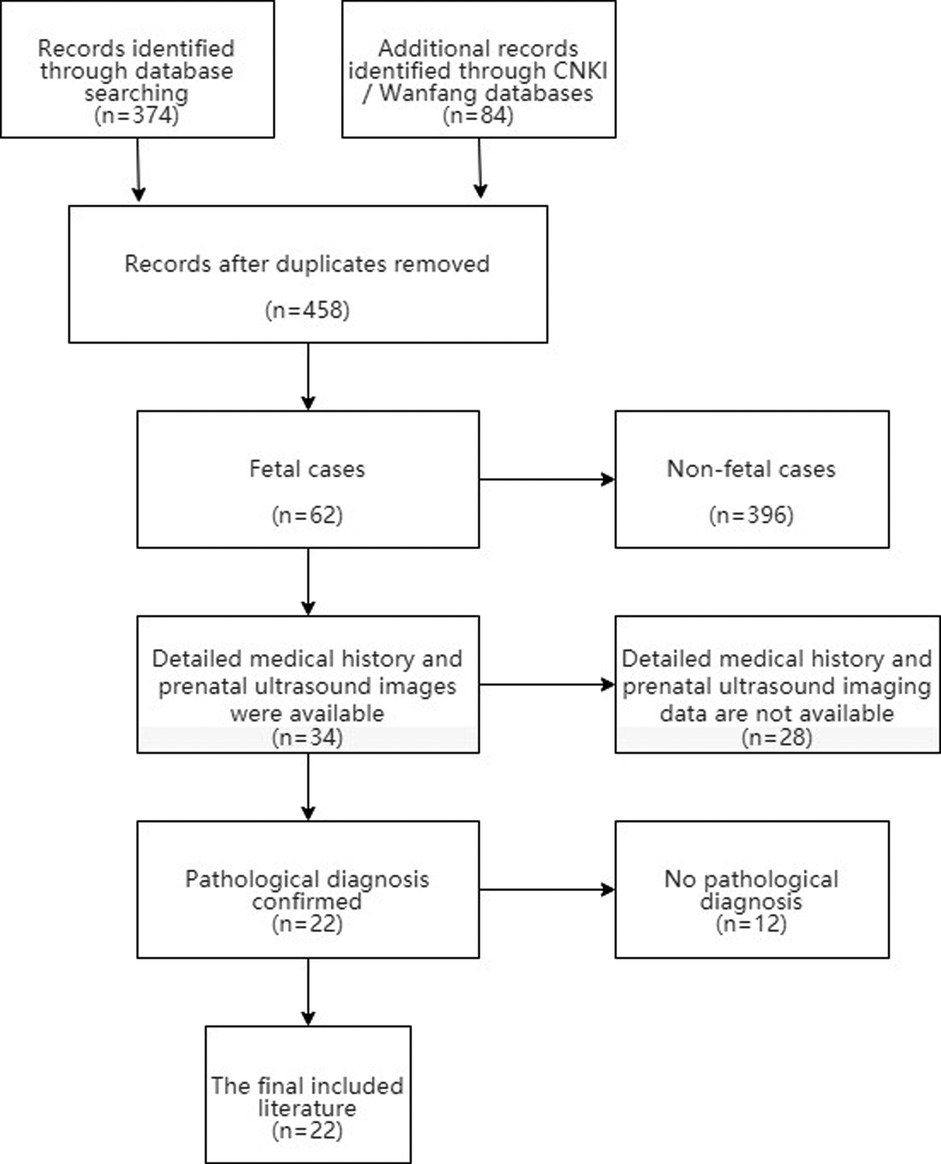
For a systematic review of the literature, PubMed, CNKI and Wanfang databases were searched to retrieve the related literature of fetal mesenchymal hamartoma of the liver from January 1980 to March 2021. We selected articles for further reading based on the title and abstract. Inclusion criteria: (1) Liver masses were found in the fetus; (2) Detailed medical history and prenatal ultrasound imaging data. (3) The pathological diagnosis was MHL. We created a PRISMA flow chart showing the results of the literature search (Figure 4).
Results
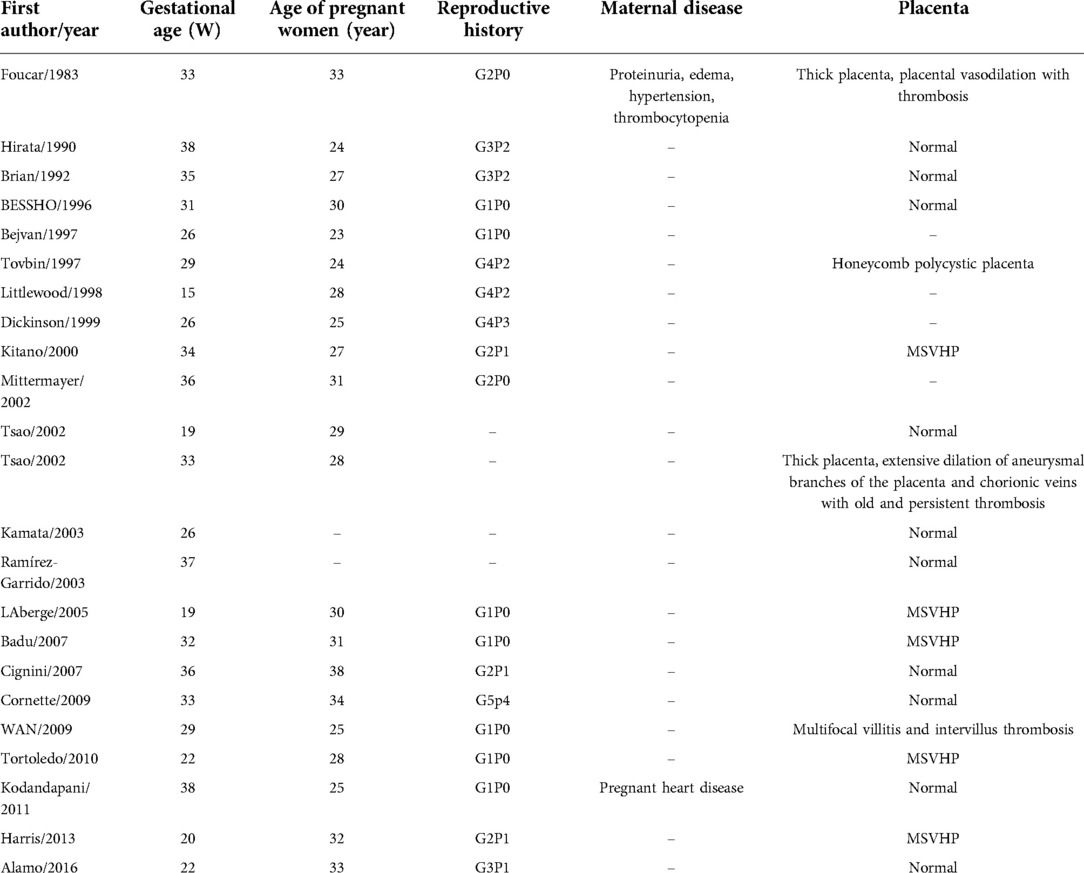
After reviewing and complying with the inclusion criteria, 22 articles were listed (4–25). Including our patient, there are 24 reported cases of fetal MHL. There were 8 male and 16 female fetuses. Nine cases (37.5%) were found in the second trimester and 15 cases (62.5%) in the third trimester. The gestational age ranged from 15 weeks to 38 weeks, and the median gestational age was 31.5 weeks. The pregnant women were 23 to 38 years old, with a median age of 28.5 years. Two pregnant women had underlying diseases. There were 9 cases (37.5%) complicated with placental abnormalities, including 5 cases of mesenchymal stem villous hyperplasia of the placenta (MSVHP), 3 cases of placental thrombosis, and 1 case of honeycomb polycystic placenta (Table 1). The diameter of the tumor ranged from 2.0 cm to 15 cm. 20 cases were cystic, 2 cases were mixed and solid respectively. 22 patients had low or no blood flow signal, and 2 patients had high blood flow signal. After surgery or autopsy, 12 cases were found to be located in the right lobe of the liver, 7 in the left lobe, and 2 in the left and right lobes. 10 cases were intrahepatic and 14 cases were exogenous. 9 cases had polyhydramnios, 5 cases had cardiac abnormalities such as pericardial effusion or ventricular dilatation, and 8 cases had pulmonary abnormalities such as diaphragm elevation, small thorax and mild pulmonary insufficiency. Only 3 cases were correctly diagnosed as MHL before operation (Table 2). Five fetuses died in utero, three died within one week after birth, and the other 16 survived long term. There were 6 cases of spontaneous delivery and 1 case died in perinatal period. 13 cases of cesarean section, 2 cases died in perinatal period. Antenatal puncture and drainage were performed in 3 cases. After birth, 18 cases underwent neonatal tumor resection, and 15 cases survived, 3 cases died during the perioperative period. Among the 18 cases, 16 cases had complete tumor resection, while 2 cases had most of the tumors removed. Two cases underwent laparoscopic surgery. Three cases performed staged surgery for the tumor. Perinatal biopsy was performed, followed by radical surgery at 7 weeks, 10 weeks and 20 months postpartum. In two cases, liver cysts recurred again after operation, and finally became smaller and subsided after observation.
Discussion
MHL was first described by Mares in 1903 and named by Edmondson in 1956 (26). Histologically, it is composed of loose mesenchymal tissue, bile duct, connective tissue and hepatocytes, accompanied by cysts formed by mesenchymal degenerative areas or dilated bile duct and lymphatic vessels. Although MHL is the second most common benign liver tumor in childhood, fetal MHL is still a rare event. In our study, 24 cases of fetal MHL diagnosed by pathology were found by prenatal ultrasound in Chinese and English literature. Only 3 cases were correctly diagnosed before delivery, and the perinatal mortality rate was as high as 37.5%. It's necessary to summarize the previous cases and discuss the diagnosis and treatment of perinatal fetal MHL.
Pathogenesis
The underlying pathogenesis of MHL is not clearly defined. Several hypotheses have been proposed for the etiology. Developmental anomalies, biliary obstruction, regional ischaemia, or disordered hyperplasia after liver injury have all been proposed as playing a role in its genesis (27, 28). However, the current discovery of the cytogenetics and molecular genetics showed that HML could be a real tumor. Recurrent genetic alterations identified in HML include androgenetic-biparental mosaicism (ABM) and chromosomal rearrangements which result in activation of chromosome 19q microRNA cluster (C19MC) (29). Meanwhile, sporadic HMH lesions are frequently associated with a translocation involving MALAT1 gene at chromosome 11q13 and C19MC gene at 19q13.4 (30). Appelaniz-Ruiz et al. reported two pediatric cases of mesenchymal hamartoma of the liver associated with germline DICER1 pathogenic variants (31). El Demellawy et al. described an atypical HML with a tandem triplication of chromosomal segment at chromosomem 1q44 (32). Meanwhile, rare cases of HML in the setting of Beckwith-Wiedemann syndrome (BWS) have been reported (33). HML may also be part of the expanding spectrum of findings of BWS (34). In addition, the other intriguing reports of rare concurrent infantile hemangioendothelioma and MHL (35, 36), and whether the two have a common pathogenesis is unclear. It is possible that MHL is etiologically heterogeneous. Overall, more cases are needed to further clarify the pathogenesis of HML.
Clinical presentation
In this group, fetal MHL was found by prenatal imaging, and the pregnant women had no obvious clinical symptoms. Fetal MHL mainly occurs in the second and third trimester of pregnancy. Littlewood et al. (10) first discovered fetal MHL at the 15th week of pregnancy. MHL is considered to be a tumor associated with the growth of hilar structures during liver development. Embryological studies showed that the portal area appears at the 12th week of the fetal liver, and begins to secrete bile at the 14th week. In this group of cases, the lesions were not discovered until 15 weeks later, corresponding to the development time of fetal liver. The median age of pregnant women was 28.5 years old (23–38 years old), and all of them were younger than 35 years old, except one who was 38 years old. Twelve of them had given birth to healthy children without liver tumors. There were no obvious underlying diseases in pregnant women except case 1, who had a history of proteinuria, edema, hypertension and thrombocytopenia before pregnancy, and case 21, who had gestational heart disease。Only two pregnant women had an increase in alpha- fetoprotein and one had an abnormal increase in β-human chorionic gonadotropin (4, 11, 12).
The male and female fetuses in this group were 8 (33.3%) and 16 (66.7%) respectively, which is different from the previous studies in which the male incidence rate of infant cases was higher than that of female (37). In this study, the male perinatal mortality rate was 25% (2 cases), while the female perinatal mortality rate was as high as 43.8% (7 cases), which may also be the reason why the proportion of male cases in infancy was higher than that of female cases. The specific mechanism needs to be further studied.
Imaging findings
Fetal MHL was mainly located in the liver or the lower margin of the liver, a small number of large exogenous masses occupied most of the abdominal cavity, and the source of the lesions could not be determined. In 6 cases, the lesions were intrahepatic, and in 11 cases, the tumors were located at the lower edge of the liver lobe, the upper end of the right kidney, and the lower end of the diaphragm. The lesion site could not be determined in 7 cases. Finally, it was confirmed by operation or autopsy that 10 cases were intrahepatic lesions, 14 cases were exogenous lesions. Among the 7 cases of tumors whose source could not be determined before delivery, 5 cases were exophytic, the diameter of tumors ranged from 7 cm to 13 cm, and some tumors occupied most of the abdominal cavity, and even extended to the pelvic cavity. Among the 7 cases, 2 cases were misdiagnosed as lymphangioma and 1 case was misdiagnosed as ovarian cyst. On the contrary, prenatal ultrasound is relatively easy to determine the source of intrahepatic lesions, but the source of mass cannot be determined in some intrahepatic lesions. Harris (25) reported a case in which the entire right lobe of the liver was occupied by a cystic mass, which was misdiagnosed as an ovarian cyst prenatal.
Ultrasonography of fetal MHL shows cystic and solid masses of varying sizes in the abdominal cavity. Most of them are cystic, including multicystic and unicystic, showing hypoechoic or anechoic, and a few are mixed or solid. Solid components can also be seen at the edge of the cystic MHL, and septa of varying thickness can be seen in the mass. Color Doppler Flow Imaging shows that most of the tumors have low blood flow or no blood flow signal, and a few have high blood flow signal. In this group, 20 cases (83.3%) were cystic, and only 2 cases (8.33%) were mixed and solid respectively. Some studies believe that MHL is solid at first. With the growth of the mass, interstitial cystic change is accompanied by secondary fluid accumulation, lymphatic obstruction and expansion in the cyst, which gradually expands the cystic area in the mass, and occupies most or even all of the mass, making the mass appear cystic solid or mainly cystic (37). Through the studies of ultrasonic and pathological on children's solid MHL, Wang Xiaoman et al. (38) believed that spongy MHL could not be identified by ultrasound due to its tiny pores, and thus showed solid changes, but its pathological findings still had numerous small cysts, still in line with the pathological characteristics of polycystic. In this group, 23 cases (92.9%) had low or no blood flow signal, and only 1 case (7.1%) had high blood flow signal. Cases with high blood flow signal are easy to be misdiagnosed. Cases with high blood flow signal are prone to misdiagnosis. WAN (21) misdiagnosed a HML with high blood flow signal as hemangioma. Because fetal ultrasound showed a vascular cystic mass in the liver, postpartum ultrasound and MRI also suggested high blood flow signal.
Although MHL is a benign tumor, fetal MHL can grow rapidly, resulting in displacement of surrounding organs or compression symptoms. In this group, 9 cases had polyhydramnios, 5 cases had cardiac abnormalities such as pericardial effusion or ventricular dilatation, and 8 cases had pulmonary abnormalities such as diaphragm elevation, small thorax and mild pulmonary insufficiency. MHL can compress the inferior vena cava and umbilical vein, which can lead to congestive heart failure, including fetal heart enlargement, cardiac dilatation, pericardial effusion, fetal edema, etc. Fluid accumulation in cysts and decreased albumin production due to compression of the liver itself further increase the risk of edema. Compression from the mass causes obstruction of the upper digestive tract, leading to polyhydramnios. In addition, the fetus is at risk of lung hypoplasia due to the compression of the diaphragm by the mass.
Fetal magnetic resonance imaging (MRI) may help to clarify the source of the lesion and the nature of the tumor. Compared with ultrasound, MRI has higher resolution in soft tissue examination. The visual field is larger, and can be scanned in any section to display the full picture of the fetus. MRI is less affected by the mother's condition, not by the fetal bone and amniotic fluid volume, and less affected by the operator's technical level. Currently, MRI has been increasingly used in the diagnosis of fetal diseases, and has become an important supplement to obstetric ultrasound, and can provide additional information that cannot be detected by ultrasound. In this group, a total of 5 fetuses received prenatal MRI examination, mainly showing high signal in T2-weighted images and low signal in T1-weighted images, suggesting cystic components. Hepatic hamartoma was diagnosed by MRI in 2 of 5 cases (40%), while prenatal ultrasound accurately diagnosed only 1 case. In another 2 cases, the tumor was derived from liver by judging the accurate relationship between the tumor and surrounding organs, and only 1 case of huge cystic mass was misdiagnosed as ovarian cyst. Therefore, MRI further examination is recommended when prenatal abdominal masses cannot be clearly diagnosed.
Concommitant placental abnormalities have been reported in 9 cases (37.5%) of HML, including 5 cases with mesenchymal stem villous hyperplasia of the placenta (MSVHP), 3 cases with placental thrombosis and 1 case with honeycomb polycystic placenta. MSVHP is a recently proposed placental disease, which is characterized by diffuse edema of stem villi, relatively normal terminal villi, and often accompanied by abnormal villi vasodilatation. The increased incidence of HML with PMD and the morphological similarities of the changes seen in both the placenta and liver, suggests a possible common developmental mechanism. The association between HML and placental mesenchymal dysplasia was initially described by Alwaidh et al. (39). Both entities share androgenetic-biparental mosaicism as an underlying mechanism; paternal uniparental disomy results in imbalanced expression of imprinted loci in androgenetic cells which may lead to abnormal tissue phenotypes (40).
Treatment of fetal MHL
The main cause of death in fetuses and neonates with mesenchymal hamartoma was progressive abdominal distension owing to a rapidly expanding, fluid-filled, cystic hepatic mass that produced severe respiratory distress and compression of normal, intraabdominal blood vessels and other structures (41). The primary task of treatment is to ensure the stability of fetuses and neonates vital signs.
In this group, 3 patients underwent biopsy and drainage of mass before delivery. Tsao et al. (14) described a large fetal intraperitoneal unilocular HML found at 19 weeks, which began to decrease after three cyst aspirations. A healthy boy was born at 35 weeks and the cyst was removed laparoscopically. Bejvan (9) reported an invasive puncture at 26 weeks for a rapidly growing polycystic mass with pericardial effusion. Due to the rapid re-accumulation of fluid after puncture, the authors re-inserted two pigtail catheters, one between the largest cyst and the other between the cyst and the amniotic cavity, but the results were not satisfactory. Premature rupture of membranes occurred at 30 weeks and the baby was delivered by cesarean section. Postnatal percutaneous drainage also had no significant effect. On the 21st day after birth, laparotomy was performed to confirm and remove a pedicled hepatic mesenchymal hamartoma. Kamata et al. (15) found a polycystic mass in the left upper abdomen at 26 weeks, and had an emergency caesarean section at 30 + 5 weeks due to maternal hypertension. The fetus underwent percutaneous cyst puncture and drainage before delivery, and bleeding was found at the puncture site after delivery, with hemoglobin as low as 5.7 g/dl. Emergency laparotomy was forced, and hemorrhage within the tumor was finally found. Thus, in polycystic MHL, drainage may not achieve the desired effect of tumor reduction due to unobstructed and incompatible communication between different cysts. Since the liquid will be quickly regenerated, the drainage is repeated frequently. And prenatal treatment is only temporary decompression, which can't reduce the need for postpartum surgical resection. Meanwhile, puncture may lead to fetal bleeding. In addition, invasive fetal treatment carries a risk of premature rupture of membranes and premature delivery. It is also worth noting that previous studies have shown that the cytology of cyst aspirates cannot be used to determine the diagnosis (38), so this invasive procedure has no role in aiding diagnosis. Therefore, from these only three puncture cases, it can be inferred that prenatal cyst puncture may be of therapeutic significance for monocystic MHL, but not for polycystic MHL, and may cause harm to the fetus.
Early cesarean section can improve the fetal survival rate. In this group, 13 cases underwent early cesarean section, and finally 3 cases died after delivery. The other 11 cases underwent observation or natural delivery. Finally, 5 cases died in utero and 1 case died after delivery. The rapid growth of fetal MHL volume can often produce compression symptoms. MHL compresses the inferior vena cava and umbilical vein, which can lead to congestive heart failure, resulting in fetal edema. There were 5 cases of fetal heart enlargement, dilatation of the heart cavity, pericardial effusion and fetal edema, among which 4 cases died in perinatal period. 8 cases suffered from chest compression, such as diaphragmatic muscle elevation, thoracic diminution and pulmonary insufficiency, of which 4 cases died in perinatal period. Cardiopulmonary compression symptoms caused by MHL may be an important cause of fetal death. Cesarean section is beneficial to fetal survival before serious cardiopulmonary dysfunction occurs. In addition, vaginal delivery is reported to carry a risk of cyst rupture and death, as well as a risk of soft tissue dystocia, so cesarean section should be the preferred method of delivery.
Since MHL has often caused compression symptoms of peripheral organs, and the tumor volume still grows rapidly, timely and effective treatment is needed. Some new genetic evidences suggest the MHL may be neoplastic ab initio. In addition, Clinical and histological evidence has strongly suggested that UES can develop within a pre-existing HML. Malignant transformation may occur several years after an incomplete resection of the lesions (42). Therefore, the preferred treatment is complete resection of the mass. Open resection or laparoscopic surgery can be performed according to the size and anatomical location of the mass. When one-stage resection is difficult, staged operation still has good effect. Regular radiological follow-up should be carried out after operation, and local recurrence may subside. But it should be noted that it is important to sample different parts of the HML to exclude areas of malignant transformation (2). For some neonates with unstable vital signs, tumor vascular interventional therapy can be considered (43). Liver transplantation is the most effective treatment for unresectable symptomatic HML (33, 44).
In conclusion, MHL is a rare benign tumor of fetal liver, which is often found in the second and third trimester of pregnancy, and there is no obvious predisposing population. The typical ultrasonographic findings of MHL are cystic and solid masses in the liver region, which are mainly cystic and rarely solid, and most of them are exophytic growth. The Color Doppler Flow Imaging shows that most of the tumors have low blood flow or no blood flow signal, and a few have high blood flow signal. MHL is often accompanied by compression symptoms such as cardiac enlargement, dilatation of cardiac cavity, pericardial effusion, fetal edema, elevation of diaphragm, diminution of thorax, pulmonary insufficiency, and increased amniotic fluid. MHL is often associated with placental mesenchymal dysplasia. Prenatal cyst puncture may have therapeutic significance for single cystic MHL, but not for polycystic MHL, and may cause damage to the fetus. Cesarean section may improve the survival rate of newborns. Complete resection of tumor after birth is an effective treatment. When one-stage operation is difficult to perform, vascular intervention or partial resection is feasible, and then re-staged operation still has a good prognosis. Liver transplantation is the most effective treatment for unresectable symptomatic HML.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
XD and XY: contributed to the conception of the study; ZZ and FP: contributed significantly to analysis and manuscript preparation; XL and ST: performed the data analyses and wrote the manuscript; These authors contributed equally to this work and should be considered co-first authors. QY: helped perform the analysis with constructive discussions. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Laboratory of Hepatobiliary and Pancreatic Diseases in Children (Construction Unit) (2022FEYJS004).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Meyers RL. Tumors of the liver in children. Surg Oncol. (2007) 16(3):195–203. doi: 10.1016/j.suronc.2007.07.002
2. Martins-Filho SN, Putra J. Hepatic mesenchymal hamartoma and undifferentiated embryonal sarcoma of the liver: a pathologic review. Hepat Oncol. (2020) 7(2):HEP19. Published 2020 Apr 7. doi: 10.2217/hep-2020-0002
3. Alamo L, Perrin L, Vial Y, Anooshiravani M, Meuli R. Prenatal imaging of congenital hepatic tumors: a report of three cases. Clin Imaging. (2017) 41:112–7. doi: 10.1016/j.clinimag.2016.10.003
4. Foucar E, Williamson RA, Yiu-Chiu V, Varner MW, Kay BR. Mesenchymal hamartoma of the liver identified by fetal sonography. AJR Am J Roentgenol. (1983) 140(5):970–2. doi: 10.2214/ajr.140.5.970
5. Hirata GI, Matsunaga ML, Medearis AL, Dixon P, Platt LD. Ultrasonographic diagnosis of a fetal abdominal mass: a case of a mesenchymal liver hamartoma and a review of the literature. Prenat Diagn. (1990) 10(8):507–12. doi: 10.1002/pd.1970100805
6. Mason BA, Hodges W, Goodman JR. Antenatal sonographic detection of a rare solid hepatic mesenchymal hamartoma. J Matern Fetal Med. (1992) 1:134–6. doi: 10.3109/14767059209161906
7. Bessho T, Kubota K, Komori S, Ohtsuka Y, Uneo Y, Uematsu K, et al. Prenatally detected hepatic hamartoma: another cause of non-immune hydrops. Prenat Diagn. (1996) 16(4):337–41. doi: 10.1002/(SICI)1097-0223(199604)16:4%3C337:AID-PD848%3E3.0.CO;2-U
8. Bejvan SM, Winter TC, Shields LE, Brock BV, Nghiem HV, Schmiedl UP, et al. Prenatal evaluation of mesenchymal hamartoma of the liver: gray scale and power Doppler sonographic imaging. J Ultrasound Med. (1997) 16(3):227–9. doi: 10.7863/jum.1997.16.3.227
9. Tovbin J, Segal M, Tavori I, Lotan G, Maymon R. Hepatic mesenchymal hamartoma: a pediatric tumor that may be diagnosed prenatally. Ultrasound Obstet Gynecol. (1997) 10(1):63–5. doi: 10.1046/j.1469-0705.1997.10010063.x
10. Littlewood Teele R, Pease PW, Rowley RS. Malrotation in newborns following antenatal diagnosis of intra-abdominal cyst. Pediatr Radiol. (1998) 28(9):717–21. doi: 10.1007/s002470050450
11. Dickinson JE, Knowles S, Phillips JM. Prenatal diagnosis of hepatic mesenchymal hamartoma. Prenat Diagn. (1999) 19(1):81–4. doi: 10.1002/(SICI)1097-0223(199901)19:1%3C81::AID-PD469%3E3.0.CO;2-H
12. Kitano Y, Ruchelli E, Weiner S, Adzick NS. Hepatic mesenchymal hamartoma associated with mesenchymal stem villous hyperplasia of the placenta. Fetal Diagn Ther. (2000) 15(3):134–8. doi: 10.1159/000020992
13. Mittermayer C, Bettelheim D, Horcher E, Fakhari M, Deutinger J, Bernaschek G. Prenatal sonographic detection of a giant multiseptate hepatic cyst in the third trimester. Ultrasound Obstet Gynecol. (2002) 20(1):97–8. doi: 10.1046/j.1469-0705.2002.00722_3.x
14. Tsao K, Hirose S, Sydorak R, Goldstein RB, Machin GA, Albanese CT, et al. Fetal therapy for giant hepatic cysts. J Pediatr Surg. (2002) 37(10):E31. doi: 10.1053/jpsu.2002.35444
15. Kamata S, Nose K, Sawai T, Hasegawa T, Kuroda S, Sasaki T, et al. Fetal mesenchymal hamartoma of the liver: report of a case. J Pediatr Surg. (2003) 38(4):639–41. doi: 10.1053/jpsu.2003.50142
16. Ramírez-Garrido F, LópezGonzález-Garrido Jde D, Ruíz-López MJ, Sabatell-López RM, Mirás-Baldó MJ, Rodróguez-Fernández A. Prenatal and post-natal imaging of an hepatic mesenchymal hamartoma. Eur J Pediatr. (2003) 162(1):57–8. doi: 10.1007/s00431-002-1058-x
17. Laberge JM, Patenaude Y, Desilets V, Cartier L, Khalife S, Jutras L, et al. Large hepatic mesenchymal hamartoma leading to mid-trimester fetal demise. Fetal Diagn Ther. (2005) 20(2):141–5. doi: 10.1159/000082439
18. Francis B, Hallam L, Kecskes Z, Ellwood D, Croaker D, Kent A. Placental mesenchymal dysplasia associated with hepatic mesenchymal hamartoma in the newborn. Pediatr Dev Pathol. (2007) 10(1):50–4. doi: 10.2350/06-03-0066.1
19. Cignini P, Coco C, Giorlandino M, Bagolan P, Morini F, Giorlandino C. Fetal hepatic mesenchymal hamartoma: a case report. J Prenat Med. (2007) 1(3):45–6.22470828
20. Cornette J, Festen S, van den Hoonaard TL, Steegers EA. Mesenchymal hamartoma of the liver: a benign tumor with deceptive prognosis in the perinatal period. Case report and review of the literature. Fetal Diagn Ther. (2009) 25(2):196–202. doi: 10.1159/000212057
21. Wan P, Susman J, Kandel J, Jan D, Jerome EH, Parravicini E. Neonatal hepatic mesenchymal hamartoma causing cardiac failure and disseminated intravascular coagulopathy. Am J Perinatol. (2009) 26(8):601–4. doi: 10.1055/s-0029-1220783
22. Tortoledo M, Galindo A, Ibarrola C. Placental mesenchymal dysplasia associated with hepatic and pulmonary hamartoma. Fetal Pediatr Pathol. (2010) 29(4):261–70. doi: 10.3109/15513811003782474
23. Kodandapani S, Pai MV, Kumar V, Pai KV. Prenatal diagnosis of congenital mesenchymal hamartoma of liver: a case report. Case Rep Obstet Gynecol. (2011) 2011:932583. doi: 10.1155/2011/932583
24. Ruhland B, Schröer A, Gembruch U, Noack F, Weichert J. Prenatal imaging and postnatal pathologic work-up in a case of fetal hepatic hamartoma and placental mesenchymal dysplasia. Ultrasound Obstet Gynecol. (2011) 38(3):360–2. doi: 10.1002/uog.9005
25. Harris K, Carreon CK, Vohra N, Williamson A, Dolgin S, Rochelson B. Placental mesenchymal dysplasia with hepatic mesenchymal hamartoma: a case report and literature review. Fetal Pediatr Pathol. (2013) 32(6):448–53. doi: 10.3109/15513815.2013.835893
26. Khan MR, Binkovitz LA, Smyrk TC, Potter D Jr, Furuya KN. Mesenchymal hamartoma in children: a diagnostic challenge. Case Rep Pediatr. (2019) 2019:4132842. Published 2019 Sep 16. doi: 10.1155/2019/4132842
27. Edmonson HA. Differential diagnosis of tumors and tumor-like lesions of liver in infancy and childhood. Am J Dis Child. (1956) 91:168–86. doi: 10.1001/archpedi.1956.02060020170015
28. Lennington WJ, Gray GF Jr, Page DL. Mesenchymal hamartoma of liver: a regional ischemic lesion of a sequestrated lobe. Am J Dis Child. (1993) 147:193–6. doi: 10.1001/archpedi.1993.02160260083027
29. Kapur RP, Berry JE, Tsuchiya KD, Opheim KE. Activation of the chromosome 19q MicroRNA cluster in sporadic and androgenetic-biparental mosaicism–associated hepatic mesenchymal hamartoma. Pediatr Dev Pathol. (2014) 17(2):75–84. doi: 10.2350/13-12-1415-OA.1
30. Keller RB, El Demellawy D, Quaglia A, Finegold M, Kapur RP. Methylation status of the chromosome arm 19q MicroRNA cluster in sporadic and androgenetic-biparental mosaicism–associated hepatic mesenchymal hamartoma. Pediatr Dev Pathol. (2015) 18(3):218–27. doi: 10.2350/15-01-1600-OA.1
31. Apellaniz-Ruiz M, Segni M, Kettwig M, Glüer S, Pelletier D, Nguyen VH, et al. Mesenchymal hamartoma of the liver and DICER1 syndrome. N Engl J Med. (2019) 380(19):1834–42. doi: 10.1056/NEJMoa1812169
32. El Demellawy D, Lee JY, McDonell L, Dyment DA, Knisely AS, McGowan-Jordan J, et al. Atypical hepatic mesenchymal hamartoma: histologic appearance, immunophenotype, and molecular findings. Pediatr Dev Pathol. (2019) 22(4):365–9. doi: 10.1177/1093526618806750
33. Pan ET, Yoeli D, Kueht ML, Galvan NTN, Cotton RT, O'Mahony CA, et al. Liver transplantation as definitive treatment of an unresectable mesenchymal hamartoma in a child with beckwith-wiedemann syndrome. J Surg Case Rep. (2017) 2017(8):rjx167. Published 2017 Aug 31. doi: 10.1093/jscr/rjx167
34. Cajaiba MM, Sarita-Reyes C, Zambrano E, Reyes-Múgica M. Mesenchymal hamartoma of the liver associated with features of beckwith-wiedemann syndrome and high serum alpha-fetoprotein levels. Pediatr Dev Pathol. (2007) 10(3):233–8. doi: 10.2350/06-07-0128.1
35. Bejarano PA, Serrano MF, Casillas J, Dehner LP, Kato T, Mitral N, et al. Concurrent infantile hemangioendothelioma and mesenchymal hamartoma in a developmentally arrested liver of an infant requiring hepatic transplantation. Pediatr Dev Pathol. (2003) 6(6):552–7. doi: 10.1007/s10024-003-3024-7
36. Hsiao KH, Lin LH, Chen DF, Huang SH. Hepatic mesenchymal hamartoma combined with infantile hepatic hemangioendothelioma in an infant. J Formos Med Assoc. (2007) 106(3 Suppl):S1–4. doi: 10.1016/s0929-6646(09)60357-0
37. Sun X, Yuan X, Yang M, Zhang X, Zou J, Guo H, et al. CT Manifestations and clinical pathology features of hepatic mesenchymal hamartoma in children. Chin J Radiol. (2013) 47(10):917–21. doi: 10.3760/cma.j.issn.1005-1201.2013.10.011
38. Wang X, Jia L, Hu Y. Ultrasonic manifestations of heptic mesenchymal hamartoma in children. Chin J Med Ultrasound (Electr Ed). (2012) 9(6):516–20. doi: 10.3877/cma.j.issn.1672-6448.2012.06.015
39. Alwaidh MH, Woodhall CR, Carty HT. Mesenchymal hamartoma of the liver: a case report. Pediatr Radiol. (1997) 27(3):247–9. doi: 10.1007/s002470050113
40. Reed RC, Beischel L, Schoof J, Johnson J, Raff ML, Kapur RP. Androgenetic/biparental mosaicism in an infant with hepatic mesenchymal hamartoma and placental mesenchymal dysplasia. Pediatr Dev Pathol. (2008) 11(5):377–83. doi: 10.2350/07-12-0401.1
41. Isaacs H Jr. Fetal and neonatal hepatic tumors. J Pediatr Surg. (2007) 42(11):1797–803. doi: 10.1016/j.jpedsurg.2007.07.047
42. Stringer MD, Alizai NK. Mesenchymal hamartoma of the liver: a systematic review. J Pediatr Surg. (2005) 40(11):1681–90. doi: 10.1016/j.jpedsurg.2005.07.052
43. Mulrooney DA, Carpenter B, Georgieff M, Angel C, Hunter D, Foker J, et al. Hepatic mesenchymal hamartoma in a neonate: a case report and review of the literature. J Pediatr Hematol Oncol. (2001) 23(5):316–7. doi: 10.1097/00043426-200106000-00017
Keywords: mesenchymal hamartoma, liver tumor, fetus, prenatal examination, treat
Citation: Liu X, Tian S, Zhu Z, Peng F, Yuan Q, Duan X and Yan X (2022) Fetal mesenchymal hamartoma of the liver: A case report and literature review. Front. Pediatr. 10:1016260. doi: 10.3389/fped.2022.1016260
Received: 11 August 2022; Accepted: 7 November 2022;
Published: 23 November 2022.
Edited by:
Francesco Morini, Meyer Children's Hospital, ItalyReviewed by:
Bo-Yee Ngan, Hospital for Sick Children, University of Toronto, CanadaEmil Radu Iacob, Victor Babes University of Medicine and Pharmacy, Romania
© 2022 Liu, Tian, Zhu, Peng, Yuan, Duan and Yan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xueqiang Yan eWFueHVlcWlhbmcxQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
Specialty Section: This article was submitted to Pediatric Surgery, a section of the journal Frontiers in Pediatrics
 Xin Liu1†
Xin Liu1† Xueqiang Yan
Xueqiang Yan