
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Pediatr., 23 January 2023
Sec. Pediatric Infectious Diseases
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.1013300
This article is part of the Research TopicCase Reports in Pediatric Infectious Diseases 2022View all 10 articles
We present the case of a 13-year-old boy who unexpectedly needed to be resuscitated at home after an assumed uncomplicated otitis media. Imaging at our clinic showed mastoiditis and a cystoid mass in the left cerebellopontine angle compressing the brainstem, as well as an Arnold-Chiari-Malformation. Both the laboratory examination of cerebrospinal fluid (CSF) and surgical biopsy with pathological evaluation of the mastoid supported the inflammatory etiology of the mass. Microbiologically, Streptococcus intermedius was detected in the blood culture and CSF. Due to brain death, which most likely already existed preclinically, the organs were released for donation during the course. Our case demonstrates a very rare lethal complication of acute otitis media on the basis of a cerebral malformation and emphasizes the need to stay alert when patients complain of symptoms after assumed resolution.
Mastoiditis, despite being the most common severe complication of acute otitis media (AOM), is a nowadays rare condition (1, 2). However, since it is a potentially life-threatening disease, it is of great importance to recognize symptoms in time. Depending on age, these include predominantly a protruding ear, retroauricular erythema, swelling or pain, ear discharge, fever, and a deteriorated general condition (3). Particularly challenging are less symptomatic or even asymptomatic courses, which have been described especially in cases in which AOM was treated with an antibiotic (4). Diagnostics include a medical history, the physical examination and laboratory diagnostics of the blood. However, there is still no consensus on the need and timing of computer tomography (CT). For treatment, research demonstrated that in uncomplicated cases, conservative management (antibiotic treatment with or without myringotomy and ventilation tubes) is an efficient first-line treatment. However, mastoidectomy should be performed in case of failure of conservative therapy after 48–72 h or in the case of complications, e.g., epidural or subdural abscess, facial paralysis, sinus thrombosis or brain abscess. As a result, with appropriate therapy, the prognosis is generally favorable with few long-time complications (5).
We present the case of a 13-year-old boy with obesity and hypertension who unexpectedly needed to be resuscitated at home by his mother (for the timeline see Figure 1). When the emergency physician arrived half an hour later, pupils were already dilated. After more than 30 minutes of professional resuscitation with intubation, administration of adrenaline and multiple defibrillations, spontaneous circulation was achieved. Intubated and ventilated, the patient was then admitted to our pediatric intensive care unit (PICU), where we performed a cranial computed tomography (CT) and, in the course, a cranial magnetic resonance imaging (MRI), in which the clinical suspicion of a lower entrapment was confirmed. The most likely cause appeared to be the caudal ectopia of the cerebellar tonsils and a cystoid mass in the left cerebellopontine angle compressing the brainstem, with prominent surrounding cerebellar edema and inflammatory changes in the area of the petrous bone and the left mastoid (see Figure 2). Laboratory results on admission are shown in Table 1.
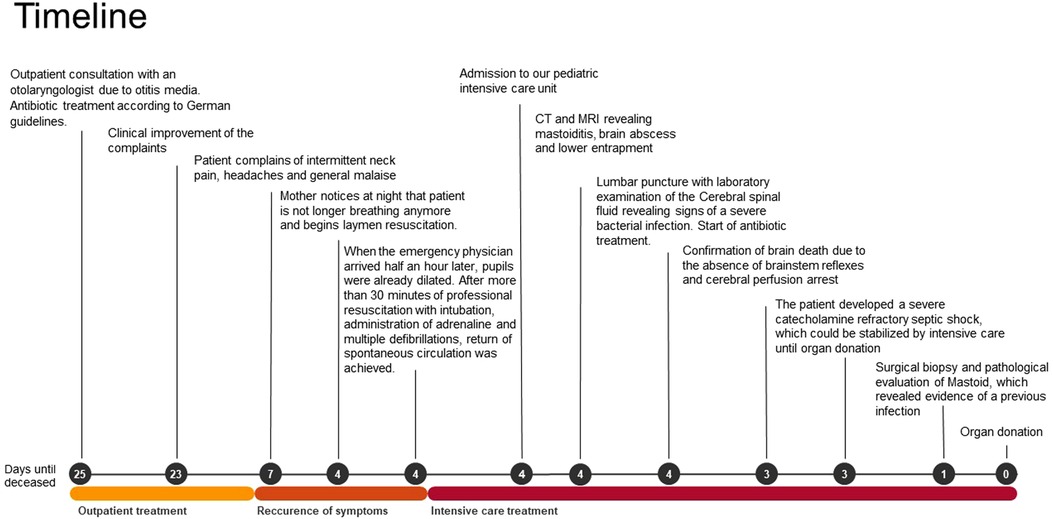
Figure 1. Timeline with chronological depiction of events from outpatient diagnosis of otitis media to organ donation.
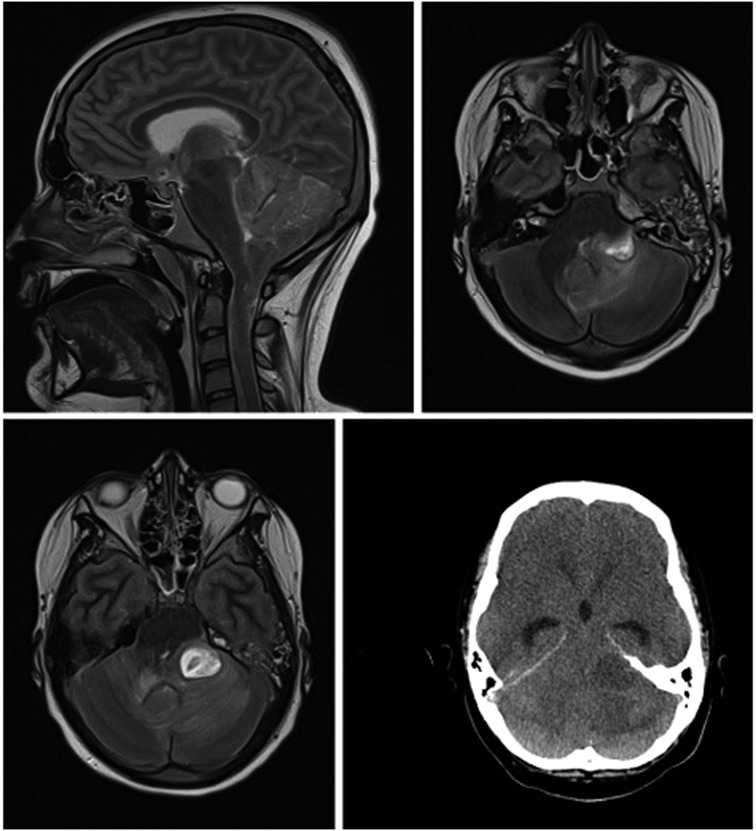
Figure 2. cMRI and cCT show lower entrapment most likely caused by caudal ectopia of the cerebellar tonsils and a cystoid mass in the left cerebellopontine angle compressing the brainstem, with prominent surrounding cerebellar edema and inflammatory changes in the area of the petrous bone and the left mastoid.
A conversation with the mother revealed, that three weeks earlier, the patient was diagnosed with Acute Otitis media (AOM) and treated with oral antibiotics according to German guidelines. As a result, there had been a clinical improvement without symptom progression or fever. However, three days before admission to our PICU, the patient complained of intermittent neck pain and headache and was not feeling well. Because of this, he had slept in his mother's bed where he had been talking while sleeping, then suddenly paused and stopped breathing. Other symptoms in relation to possible neurological, oncological or infectious diseases were negated. The patient's medical history only revealed arterial hypertension treated with metoprolol, and a pronounced obesity of 110 kg body weight. In addition, the patient had repeatedly complained of headaches and decreased physical resilience for the last eight weeks. The physical examination on our PICU showed a mildly inflamed left tympanic membrane, consistent with an otitis media that had subsided a few weeks earlier. The patient was completely vaccinated according to the recommendations of the Robert Koch institute including two vaccinations with Comirnaty.
In the absence of brainstem reflexes and cerebral perfusion arrest, we confirmed brain death one day after admission according to the guidelines of the German medical association. We discussed the possibility of organ donation with the parents, who consented.
For differential diagnostic clarification of the etiology of the mass, we performed a lumbar puncture. Laboratory examination of the Cerebral spinal fluid (CSF) revealed signs of a severe bacterial infection, which supported the inflammatory etiology of the mass. Microbiologically, Streptococcus intermedius was detected both in the blood culture and in the CSF, which showed no resistances against standard antibiotics. Despite extensive diagnostics, there was no evidence of an acute viral infection. Myocarditis could be excluded by further investigations. Antibiotic treatment was started right after the diagnostic work-up.
In the meantime, the patient developed a severe catecholamine refractory septic shock, which could be stabilized by intensive care until organ donation. To rule out a malignant process of the left mastoid before organ transplantation, we performed surgical biopsy and pathological evaluation, which revealed evidence of a previous infection of the mastoid with fibrosed mucosa. A neoplastic process could be excluded. After clarification of the microbiological etiology of the cerebellar abscess, organ donation could proceed without complications.
This case illustrates the rare but life-threatening presentation of mastoiditis with subsequent subdural empyema and cerebral herniation after AOM.
With a cumulative prevalence of more than 60% by the age of seven, AOM is not only a highly common disease (6) but it is also the most prevalent reason for antibiotic therapy in young children (7). Despite the high incidence, severe complications are rare (8). These include, with decreasing probability, mastoiditis, sub-periosteal abscess, facial nerve palsy, epidural abscess, sigmoid sinus and internal jugular vein thrombosis. Other complications such as elevated intracranial pressure, cerebral stroke and suppurative meningitis are rare and have been described only in single cases (1). Mastoiditis, as the most common complication, was observed in the United Kingdom in 1.8 per 10,000 AOM episodes in which antibiotics were administered and in 3.8 per 10,000 AOM episodes in which no antibiotic was administered (9), whereas a meta-analysis described 23.7 cases of mastoiditis per 10,000 episodes of AOM (10). Depending on the investigated country and time period, the incidence rate of Mastoiditis is reported to be 1.2–4.8/100,000 person-years (2, 11).
Regarding the dynamics of incidence in the last years and decades, there are conflicting findings. Several studies indicate an increase in incidence and an increase in surgical intervention and attribute this to the rising number of antibiotic resistances (12, 13). On the other hand, numerous studies report a constant or even decreasing incidence (2, 9, 11, 14). It should be noted, however, that an increase in the complication rate and the need for surgical intervention was observed here as well (14).
However, precise data on changes in incidence over the last two decades is important, as a possible rise in incidence has been associated with a more restrictive usage of antibiotics (2). This is supported by the fact, that Thompson and colleagues observed in their cohort study that antibiotic treatment for AOM reduced the risk of future mastoiditis by 50% (9). Arguing against the more liberal use of antibiotics in AOM, other studies have not only observed no effect of antibiotic administration on the risk of mastoiditis (15) but in some cases prior antibiotic administration was even associated with an increased risk for the need for surgical intervention (16). In addition, given the rarity of mastoiditis, the number needed to treat with an antibiotic to prevent one case of mastoiditis is estimated to be between 2,500 and 4,381 (9, 17). Here, however, the benefits bear no relation to the financial costs, the possible side effects and the development of bacterial resistance. Furthermore, our case supports the studies showing that despite antibiotic treatment, complete prevention is not provided and prior antibiotic administration may even mask mastoiditis (4, 18). In our case, however, it should be noted that the potential standard dosage of the antibiotic in an obese patient may have been a contributing factor to treatment failure.
The most common pathogens of mastoiditis include Streptococcus Pneumoniae, Pseudomonas aeruginosa, Streptococcus Pyogenes group A, Staphylococcus aureus, and Haemophilus influenzae (1, 11, 19). In addition, Fusobacterium Necrophorum has been detected more frequently in mastoiditis in recent years (14, 20), particularly in cases previously treated with antibiotics or where complications occurred and surgery was required (14, 19). Concerningly, a rising number of resistant infections have been found in mastoiditis in recent years as well (1, 13).
Streptococcus intermedius, a commensal microorganism of the oral flora and the bacterium we consider to be the most likely causative, does not appear to be a typical pathogen of mastoiditis. It does, however, appear to be a common pathogen associated with brain abscesses (21). There are case reports describing brain abscesses in children after mastoiditis caused by Streptococcus intermedius (22). Pathophysiologically, the brain abscess formation occurs after tissue damage, which is followed by bacterial colonization and subsequent tissue liquefaction and pus formation due to hyaluronidase activity of Streptococcus intermedius (21).
It is also worth mentioning that on MRI, in addition to the mass compressing the brainstem, there was a descent of the cerebellar tonsils, indicating a previously unknown Arnold-Chiari malformation Type 1. This as a rare craniovertebral junction malformation with caudal ectopia of the cerebellar tonsils through the Foramen Magnum that can lead to slowly progressive symptoms like headaches, neck pain, motor deficits, cranial nerve palsy, oropharyngeal dysfunctions and sleep disorders (23, 24). Epidemiologic data are scarce and with wide discrepancies. For instance, two retrospective analyses of MRI images reported incidences of 0.77 and 3.6%, respectively (25, 26). Matching our case, the disease was observed more frequently in children and young adults and the male sex (23). However, there is a minority of asymptomatic patients with an acute fatal onset (27, 28). Although, comparable to the case described by Stephany et al. the patient headaches, which he had described for several weeks can retrospectively be considered as a possible prodrome (29). Due to the ectopic position of the cerebellar tonsils through the foramen magnum into the spinal canal and the thus chronically constricted brainstem, patients with Arnold-Chiari malformation are particularly susceptible to cerebellar tonsillar impaction. Minor trauma, or as in our case, an intracerebral mass, can promptly decompensate the vulnerable system. Pathophysiologically, both direct mechanical entrapment of brain tissue and compression of cerebral vessels with subsequent ischemia can impair the function of the respiratory and circulator as well as the ascending reticular activating system with direct catastrophic consequences (23). In our case, this might have contributed to the rapid clinical deterioration and ultimately cardiovascular failure.
Although the coincidence of an Arnold-Chiari malformation and mastoiditis is rare, this case illustrates that even nowadays otitis media can have a severe and sometimes lethal outcome. Especially, recurring complaints such as headache, fever or neck pain should always raise the suspicion of unsuccessful treatment and require a conscientious investigation to initiate the appropriate diagnostics and subsequent therapy in time. To prevent a fatal course, as in our case, it is, therefore, crucial to educate the patient and parents about the need for immediate reappearance in the event of the above-mentioned complaints, some of which may occur after a latency of 8–12 weeks. Finally, this case also highlights that antibiotic therapy never reliably protects against serious complications and, in the worst case, can even lead to a delay in diagnosis because the treating physician is under a false sense of security.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
OF: wrote the manuscript in consultation with JB. RH: supervised the treatment of the patient and the elaboration of the manuscript. JB and RH: provided critical feedback. All authors contributed to the article and approved the submitted version.
We acknowledge support by the Open Access Publication Funds of the Martin-Luther University Halle-Wittenberg.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Mattos JL, Colman KL, Casselbrant ML, Chi DH. Intratemporal and intracranial complications of acute otitis media in a pediatric population. Int J Pediatr Otorhinolaryngol. (2014) 78(12):2161–4. doi: 10.1016/j.ijporl.2014.09.032
2. Van Zuijlen DA, Schilder AGM, Van Balen FAM, Hoes AW. National differences in incidence of acute mastoiditis: relationship to prescribing patterns of antibiotics for acute otitis media? Pediatr Infect Dis J. (2001) 20(2): 140–4. doi: 10.1097/00006454-200102000-00004 Verfügbar unter: https://journals.lww.com/pidj/Fulltext/2001/02000/National_differences_in_incidence_of_acute.4.aspx11224830
3. Groth A, Enoksson F, Hultcrantz M, Stalfors J, Stenfeldt K, Hermansson A. Acute mastoiditis in children aged 0–16 years—a national study of 678 cases in Sweden comparing different age groups. Int J Pediatr Otorhinolaryngol. (2012) 76(10):1494–500. doi: 10.1016/j.ijporl.2012.07.002
4. Gregers Petersen C, Ovesen T, Brahe Pedersen C. Acute mastoidectomy in a danish county from 1977 to 1996 with focus on the bacteriology. Int J Pediatr Otorhinolaryngol. (1998) 45(1):21–9. doi: 10.1016/S0165-5876(98)00080-9
5. Loh R, Phua M, Shaw CKL. Management of paediatric acute mastoiditis: systematic review. J Laryngol Otol. (2018) 132(2):96–104. doi: 10.1017/S0022215117001840
6. Plasschaert AIO, Rovers MM, Schilder AGM, Verheij TJM, Hak E. Trends in doctor consultations, antibiotic prescription, and specialist referrals for Otitis Media in children: 1995–2003. Pediatrics. (2006) 117(6):1879–86. doi: 10.1542/peds.2005-2470
7. Grijalva CG. Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings. JAMA. (2009) 302(7):758. doi: 10.1001/jama.2009.1163
8. Rosenfeld RM, Casselbrant ML, Hannley MT. Implications of the AHRQ evidence report on acute Otitis Media. Otolaryngol Neck Surg. (2001) 125(5):440–8. doi: 10.1016/S0194-5998(01)00309-6
9. Thompson PL, Gilbert RE, Long PF, Saxena S, Sharland M, Wong ICK. Effect of antibiotics for Otitis Media on mastoiditis in children: a retrospective cohort study using the United Kingdom general practice research database. Pediatrics. (2009) 123(2):424–30. doi: 10.1542/peds.2007-3349
10. Rosenfeld RM, Kay D. Natural history of untreated otitis media. Laryngoscope. (2010) 113(10):1645–57. doi: 10.1097/00005537-200310000-00004
11. Anthonsen K, Høstmark K, Hansen S, Andreasen K, Juhlin J, Homøe P. Acute mastoiditis in children: a 10-year retrospective and validated multicenter study. Pediatr Infect Dis J. (2013) 32(5):436–40. doi: 10.1097/INF.0b013e31828abd13
12. Benito MB, Gorricho BP. Acute mastoiditis: increase in the incidence and complications. Int J Pediatr Otorhinolaryngol. (2007) 71(7):1007–11. doi: 10.1016/j.ijporl.2007.02.014
13. Thorne MC, Chewaproug L, Elden LM. Suppurative complications of acute Otitis media: changes in frequency over time. Arch Otolaryngol Neck Surg. (2009) 135(7):638. doi: 10.1001/archoto.2009.75
14. Stern Shavit S, Raveh E, Levi L, Sokolov M, Ulanovski D. Surgical intervention for acute mastoiditis: 10 years experience in a tertiary children hospital. Eur Arch Otorhinolaryngol. (2019) 276(11):3051–6. doi: 10.1007/s00405-019-05606-2
15. Ho D, Rotenberg BW, Berkowitz RG. The relationship between acute mastoiditis and antibiotic use for acute Otitis Media in children. Arch Otolaryngol Neck Surg. (2008) 134(1):45. doi: 10.1001/archotol.134.1.45
16. Grossman Z, Zehavi Y, Leibovitz E, Grisaru-Soen G, Shachor Meyouhas Y, Kassis I. Severe acute mastoiditis admission is not related to delayed antibiotic treatment for antecedent acute Otitis media. Pediatr Infect Dis J. (2016) 35(2):162–5. doi: 10.1097/INF.0000000000000951
17. Petersen I, Johnson AM, Islam A, Duckworth G, Livermore DM, Hayward AC. Protective effect of antibiotics against serious complications of common respiratory tract infections: retrospective cohort study with the UK general practice research database. Br Med J. (2007) 335(7627):982. doi: 10.1136/bmj.39345.405243.BE
18. Keith T, Saxena S, Murray J, Sharland M. Risk–benefit analysis of restricting antimicrobial prescribing in children: what do we really know? Curr Opin Infect Dis. (2010) 23(3):242–8. doi: 10.1097/QCO.0b013e328338c46d Verfügbar unter: https://journals.lww.com/co-infectiousdiseases/Fulltext/2010/06000/Risk_benefit_analysis_of_restricting_antimicrobial.8.aspx20375892
19. Mansour T, Yehudai N, Tobia A, Shihada R, Brodsky A, Khnifies R. Acute mastoiditis: 20 years of experience with a uniform management protocol. Int J Pediatr Otorhinolaryngol. (2019) 125:187–91. doi: 10.1016/j.ijporl.2019.07.014
20. Yarden-Bilavsky H, Raveh E, Livni G, Scheuerman O, Amir J, Bilavsky E. Fusobacterium necrophorum mastoiditis in children – emerging pathogen in an old disease. Int J Pediatr Otorhinolaryngol. (2013) 77(1):92–6. doi: 10.1016/j.ijporl.2012.10.003
21. Issa E, Salloum T, Tokajian S. From normal Flora to brain abscesses: a review of Streptococcus intermedius. Front Microbiol. (2020) 11:826. doi: 10.3389/fmicb.2020.00826
22. Yakut N, Kadayifci EK, Karaaslan A, Atici S, Akkoc G, Ocal Demir S. Braın abscess due to Streptococcus intermedius secondary to mastoiditis in a child. SpringerPlus. (2015) 4(1):809. doi: 10.1186/s40064-015-1608-0
23. Massimi L, Della Pepa GM, Caldarelli M, Di Rocco C. Abrupt clinical onset of chiari type I/syringomyelia complex: clinical and physiopathological implications. Neurosurg Rev. (2012) 35(3):321–9. doi: 10.1007/s10143-012-0391-4
24. Ferré Masó A, Poca MA, de la Calzada MD, Solana E, Romero Tomás O, Sahuquillo J. Sleep disturbance: a forgotten syndrome in patients with chiari I malformation. Neurol Engl Ed. (2014) 29(5):294–304. doi: 10.1016/j.nrl.2011.01.008
25. Meadows J, Kraut M, Guarnieri M, Haroun RI, Carson BS. Asymptomatic chiari type I malformations identified on magnetic resonance imaging. J Neurosurg. (2000) 92(6):920–6. doi: 10.3171/jns.2000.92.6.0920
26. Strahle J, Muraszko KM, Kapurch J, Bapuraj JR, Garton HJL, Maher CO. Chiari malformation type I and syrinx in children undergoing magnetic resonance imaging: clinical article. J Neurosurg Pediatr. (2011) 8(2):205–13. doi: 10.3171/2011.5.PEDS1121
27. Martinot A, Hue V, Leclerc F, Vallee L, Closset M, Pruvo JP. Sudden death revealing chiari type 1 malformation in two children. Intensive Care Med. (1993) 19(2):73–4. doi: 10.1007/BF01708364
28. Yarbrough CK, Powers AK, Park TS, Leonard JR, Limbrick DD, Smyth MD. Patients with chiari malformation type I presenting with acute neurological deficits: case series: clinical article. J Neurosurg Pediatr. (2011) 7(3):244–7. doi: 10.3171/2010.11.PEDS1097
Keywords: mastoiditis, otitis media, arnold-chiari-malformation, sudden death, brain absces
Citation: Feussner O, Haase R and Baier J (2023) Case report: Otitis media with subsequent mastoiditis and cerebral herniation in a patient with Arnold chiari malformation. Front. Pediatr. 10:1013300. doi: 10.3389/fped.2022.1013300
Received: 6 August 2022; Accepted: 28 December 2022;
Published: 23 January 2023.
Edited by:
Hans Van Rostenberghe, Universiti Sains Malaysia (USM), MalaysiaReviewed by:
Saba Battelino, University Medical Centre Ljubljana, Slovenia© 2023 Feussner, Haase and Baier. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Oskar Feussner b3NrYXIuZmV1c3NuZXJAZ214LmRl
Specialty Section: This article was submitted to Pediatric Infectious Diseases, a section of the journal Frontiers in Pediatrics
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.