- 1Division of Pediatric Critical Care, Department of Pediatrics, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand
- 2Division of Allergy and Immunology, Department of Pediatrics, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand
- 3Pediatric Translational Research Unit, Department of Pediatrics, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand
- 4Department of Clinical Epidemiology and Biostatistics, Department of Pediatrics, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand
- 5Chakri Naruebodindra Medical Institute, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Samut Prakan, Thailand
- 6Department of Rheumatic and Immunologic Diseases, Cleveland Clinic, Cleveland, OH, United States
Objective: Fluid administration is the initial step of treatment of unstable pediatric patients. Evaluation of fluid responsiveness is crucial in mechanically ventilated children to avoid fluid overload, which increases mortality. We aim to review and compare the diagnostic performance of dynamically hemodynamic parameters for predicting fluid responsiveness in mechanically ventilated children.
Design: A systematic review was performed using four electronic databases, including PubMed, EMBASE, Scopus, and Central, for published articles from 1 January 2010 to 31 December 2020. Studies were included if they described diagnostic performance of dynamic parameters after fluid challenge was performed in mechanically ventilated children.
Settings: Pediatric intensive and cardiac intensive care unit, and operative room.
Patients: Children aged 1 month to 18 years old who were under mechanical ventilation and required an intravenous fluid challenge.
Measurements and Main Results: Twenty-seven studies were included in the systematic review, which included 1,005 participants and 1,138 fluid challenges. Respiratory variation in aortic peak velocity was reliable among dynamic parameters for predicting fluid responsiveness in mechanically ventilated children. All studies of respiratory variation in aortic peak velocity showed that the area under the receiver operating characteristic curve ranged from 0.71 to 1.00, and the cutoff value for determining fluid responsiveness ranged from 7% to 20%. Dynamic parameters based on arterial blood pressure (pulse pressure variation and stroke volume variation) were also used in children undergoing congenital heart surgery. The plethysmography variability index was used in children undergoing neurological and general surgery, including the pediatric intensive care patients.
Conclusions: The respiratory variation in aortic peak velocity exhibited a promising diagnostic performance across all populations in predicting fluid responsiveness in mechanically ventilated children. High sensitivity is advantageous in non-cardiac surgical patients and the pediatric intensive care unit because early fluid resuscitation improves survival in these patients. Furthermore, high specificity is beneficial in congenital heart surgery because fluid overload is particularly detrimental in this group of patients.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=206400
Introduction
Fluid administration is the first line of treatment for critically ill children who are admitted to the pediatric intensive care unit (PICU) with unstable hemodynamics. However, only 40% to 69% of these children show a response to fluid administration (1). Fluid responsiveness is defined as an increase in cardiac output of more than 10% to 15% after an intravenous fluid challenge (1–3). Early administration of fluid in patients who are responsive improves survival. However, fluid administration to those who are unresponsive may cause fluid overload, leading to longer ventilator days and higher morbidity and mortality rates (4–6).
Many hemodynamic parameters have been used to predict fluid responsiveness in critically ill children. These parameters can be divided into static and dynamic parameters (Supplementary Table S1). Static parameters are measured at a specific time point during observation. Dynamic parameters are measured by monitoring changes in physiological responses based on cardiopulmonary interaction (e.g., variability change in preload during mechanical ventilation). Most studies have suggested that dynamic parameters are more accurate than static parameters for predicting fluid responsiveness (1, 7–9).
Dynamic parameters can be measured in an invasive or non-invasive manner. Ultrasonic cardiac output monitoring and electrical cardiometry are non-invasive methods that are commonly used to assess dynamic parameters in the intensive care unit (ICU) setting.
Previous studies of dynamic parameters were conducted in different circumstances and populations (10–36). To date, there are no standard parameters that can be used across all critically ill children, especially in mechanically ventilated children, who are prone to fluid overload. This systematic review aimed to compare the diagnostic performance of dynamic parameters for predicting fluid responsiveness in mechanically ventilated children.
Materials and methods
This study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) reporting guideline (37). The protocol was registered and approved by the international prospective register of systematic reviews PROSPERO (CRD42020206400) on 1 October 2020. Inclusion criteria included the following: (i) children aged 1 month to 18 years old who were under mechanical ventilation and required an intravenous fluid challenge; (ii) diagnostic accuracy studies of dynamic parameters for predicting fluid responsiveness compared with the gold standard definition of fluid responsiveness (10%–15% increase in cardiac output after a fluid challenge as measured by the pressure recording analytic method, an echocardiogram, or non-invasive cardiac output monitoring), and the measurements needed to be performed before and after a fluid challenge; and (iii) the diagnostic performance included the cutoff value, sensitivity, specificity, and area under the receiver operating characteristic (ROC) curve. Meta-analyses, systematic reviews, narrative reviews, clinical practice guidelines, conference proceedings, case series and case reports with a sample size < 10, and non-English articles were excluded.
Outcome
The primary outcome was to study the diagnostic performance of dynamic hemodynamic parameters, including sensitivity, specificity, and the area under ROC curve, for the prediction of fluid responsiveness in mechanically ventilated children. The secondary outcome was to identify the reliable dynamic parameters among mechanically ventilated children in different clinical circumstances.
Search strategy
A systematic review was performed using four electronic databases, including PubMed, EMBASE, Scopus, and Central, for published articles from 1 January 2010 to 31 December 2020. The last search was conducted on 15 January 2021. The search terms were fluid, volume, response, challenge, bolus, and guided. These words were combined with the medical subject heading (MeSH) terms hemodynamics, hemodynamic monitoring, fluid therapy, cardiac output, infant, child, adolescent, and pediatrics. An additional search for potentially eligible articles was carried out using references of selected retrieved articles.
Study selection and risk of bias assessment
Two authors (P.Y. and W.K.) independently reviewed abstracts of the retrieved articles for their eligibility. Articles that clearly did not fulfill the inclusion criteria were excluded at this stage. The remaining articles underwent a full-text review for final determination of their eligibility Any disagreements were resolved by conference with a third author (R.L.). The risk of bias was assessed using the Quality Assessment of Studies of Diagnostic Accuracy tool (38, 39), which is composed of the following 4 domains: patient selection, index test, reference standard, and flow-timing, while the applicability concern was assessed through 3 domains: patient selection, index test, and reference standard. The risk of bias and applicability concern was judged as “low”, “high”, or “unclear.” If a study was judged as “low” in all domains relating to bias or applicability, then the overall judgment of a “low risk of bias” was assigned for that study. If a study was judged as “high” in one or more domains, it was judged as a “high risk of bias”. The term “unclear” was assigned only when there were missing data that could not be retrieved.
Data extraction and data synthesis
Two authors (P.Y. and R.L.) independently extracted data from the included articles using a standardized data extraction form derived from the Cochrane Public Health Group Data Extraction and Assessment Template. We contacted the corresponding author of the included articles for missing data. However, only 2 of 10 corresponding authors replied. Those missing data were labeled as not reported.
The following data were collected for systematic review: sample size, age, specific circumstance of participants, definition and percentage of fluid responsiveness, cutoff value, and diagnostic performance of dynamic parameters.
Results
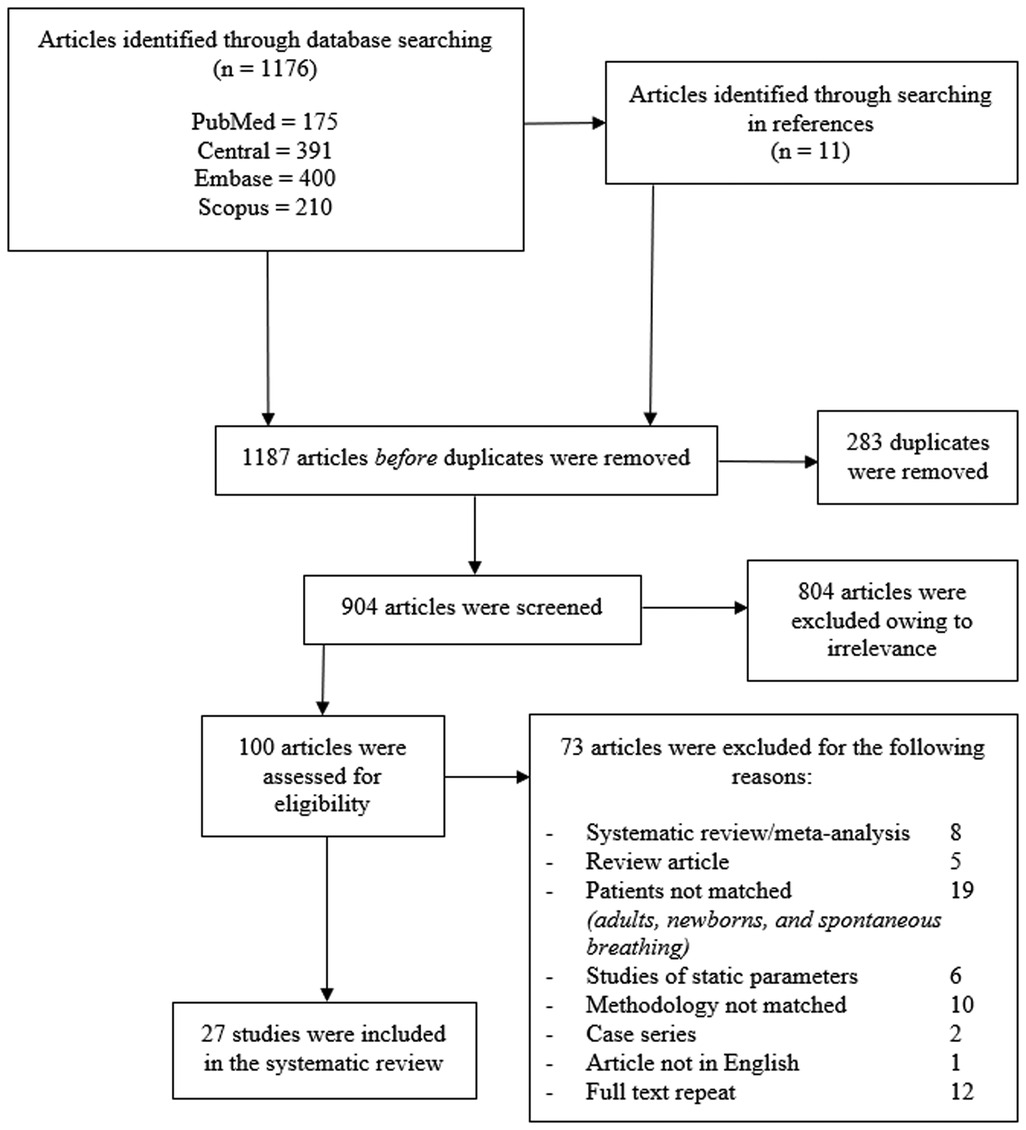
The identification and selection of studies are shown in Figure 1. A total of 27 studies were included in the final systematic review (10–36), which comprised 1,005 participants and 1,138 intravenous fluid challenges. A total of 77% (21/27) of studies were published after the last systematic review (1). Twenty-five studies were conducted as prospective observational cohorts (10–16, 18–36), and only 1 study was retrospective cohort study (17). There were 4 major groups of patients in different clinical settings as follows: (i) the congenital heart surgery group in 14 studies; (ii) the general surgery group in 5 studies; (iii) the neurological surgery group in 4 studies; and (iv) the general PICU group in 4 studies. Among the subgroups of participants, different fluid types and volumes were administered. Patients with congenital heart surgery mostly received colloid or blood components; only 2 of 14 studies used isotonic crystalloids. The other 3 groups of participants mostly received crystalloids with larger bolus volumes.
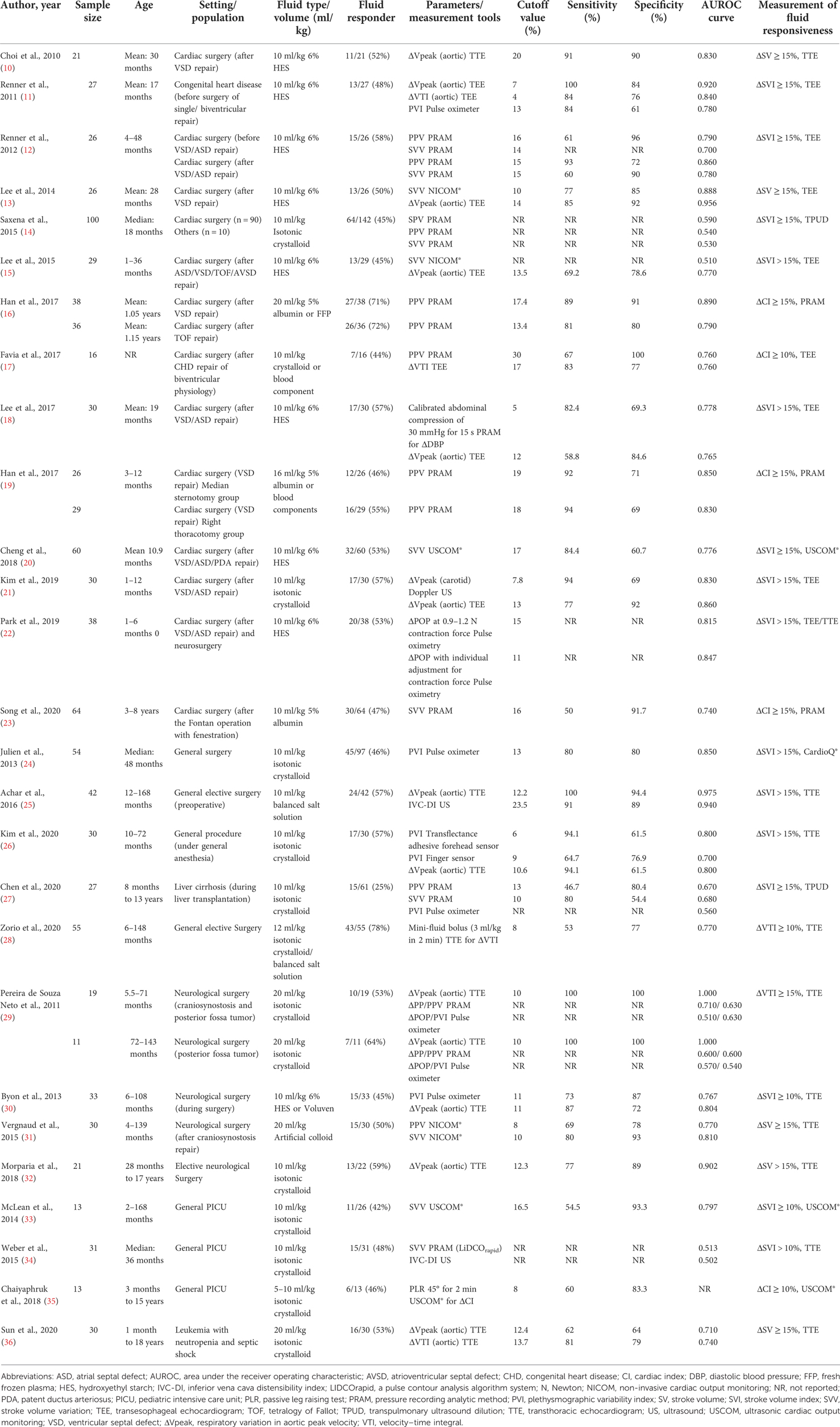
Table 1 shows the diagnostic performance of dynamic parameters compared with the gold standard measurement of fluid responsiveness. The gold standard measurement was an increase in cardiac output of 10%–15% after fluid administration, which was represented by multiple parameters as follows: the stroke volume index in 15 studies, stroke volume in 5 studies, the cardiac index in 4 studies, and the velocity–time integral in 2 studies. Eleven dynamic parameters (see Supplementary Table S2 with equations) were investigated in the 27 included studies.
The respiratory variation in aortic peak velocity (ΔVpeak) was the most common dynamic parameter examined (12/27 studies). Moreover, ΔVpeak provided a reliable diagnostic performance. All studies of ΔVpeak showed that the area under the ROC curve ranged from 0.71 to 1.00, and the cutoff value of ΔVpeak for determining fluid responsiveness ranged from 7% to 20%.
Because patients with congenital heart surgery were included in approximately half of all studies, we allocated participants to 2 new subgroups as follows: the congenital heart surgery subgroup (10–23) and the non-cardiac surgery subgroup (general surgery, neurological surgery, and general PICU patients) (24–36). In congenital heart surgery subgroup, ΔVpeak showed the best sensitivity of 100% at the cutoff value of 7% when performed by transesophageal echocardiogram (TEE) (11). The best specificity of ΔVpeak was 92% at the cutoff values 13%–14% by TEE (13, 21). Another reliable dynamic was the pulse pressure variation (PPV), with the sensitivity of 94% (at the cutoff value of 18%) and the specificity of 100% (at the cutoff value of 30%) (17). In the non-cardiac surgery subgroup, ΔVpeak performed by transthoracic echocardiogram (TTE) showed the best sensitivity of 100% (at the cutoff values 10% and 12.2%) (25, 29) with the best specificity of of 100% (at the cutoff value 10%) (29). Note that plethysmographic variability index (PVI) measured by the transflectance adhesive forehead sensor exhibited the second-best sensitivity of 94.1% (at the cutoff value of 6%) (26), while stroke volume variation (SVV) provided the second-best specificity of 93.3% (at cutoff values 16.5%) (33).
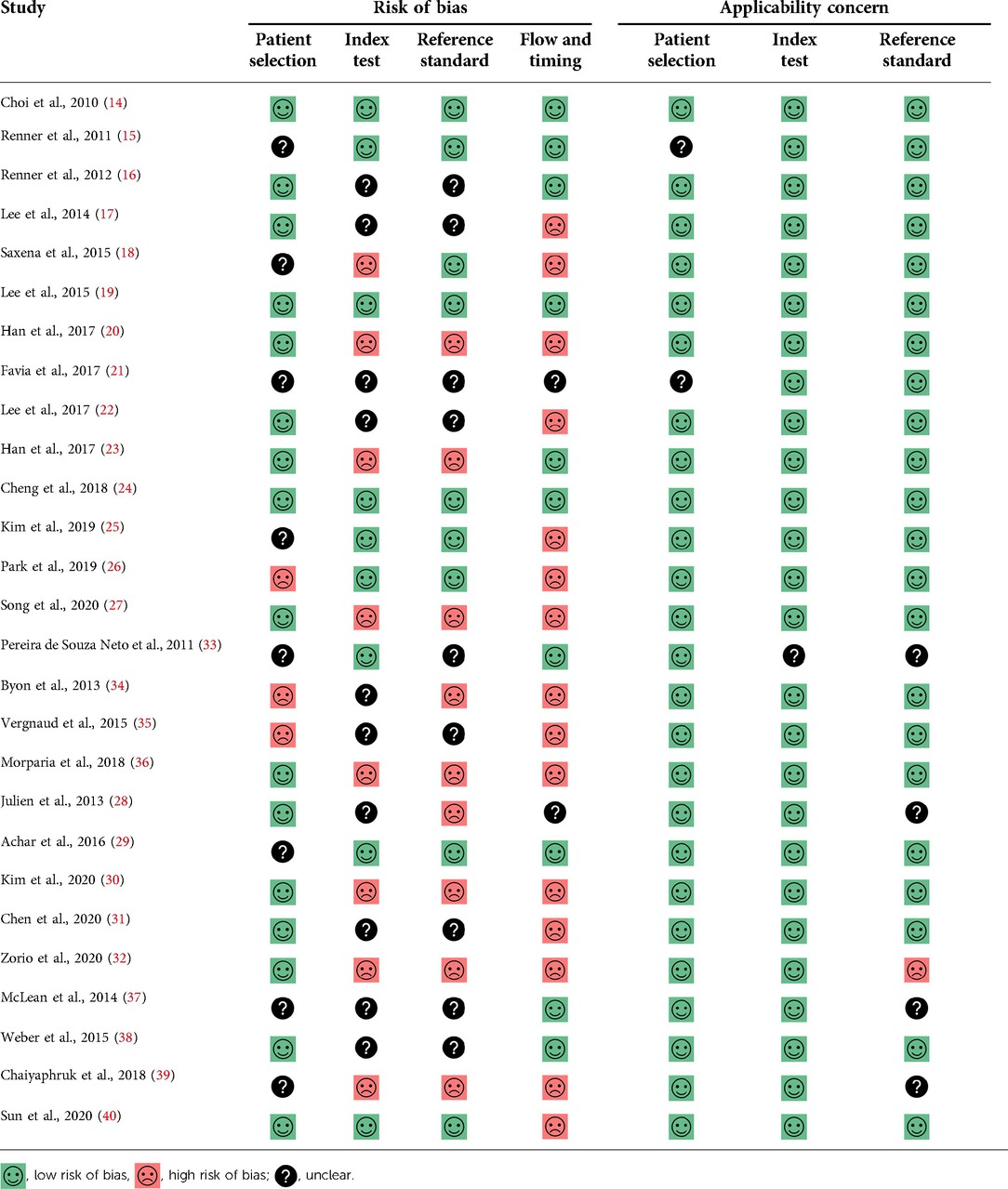
The risk of bias assessment of all included studies is shown in Table 2. The reference standard domain was judged to have a high risk of bias in 9 studies because the interpretation of the reference standard test was made with knowledge of index test results. The flow and timing domain were also judged to have a high risk of bias in 15 studies because all included patients were not in the final analysis (per-protocol analysis).
Discussion
In 2013, Gan et al. (1) studied static and dynamic parameters, and found that dynamic parameters were more reliable in predicting fluid responsiveness in children. Several new dynamic parameters have since been introduced and studied in the pediatric population during the last 10 years. Therefore, we conducted this review to extend the work of Gan et al. (1) on dynamic parameters and to provide an update with newly examined parameters.
New dynamic parameters from non-invasive ultrasonic cardiac output monitoring, electrical cardiometry, and ultrasound are easily accessible and widely used in the PICU. These new parameters are reliable and can be measured by non-experienced physicians in a few minutes (40, 41). Therefore, they could be useful tools for clinicians to determine whether patients should undergo a fluid challenge.
This systematic review showed that ΔVpeak had a promising diagnostic performance across all populations. The ΔVpeak was studied as a single parameter or together with other dynamic parameters. The cutoff values for predicting fluid responsiveness ranged from 7% to 20%, while the average values ranged from 12% to 14%. In group of congenital heart surgery, the echocardiogram performed by transesophageal technique but in other groups, mostly performed by transthoracic technique. A major disadvantage of ΔVpeak is that this parameter requires an experienced operator of echocardiography.
The highest sensitivity of ΔVpeak in patients who had congenital heart surgery is advantage because fluid overload can increase the risk of acute kidney injury and poor postoperative outcomes in patients with congenital heart disease (42, 43). Therefore, a parameter with high specificity, such as ΔVpeak, could reduce such adverse events and complications by decreasing an unnecessary fluid challenge in this patient subgroup. When ΔVpeak is not accessible, new dynamic parameters from non-invasive methods such as ultrasonic cardiac output monitoring, electrical cardiometry, and arterial line variable parameters should be considered, because of easy accessibility and mostly non-operator dependent methods. Pulse pressure variation could be used as alternative because it also had a high specificity. Patients in the non-cardiac subgroup are most likely to benefit from early fluid resuscitation. The ΔVpeak and PVI should be considered in this context because they have a high sensitivity.
Each study with patients in the congenital heart surgery group reported inotropic and vasopressor administration in various forms, including the percentage of inotrope use in the population and the Vasoactive Inotropic Score, and some studies did not report inotropic or vasopressor data. Therefore, we did not perform analysis for specific dynamic parameters based on inotropic status.
There are some limitations to our study. First, our search strategy was limited to the last 10 years. The reason for his limitation was to focus on new dynamic parameters that appeared after the systematic review in 2013 by Gan et al. (1) Second, there was heterogeneity of the study design, including multiple participant groups in different clinical settings, different fluid types, varying amounts of volume (5–20 ml/kg), and the definition of fluid responsiveness using different parameters across the studies.
The findings from this systematic review suggest some future research opportunities. The ΔVpeak, which is the most reliable parameter for predicting fluid responsiveness in mechanically ventilated children, has not been investigated in children with spontaneous breathing. Preload challenge maneuvers (e.g., calibrated abdominal compression, mini-fluid bolus, the passive leg raising test, and the end-expiratory occlusion test) have been extensively studied in the adult population for predicting fluid responsiveness (44). However, these maneuvers have not been well investigated in pediatric population.
Conclusions
The ΔVpeak exhibited a promising diagnostic performance in predicting fluid responsiveness in mechanically ventilated children. The sensitivity of ΔVpeak is advantageous in non-cardiac surgical patients and the PICU setting because early fluid resuscitation improves survival in these patients. Furthermore, the specificity of ΔVpeak is beneficial in congenital heart surgery because fluid overload is particularly detrimental in this group of patients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
Conceptualization: RL; methodology: PY, WK, SC, RL, and PU; investigation: PY and WK; data curation: PY and WK; validation: SC, RL, and PU; writing—original draft preparation: PY; writing—review and editing: WK, SC, RL, and PU; visualization: PY; supervision: SC, RL, and PU. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Department of Pediatrics, Faculty of Medicine Ramathibodi Hospital, Mahidol University.
Acknowledgments
We thank Ellen Knapp, PhD, from Edanz (https://edanz.com/ac) for editing a draft of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.1010600/full#supplementary-material.
References
1. Gan H, Cannesson M, Chandler JR, Ansermino JM. Predicting fluid responsiveness in children: a systematic review. Anesth Analg. (2013) 117:1380–92. doi: 10.1213/ANE.0b013e3182a9557e
2. Marlik PE, Monnet X, Teboul JL. Hemodynamic parameters to guide fluid therapy. Ann Intensive Care. (2011) 1(1):1. doi: 10.1186/2110-5820-1-1
3. Toscani L, Aya HD, Antonakaki D, Bastoni D, Watson X, Arulkumaran N, et al. What is the impact of the fluid challenge technique on diagnosis of fluid responsiveness? A systematic review and meta-analysis. Crit Care. (2017) 21:207. doi: 10.1186/s13054-017-1796-9
4. Sutherland SM, Zappitelli M, Alexander SR, Chua AN, Brophy PD, Bunchman TE, et al. Fluid overload and mortality in children receiving continuous renal replacement therapy: the prospective pediatric continuous renal replacement therapy registry. Am J Kidney Dis. (2010) 55:316–25. doi: 10.1053/j.ajkd.2009.10.048
5. Li Y, Wang J, Bai Z, Chen J, Wang X, Pan J, et al. Early fluid overload is associated with acute kidney injury and PICU mortality in critically ill children. Eur J Pediatr. (2016) 175(1):39–48. doi: 10.1007/s00431-015-2592-7
6. Raina R, Sethi SK, Wadhwani N, Vemuganti M, Krishnappa V, Bansal SB. Fluid overload in critically ill children. Front Pediatr. (2018) 6:306. doi: 10.3389/fped.2018.00306
7. Yi L, Liu Z, Qiao L, Wan C, Mu D. Does stroke volume variation predict fluid responsiveness in children: a systematic review and meta-analysis. PLoS One. (2017) 12:e0177590. doi: 10.1371/journal.pone.0177590
8. Desgranges FP, Desebbe O, Pereira de Souza Neto E, Raphael D, Chassard D. Respiratory variation in aortic blood flow peak velocity to predict fluid responsiveness in mechanically ventilated children: a systematic review and meta-analysis. Paediatr Anaesth. (2016) 26:37–47. doi: 10.1111/pan.12803
9. Wang X, Jiang L, Liu S, Ge Y, Gao J. Value of respiratory variation of aortic peak velocity in predicting children receiving mechanical ventilation: a systematic review and meta-analysis. Crit Care. (2019) 23(1):372. doi: 10.1186/s13054-019-2647-7
10. Choi DY, Kwak HJ, Park HY, Kim YB, Choi CH, Lee JY. Respiratory variation in aortic blood flow velocity as a predictor of fluid responsiveness in children after repair of ventricular septal defect. Pediatr Cardiol. (2010) 31:1166–70. doi: 10.1007/s00246-010-9776-8
11. Renner J, Broch O, Gruenewald M, Scheewe J, Francksen H, Jung O, et al. Non-invasive prediction of fluid responsiveness in infants using pleth variability index. Anaesthesia. (2011) 66:582–9. doi: 10.1111/j.1365-2044.2011.06715.x
12. Renner J, Broch O, Duetschke P, Scheewe J, Höcker J, Moseby M, et al. Prediction of fluid responsiveness in infants and neonates undergoing congenital heart surgery. Br J Anaesth. (2011) 108:108–15. doi: 10.1093/bja/aer371
13. Lee JY, Kim JY, Choi CH, Kim HS, Lee KC, Kwak HJ. The ability of stroke volume variation measured by a noninvasive cardiac output monitor to predict fluid responsiveness in mechanically ventilated children. Pediatr Cardiol. (2014) 35:289–94. doi: 10.1007/s00246-013-0772-7
14. Saxena R, Durward A, Steeley S, Murdoch IA, Tibby SM. Predicting fluid responsiveness in 100 critically ill children: the effect of baseline contractility. Intensive Care Med. (2015) 41:2161–9. doi: 10.1007/s00134-015-4075-8
15. Lee JH, No HJ, Song IK, Kim HS, Kim CS, Kim JT. Prediction of fluid responsiveness using a non-invasive cardiac output monitor in children undergoing cardiac surgery. Br J Anaesth. (2015) 115:38–44. doi: 10.1093/bja/aev109
16. Han D, Pan S, Wang X, Jia Q, Luo Y, Li J, et al. Different predictivity of fluid responsiveness by pulse pressure variation in children after surgical repair of ventricular septal defect or tetralogy of fallot. Paediatr Anaesth. (2017) 27:1056–63. doi: 10.1111/pan.13218
17. Favia I, Romagnoli S, Di Chiara L, Ricci Z. Predicting fluid responsiveness in children undergoing cardiac surgery after cardiopulmonary bypass. Pediatr Cardiol. (2017) 38:787–93. doi: 10.1007/s00246-017-1582-0
18. Lee JH, Song IK, Kim EH, Kim HS, Kim JT. Prediction of fluid responsiveness based on liver compression-induced blood pressure changes in children after cardiac surgery. Minerva Anestesiol. (2017) 83:939–46. doi: 10.23736/S0375-9393.17.11544-0
19. Han D, Liu YG, Luo Y, Li J, Ou-Yang C. Prediction of fluid responsiveness using pulse pressure variation in infants undergoing ventricular septal defect repair with median sternotomy or minimally invasive right thoracotomy. Pediatr Cardiol. (2017) 38(1):184–90. doi: 10.1007/s00246-016-1500-x
20. Cheng YW, Xu F, Li J. Identification of volume parameters monitored with a noninvasive ultrasonic cardiac output monitor for predicting fluid responsiveness in children after congenital heart disease surgery. Medicine. (2018) 97:e12289. doi: 10.1097/MD.0000000000012289
21. Kim EH, Lee JH, Song IK, Kim HS, Jang YE, Kim JT. Respiratory variation of internal carotid artery blood flow peak velocity measured by transfontanelle ultrasound to predict fluid responsiveness in infants: a prospective observational study. Anesthesiology. (2019) 130:719–27. doi: 10.1097/ALN.0000000000002526
22. Park J, Yang S, Lee JH, Kim JT, Kim HS, Kim HC. The importance of sensor contacting force for predicting fluid responsiveness in children using respiratory variations in pulse oximetry plethysmographic waveform. J Clin Monit Comput. (2019) 33:393–401. doi: 10.1007/s10877-018-0183-7
23. Song Y, Hou H, Bai J, Gu H. Prediction of fluid responsiveness by stroke volume variation in children undergoing fontan operation. Biomed Res Int. (2020) 2020:2595960. doi: 10.1155/2020/2595960
24. Julien F, Hilly J, Sallah TB, Skhiri A, Michelet D, Brasher C, et al. Plethysmographic variability index (PVI) accuracy in predicting fluid responsiveness in anesthetized children. Paediatr Anaesth. (2013) 23:536–46. doi: 10.1111/pan.12139
25. Achar SK, Sagar MS, Shetty R, Kini G, Samanth J, Nayak C, et al. Respiratory variation in aortic flow peak velocity and inferior vena cava distensibility as indices of fluid responsiveness in anaesthetised and mechanically ventilated children. Indian J Anaesth. (2016) 60:121–6. doi: 10.4103/0019-5049.176285
26. Kim EH, Kim H, Lee JH, Kim JT, Jang YE, Ji SH, et al. Role of TFA-1 adhesive forehead sensors in predicting fluid responsiveness in anaesthetized children. Eur J Anaesthesiol. (2020) 37:713–8. doi: 10.1097/EJA.0000000000001235
27. Chen PH, Chan KC, Liao MH, Wu CY. Accuracy of dynamic preload variables for predicting fluid responsiveness in patients with pediatric liver cirrhosis: a prospective study. Paediatr Anaesth. (2020) 30:455–61. doi: 10.1111/pan.13819
28. Zorio V, Lebreton T, Desgranges FP, Bochaton T, Desebbe O, Chassard D, et al. Does a two-minute mini-fluid challenge predict fluid responsiveness in pediatric patients under general anesthesia? Paediatr Anaesth. (2020) 30(2):161–7. doi: 10.1111/pan.13793
29. Pereira de Souza Neto E, Grousson S, Duflo F, Ducreux C, Joly H, Convert J, et al. Predicting fluid responsiveness in mechanically ventilated children under general anaesthesia using dynamic parameters and transthoracic echocardiography. Br J Anaesth. (2011) 106:856–64. doi: 10.1093/bja/aer090
30. Byon HJ, Lim CW, Lee JH, Park YH, Kim HS, Kim CS, et al. Prediction of fluid responsiveness in mechanically ventilated children undergoing neurosurgery. Br J Anaesth. (2013) 110:586–91. doi: 10.1093/bja/aes467
31. Vergnaud E, Vidal C, Verchère J, Miatello J, Meyer P, Carli P, et al. Stroke volume variation and indexed stroke volume measured using bioreactance predict fluid responsiveness in postoperative children. Br J Anaesth. (2015) 114:103–9. doi: 10.1093/bja/aeu361
32. Morparia KG, Reddy SK, Olivieri LJ, Spaeder MC, Schuette JJ. Respiratory variation in peak aortic velocity accurately predicts fluid responsiveness in children undergoing neurosurgery under general anesthesia. J Clin Monit Comput. (2018) 32:221–6. doi: 10.1007/s10877-017-0013-3
33. McLean JR, Inwald DP. The utility of stroke volume variability as a predictor of fluid responsiveness in critically ill children: a pilot study. Intensive Care Med. (2014) 40(2):288–9. doi: 10.1007/s00134-013-3171-x
34. Weber T, Wagner T, Neumann K, Deusch E. Low predictability of three different noninvasive methods to determine fluid responsiveness in critically ill children. Pediatr Crit Care Med. (2015) 16:e89. doi: 10.1097/PCC.0000000000000364
35. Chaiyaphruk M, Boonjindasarp W, Sritippayawan S, Deerojanawong J, Samransamruajkit R. Accuracy of passive leg raising test in predicting of fluid responsiveness in children on ultrasonic cardiac output monitoring. Pediatr Crit Care Med. (2018) 19(65):58. doi: 10.1097/01.pcc.0000537490.01142.20
36. Sun S, Ren H, Wang Y, Zhang J, Li B, Ning B, et al. Respiratory variations in aortic blood flow to predict volume responsiveness in ventilated children with leukemia and neutropenic septic shock. Pediatr Crit Care Med. (2020) 21(5):e247–52. doi: 10.1097/PCC.0000000000002260
37. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J. (2021) 372:n71. doi: 10.1136/bmj.n71
38. Leeflang MM, Deeks JJ, Gatsonis C, Bossuyt PM, Cochrane Diagnostic Test Accuracy Working Group. Systematic reviews of diagnostic test accuracy. Ann Intern Med. (2008) 149:889–97. doi: 10.7326/0003-4819-149-12-200812160-00008
39. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2 Group. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. (2011) 155(8):529–36. doi: 10.7326/0003-4819-155-8-201110180-00009
40. Suehiro K, Joosten A, Murphy LS, Desebbe O, Alexander B, Kim SH, et al. Accuracy and precision of minimally invasive cardiac output monitoring in children: a systematic review and meta-analysis. J Clin Monit Comput. (2016) 30:603–20. doi: 10.1007/s10877-015-9757-9
41. Chaiyakulsil C, Chantra M, Katanyuwong P, Khositseth A, Anantasit N. Comparison of three non-invasive hemodynamic monitoring methods in critically ill children. PLoS One. (2018) 13:e0199203. doi: 10.1371/journal.pone.0199203
42. Mah KE, Hao S, Sutherland SM, Kwiatkowski DM, Axelrod DM, Almond CS, et al. Fluid overload independent of acute kidney injury predicts poor outcomes in neonates following congenital heart surgery. Pediatr Nephrol. (2018) 33(3):511–20. doi: 10.1007/s00467-017-3818-x
43. Kwiatkowski DM, Krawczeski CD. Acute kidney injury and fluid overload in infants and children after cardiac surgery. Pediatr Nephrol. (2017) 32(9):1509–17. doi: 10.1007/s00467-017-3643-2
44. Messina A, Dell'Anna A, Baggiani M, Torrini F, Maresca GM, Bennett V, et al. Functional hemodynamic tests: a systematic review and a metanalysis on the reliability of the end-expiratory occlusion test and of the mini-fluid challenge in predicting fluid responsiveness. Crit Care. (2019) 23:264. doi: 10.1186/s13054-019-2545-z
Keywords: fluid therapy, cardiac output, predict, pediatric, hemodynamic
Citation: Yenjabog P, Kanchongkittiphon W, Chutipongtanate S, Lertbunrian R and Ungprasert P (2022) Dynamic parameters for fluid responsiveness in mechanically ventilated children: A systematic review. Front. Pediatr. 10:1010600. doi: 10.3389/fped.2022.1010600
Received: 3 August 2022; Accepted: 28 September 2022;
Published: 21 October 2022.
Edited by:
Bonnie Harbaugh, University of Southern Mississippi, United StatesReviewed by:
Phuc Huu Phan, Vietnam National Children's Hospital, VietnamNora Bruns, Essen University Hospital, Germany
Fulya Kamit, Yeni Yüzyıl University, Turkey
E. Scott Halstead, The Pennsylvania State University, United States
© 2022 Yenjabog, Kanchongkittiphon, Chutipongtanate, Lertbunrian and Ungprasert. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rojjanee Lertbunrian bHJvamphbmVlQGhvdG1haWwuY29t
Specialty Section: This article was submitted to Pediatric Critical Care, a section of the journal Frontiers in Pediatrics
 Patcha Yenjabog
Patcha Yenjabog Wacharoot Kanchongkittiphon2
Wacharoot Kanchongkittiphon2 Somchai Chutipongtanate
Somchai Chutipongtanate Rojjanee Lertbunrian
Rojjanee Lertbunrian