- 1Department of Pediatrics, Women and Children's Hospital of Chongqing Medical University, Chongqing, China
- 2Department of Pediatrics, Chongqing Health Center for Women and Children, Chongqing, China
Objective: Although nasal continuous positive airway pressure (nCPAP) is recommended in delivery room (DR) management for preterm infants, the effect of delivering nCPAP at 6–8 cmH2O is not satisfactory. Therefore, we conducted this retrospective cohort study to compare the effects of individualized dynamic positive end-expiratory pressure (dynPEEP) vs. positive pressure ventilation (PPV) in the DR on clinical outcomes.
Methods: Preterm infants with a gestational age (GA) less than 30 weeks who received PPV (peak inspiratory pressure, PIP/PEEP 15–25/6–8 cmH2O) from August 2018 to July 2020 were included as Cohort 1 (PPV group, n = 55), and those who received dynPEEP (nCPAP 8–15 cmH2O) from June 2020 to April 2022 were included as Cohort 2 (dynPEEP group, n = 62). Primary outcomes included the DR intubation rate and the bronchopulmonary dysplasia (BPD) rate. The secondary outcomes included DR stabilization, transfer, admission, respiratory function, and other outcomes.
Results: The percentage of singleton infants was higher in the PPV group (63.6%) than in the dynPEEP group (22.6%, p = 0.000). The DR intubation and chest compression rates were higher in the PPV group (80.0% and 18.2%, respectively) than in the dynPEEP group (45.2%, p = 0.000; 3.0%, p = 0.008, respectively). The percentage of patients with 5-min Apgar scores < 5 was higher in the PPV group (9.1%) than in the dynPEEP group (0%, p = 0.016). The partial pressure of carbon dioxide was lower in the PPV group (49.77 ± 11.28) than in the dynPEEP group (56.44 ± 13.17, p = 0.004), and lactate levels were higher in the PPV group (3.60 (2.10, 5.90)) than in the dynPEEP group (2.25 (1.38, 3.33), p = 0.002). No significant differences in the BPD rate or other secondary outcomes were noted.
Conclusions: In this retrospective cohort study, the dynPEEP strategy reduced the need for DR intubation compared with PPV. The dynPEEP strategy is feasible and potentially represents an alternative respiratory strategy to PPV. Nevertheless, a randomized control trial is needed to evaluate the dynPEEP strategy.
1. Background
Some preterm infants require respiratory support at birth to adequately aerate their lungs (1–5). Lung aeration plays an important role in the transition from intrauterine to extrauterine life. However, studies have demonstrated a direct relationship between exposure to intubation (and/or mechanical ventilation, MV) and an increased risk of developing bronchopulmonary dysplasia (BPD) (6, 7). Therefore, noninvasive respiratory support in the form of nasal continuous positive airway pressure (nCPAP) of 6–8 cmH2O is recommended in the international guidelines for delivery room (DR) management (8, 9). Theoretically, nCPAP could contribute to early physiological transition by facilitating alveolar recruitment and establishing functional residual capacity in preterm infants. However, although we performed nCPAP for all very preterm infants (VPI: gestational age less than 32 weeks) immediately after birth, 17% of VPIs and 36% of extremely preterm infants (EPI: gestational age less than 28 weeks) required intubation in the DR (10).
Using higher pressures up to 20–25 cm H2O for a period of approximately 10–15 s at the initiation of respiration (sustained lung inflation, SLI) has been reported as a method to avoid intubation (11). However, the Sustained Inflation of Infants Lung (SAIL) trial was suspended early because it detected higher rates of early death, possibly attributable to receiving SLI (20–25 cmH2O, 10–15 s) (12). The European Guidelines on Respiratory Distress Syndrome (RDS) management recommend that SLI should only be used in clinical trials until further analysis of available data (8). Preclinical studies among premature lambs have shown that rapidly aerating the preterm lung at birth produces distinct regional injury patterns that affect subsequent tidal ventilation (13). The ongoing development and heterogeneity of the preterm lung are not conducive to rapid forced aeration (e.g., SLI) (14). On the other hand, SLI before tidal ventilation is not physiological in the preterm lung, and SLI at birth blunted the effect of surfactant on lung compliance (15).
However, to date, the ideal level of nCPAP in the DR remains unknown. Clinical studies regarding the appropriate positive end-expiratory pressure (PEEP) levels, which could be provided by nCPAP, are lacking. Te Pas et al. reported that immature rabbits required higher starting pressures and longer sustained inflation durations to achieve a set inflation volume (16). Higher PEEP levels improve lung aeration and pulmonary blood flow and reduce the need for positive pressure ventilation (PPV) (17). A retrospective study on EPIs showed that nCPAP with higher pressures (12–35 cmH2O) at birth may require less oxygen and decrease intubation rates compared to pressures of 5–8 cmH2O, whereas the pneumothorax rates (19 vs. 4%, p = 0.125) and the occurrence of spontaneous intestinal perforations (15 vs. 0%, p = 0.125) were increased (18). This study indicated that initial respiratory support for EPIs with high nCPAP levels might decrease intubation rates. However, the adverse event rates increased, and the optimal nCPAP pressure was not specified.
A longitudinal study indicated that after the introduction of a revised protocol to assist EPIs with a GA of 22–26 weeks, the rates of infants intubated in the DR and BPD were significantly decreased (19). In this study, the revised PEEP protocol used in the DR was between 8 and 14 cm H2O, and no harmful effects were noted. Instead, the overall mortality in the revised study group was lower than that in the control group. This study may imply that using a PEEP level of 8–14 cm H2O would be beneficial for EPIs. However, the protocol includes several interventions [e.g., prenatal management and delayed cord clamping (DCC)]; thus, the beneficial outcomes are not only associated with the revised PEEP protocol.
Above all, we hypothesize that different preterm infants may have different lung development conditions; thus, the PEEP required at birth to adequately aerate their lungs is different. In addition, the intubation rate is still high in the DR, which may also indicate that the effect of delivering nCPAP of 6–8 cmH2O is not satisfactory. Therefore, an individualized dynamic PEEP (dynPEEP) is proposed. DynPEEP using an optimal PEEP strategy might be more lung protective than SLI because it adjusts the pressure required according to the vital signs of preterm infants. In preterm lambs, dynPEEP at birth with tidal ventilation and PEEP results in more uniform aeration and ventilation and less lung injury than SLI (13) and improves the surfactant response (15). We inferred that the improved surfactant response using the dynPEEP strategy, which increased functional residual capacity and improved lung compliance/homogeneity compared with SLI, would be more likely to reduce the incidence of PPV and intubation. A recent study conducted in France showed that dynPEEP combined with the SLI strategy was beneficial. However, it is difficult to explain whether the benefit is attributable to dynPEEP or SLI (20).
The dynPEEP strategy was implemented in our hospital in June 2020. We conducted this retrospective cohort study to compare the dynPEEP strategy with PPV in the DR on the clinical outcomes of preterm infants with a gestational age (GA) less than 30 weeks who did not respond to an initial CPAP at 6–8 cmH2O.
2. Methods
2.1. Inclusion and exclusion criteria
All inborn infants < 30 weeks of gestation admitted to the neonatal intensive care unit (NICU) of the Women and Children's Hospital of Chongqing Medical University, which is a tertiary hospital in southwestern China, were initially included in this study. The study period was between August 2018 and April 2022.
The inclusion criteria were as follows: 1. Infants delivered at <30 weeks of gestation who did not respond to an initial CPAP at 6–8 cmH2O; and 2. noninvasive respiratory support was provided immediately after birth in the DR.
The exclusion criteria were as follows: 1. Refusal of consent for the data to be analyzed; 2. there was no need for any respiratory support or only support with a PEEP of 6–8 cmH2O in the DR, but dynPEEP or PPV was not provided; 3. known major congenital anomalies or inherited metabolic diseases that might have an adverse effect on breathing or ventilation; 4. maternal factors, such as general anesthesia, placental abruption, placenta previa, and monochorionic twins; and 5. outborn infants.
2.2. Participants
Two cohorts of preterm infants born at < 30 weeks of gestation and observed for 4 years were compared retrospectively before (Cohort 1: PPV group) and after the renovation of respiratory support management in the DR (Cohort 2: dynPEEP group). In Cohort 1, preterm infants who received PPV (peak inspiratory pressure, PIP/PEEP 15–25/6–8 cmH2O) from August 2018 to July 2020 were included (the PPV group, n = 55). In Cohort 2, preterm infants who received dynPEEP (nCPAP 8–15 cmH2O) from June 2020 to April 2022 were included (the dynPEEP group, n = 62).
2.3. Stabilization and respiratory support in the DR
All team members were trained to deliver noninvasive respiratory support. At delivery, DCC was performed first. The obstetricians and/or midwife were the DCC providers, and they simultaneously gently rubbed the backs of the infants. The delay time of DCC was based on the recommendation made by the European Consensus Guidelines and the American Heart Association to wait for at least 30–60 s (8,9). Then, eligible infants (230/7 to 296/7 weeks GA) were placed onto a radiant warmer (Giraffe Omnibed) wrapped in plastic wrap. Then, nCPAP was given via a face mask or nasal prong using a T-piece device (Neopuff Infant Resuscitator, Fisher & Paykel Healthcare, Auckland, New Zealand) with pressure at 6–8 cmH2O and a fraction of inspired oxygen (FiO2) of 21%–30% (30% for babies < 28 weeks gestation and 21%–30% for those 28–30 weeks gestation) (8). A pulse oximeter probe (L-NOPNeo; Masimo Corp, Irvine, CA) was placed on the right hand, and 3 ECG chest leads were applied on the chest. The oximeter was set to acquire data with maximum sensitivity and a 5 s average interval.
In the PPV group, if the infant was apneic (no spontaneous breathing with stimulation by rubbing the soles of the feet or the back of the chest for more than 10 s) and/or bradycardic (heart rate less than 100 bpm), PPV (peak inspiratory pressure, PIP/PEEP 25/6%–8% cmH2O, 40–60/min) was administered. The FiO2 was initially set at 30% and could be adjusted to 100% based on the 25th percentile of the Dawson criteria (21). Respiratory support was provided using the Neopuff TM T-piece resuscitator (Neopuff Infant Resuscitator, Fisher & Paykel Healthcare Ltd., Auckland, New Zealand) via face mask or prongs.
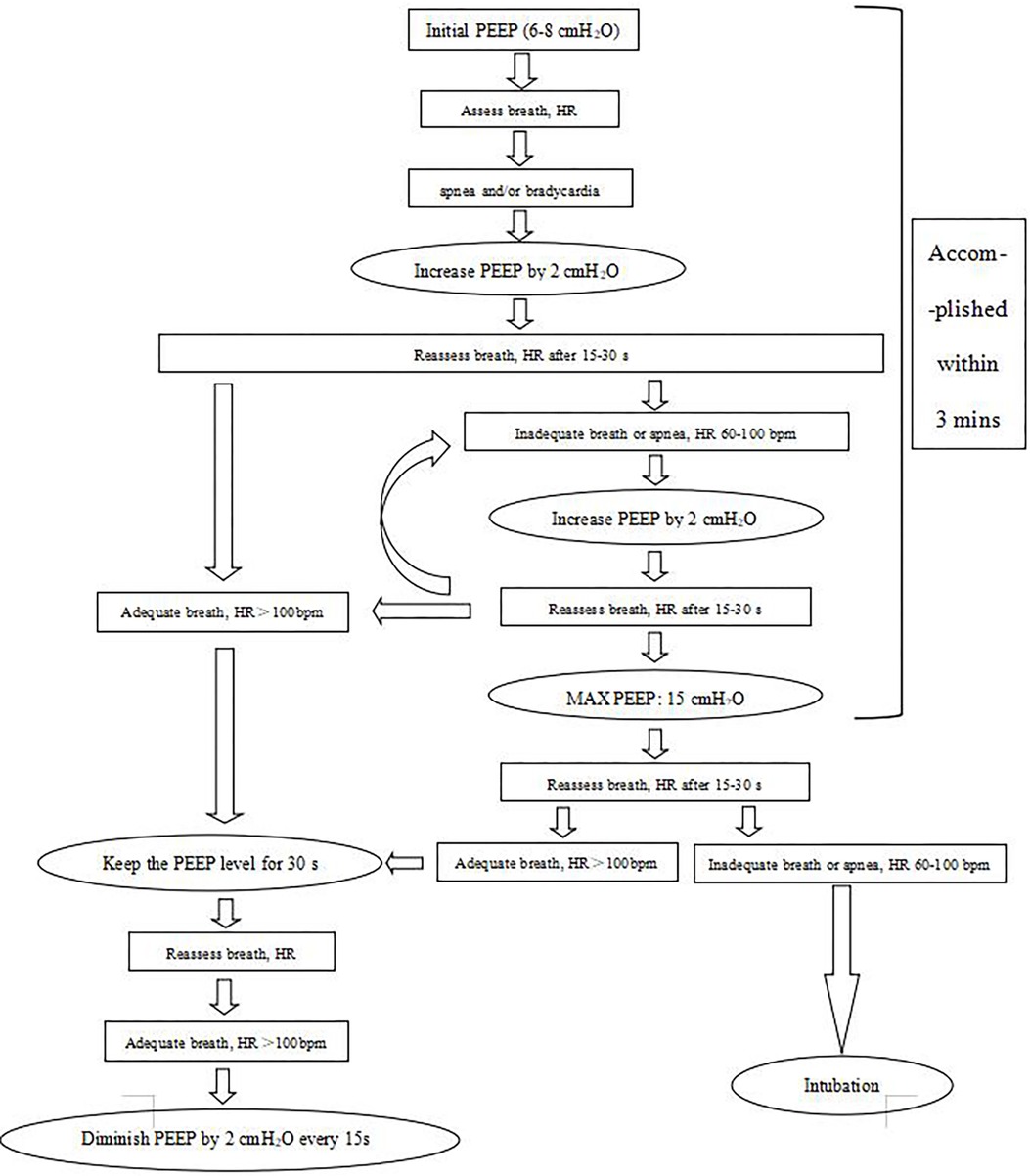
In the dynPEEP group, if the infant was apneic and/or bradycardic (same definition as mentioned above), PEEP was increased by 2 cmH2O/15–30 s to a maximum of 15 cmH2O without any PIP inflation. The FiO2 was initiated at 30% and could be increased to 100% based on the 25th percentile of the Dawson criteria. The PEEP was increased prior to FiO2 initiation. PEEP was allowed to be decreased if a baby improved. Figure 1 shows the dynPEEP algorithms in DR management. To ensure that this new method would be adopted by our staff, we trained the staff first at the beginning of the implementation. It took approximately 2 months (from the beginning of May 2020 to the end of June) for all the staff to adhere to this new method. At the beginning, all the stabilization processes of preterm infants with GAs less than 30 weeks were videotaped (the first 3 weeks in May), and the resuscitation records and videos were used to check whether the dynPEEP algorithms were carried out. We were then debriefed on the stabilization process and gave feedback to the staff who were in charge, and we trained the staff repeatedly. At the end of May 2020, the video playback and debrief process were performed approximately once or twice a week with the gradual acceptance of this new intervention. At the end of June 2020, approximately 90% of the providers stabilized the infants according to the dynPEEP algorithms in the DR.
If PPV or dynPEEP was ineffective, then the infant was intubated. The DR intubation criteria were as follows: (1) heart rate less than 60 bpm after 30 s of effective respiratory support (dynPEEP or PPV; in this case, dynPEEP was interrupted at 10–12 cmH2O), (2) heart rate remaining at less than 100 bpm 3 min after birth, and (3) still apneic 3 min after birth. Intubation was performed at the discretion of the neonatologist in charge.
The less invasive surfactant administration (LISA) method was used for pulmonary surfactant administration in the DR for all infants in case intubation was not needed. The LISA method was introduced in our unit in 2017 as a priority method for all preterm infants less than 1500 grams or with a GA less than 32 weeks. All the doctors who used this method were highly trained and experienced. If the infant was already intubated, then the intubation-surfactant-extubation (INSURE) procedure or intubation-surfactant (INSUR) without the extubation method was used, which were introduced in our unit in 2010. Surfactant was administered through the endotracheal tube; if the FiO2 dropped to 30% in a short time without dyspnea, then extubation and noninvasive respiratory support were continued. If not, MV was implemented. Pulmonary surfactant was used in the DR or in the NICU.
After stabilization in the DR, the infants were transported to the NICU in incubators. We used a Hamilton ventilator (Hamilton Medical AG, Switzerland) during the transfer for both noninvasive and mechanical ventilation. If the infant was on noninvasive respiratory support (nCPAP or nasal intermittent positive pressure ventilation, NIPPV), then a binasal prong was used as the interface. The methods and settings of respiratory support during the transfer were recorded in the resuscitation records.
In the NICU, the criteria for intubation were as follows: (1) for infants <32 weeks gestation with severe apnea (defined as recurrent apnea with >3 episodes/h associated with a heart rate < 100/min, a single episode of apnea that required PPV, or saturation of pulse oxygen (SpO2) < 85% and FiO2 > 0.6); (2) for infants with RDS or dyspnea that rapidly progressed and/or persisted after noninvasive ventilation and/or pulmonary surfactant treatment, and FiO2 ≥ 40%, PaO2 < 50–60 mmHg or SpO2 < 90% (except for cyanotic heart disease), or partial pressure of carbon dioxide (PCO2) > 60–65 mmHg, pH <7.25; and (3) if there was some instability in the hemodynamics of the infants. The extubation criteria were as follows: extubation was recommended within 24 h of meeting all the following criteria: PCO2 of 55 mmHg or less, pH of 7.25 or greater, FiO2 of 0.40 or less with oxygen saturation as measured by pulse oximetry (SpO2) of 90% or greater, and mean airway pressure (MAP) of 8 cmH2O or less with hemodynamic stability. High-frequency oscillation (HFO) ventilation was recommended if one of the following criteria was met: (1) PCO2 > 60–65 mmHg and pH <7.25 even when the tidal volume reached 6 ml/kg and the minute volume reached 0.3 L/min under MV, (2) excess secretion in the airway, and (3) air leakage, including pneumothorax and mediastinal emphysema. Permissive hypercapnia (pH >7.25, pCO2 50–65 mmHg) was allowed in our unit. The criteria to start systemic corticosteroids were as follows: (1) for infants with severe BPD according to the National Institute of Child Health and Human Development (NICHD) workshop definition (22), (2) for infants who required MV for more than 7–10 days since birth, and for those who had FiO2 > 0.4. Systematic sedation-analgesia (fentanyl and/or midazolam) was used only for mechanically ventilated infants who were irritated. The doses of caffeine citrate were as follows: a loading dose of 20 mg/kg and a maintenance dose of 5 mg/kg q24 h or q12 h.
2.4. Demographic characteristics and clinical outcomes
2.4.1. Demographic characteristics
All data on neonatal and maternal demographics were collected via resuscitation and electronic medical records.
2.4.2. Primary outcomes
The primary outcomes were the DR intubation rate and bronchopulmonary dysplasia (BPD) rate.
The definition of BPD was as follows: A premature infant (<32 weeks gestational age) with BPD had persistent parenchymal lung disease, radiographic confirmation of parenchymal lung disease, and required respiratory support or oxygen mode for ≥ 3 consecutive days to maintain arterial oxygen saturation in the 90%–95% range at 36 weeks PMA (22).
2.4.3. Secondary outcomes
1) DR outcomes
The DR chest compression rate; Apgar scores at 1, 5, and 10 min; Apgar score < 1 at 1 min and < 5 at 1, 5, and 10 min; and DR maximum fraction of inspired oxygen (FiO2) were the DR outcomes.
2) Transfer outcomes: Respiratory support during the transfer to the NICU
The methods and settings of respiratory support during the transfer were collected from the resuscitation records.
3) Admission outcomes
Admission arterial blood gas, including pH values, partial pressure of oxygen, partial pressure of carbon dioxide, base excess, lactate, maximum FiO2, and P/F (formula: PaO2 ÷ FiO2).
4) Respiratory outcomes
Mortality within 48 h after birth, surfactant therapy, surfactant administration ≥ 2 times, pneumothorax rate within 72 h of age, MV within 72 h of age, MV during hospitalization, time of start on MV (hours), duration of MV (hours), duration of noninvasive respiratory support (hours), duration of oxygen therapy (days), systematic dexamethasone and treated PDA (ibuprofen), mortality and composite BPD and/or mortality rate were the respiratory outcomes.
5) Other outcomes
Early-onset sepsis (EOS) (23), late-onset sepsis (LOS) (23), necrotizing enterocolitis (NEC) (≥ phase 2) (24), intraventricular hemorrhage (IVH) (≥ grade 3) (25), and retinopathy of prematurity (ROP) (> phase 2) were the other outcomes (26).
All data on outcomes were collected via resuscitation and electronic medical records. Outcomes with missing data due to transfer or death prior to assessment were excluded. Both the number of infants with the outcome and the number assessed are shown.
2.5. Data analysis
Data were analyzed using IBM SPSS Statistics version 23.0 (IBM Software, Chicago, Illinois, United States, 2016). The normality test and the homogeneity of variance test were performed for continuous data. If the data were normally distributed and homogenous in variance, the data were expressed as the means ± standard deviations (X ± SDs), and Student's t test was used for comparisons between the two groups. If the data were not normally distributed or the variance was not uniform, the rank-sum test (Mann‒Whitney U test) was used, and the data were presented as the medians (interquartile ranges, IQRs). The categorical data were expressed as percentages (%), and the rates were compared between the two groups by the chi-square test or Fisher's exact probability method and are presented as counts (n) and percentages (%). To control for the confounders for DR intubation, the demographic characteristics and variables with a P value < 0.05 in the univariate analysis were applied to the multivariate analysis based on logistic regression. The power of the association was represented by χ2 in the logistic regression model. Furthermore, a subgroup analysis was performed for infants with a GA less than 28 weeks. P values < 0.05 were considered statistically significant, and reported P values were two sided. No adjustment of P values was performed to account for multiple comparisons because subgroup analyses were considered exploratory.
2.6. Ethics
This study was approved by the Ethics Committee of Chongqing Health Center for Women and Children (No. 2021–022).
3. Results
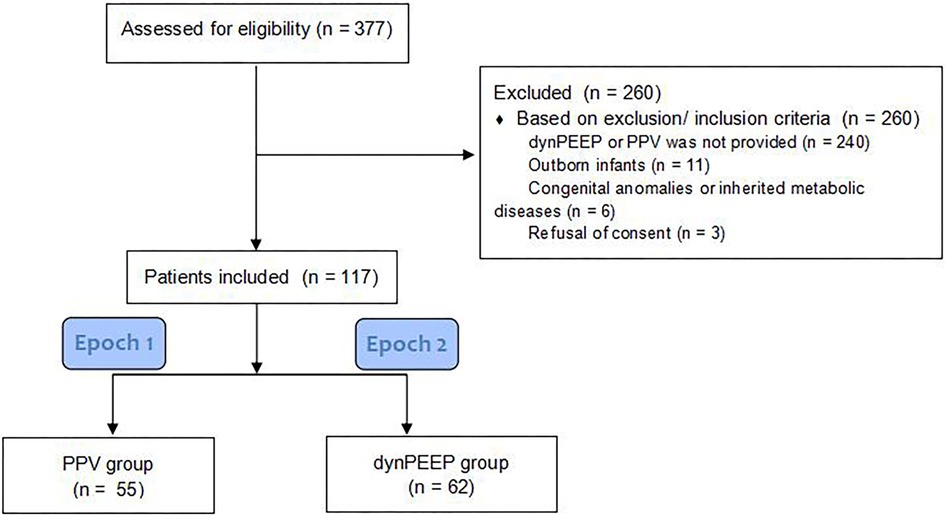
Of the 377 preterm infants who were potentially eligible to participate, 117 were included in this study, and 260 did not meet our inclusion criteria. In total, 55 infants were supported with PPV, and 62 infants were supported with dynPEEP.
The flowchart shows the inclusion and exclusion criteria for the patients (Figure 2).
3.1. Maternal and neonatal demographics
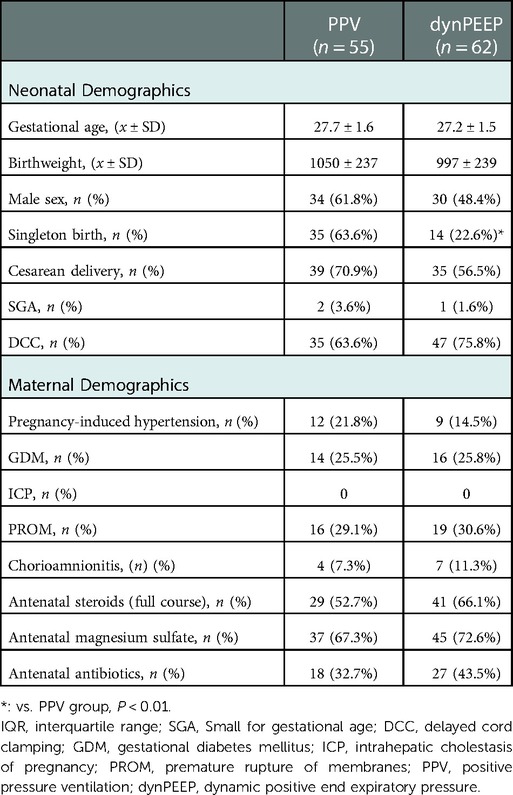
The maternal and neonatal demographics are shown in Table 1. The percentage of singleton infants in the PPV group (63.6%) was significantly higher than that in the dynPEEP group (22.6%, p = 0.000). There were no significant differences in the other demographics between the two groups.
The DR maximum PEEP was significantly higher in the dynPEEP group (12, (10, 15)) than in the PPV group (6, (6, 8)) (p = 0.000). In the dynPEEP group, the number and percentage of cases with different PEEP levels were 21 cases (33.87%) at 9–10 cmH2O, 25 cases (40.32%) at 11–14 cmH2O, and 16 cases (25.81%) at 15 cmH2O.
3.2. Primary outcomes
The DR intubation rate was higher in the PPV group (80.0%) than in the dynPEEP group (45.2%, p = 0.000) (Table 2). No significant difference in the BPD rate was noted between the two groups.
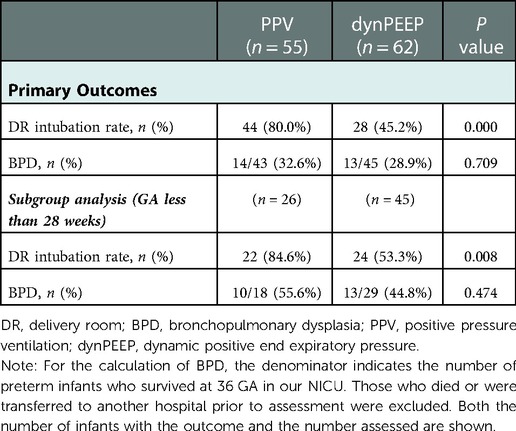
Table 2. The primary outcomes of preterm infants with gestational Age less than 30 weeks who received PPV or DynPEEP in the DR.
3.3. Secondary outcomes
3.3.1. DR, transfer, and admission outcomes
Table 3 displays the DR, transfer, and admission outcomes.

Table 3. The secondary outcomes (DR, transfer, and admission) of preterm infants with gestational Age less than 30 weeks who received PPV or DynPEEP in the DR.
For the DR outcomes, the DR chest compression rate was higher in the PPV group (18.2%) than in the dynPEEP group (3.2%, p = 0.008), and the percentage of patients with 5 min Apgar scores < 5 at was higher in the PPV group (9.1%) than in the dynPEEP group (0%, p = 0.016).
With regard to the transfer outcomes, the percentage of infants with noninvasive respiratory support in the dynPEEP group was higher than that in the PPV group. The settings during the transfer also exhibited significant differences in PEEP levels (nasal or MV) and PIP levels (MV) between the two groups.
Regarding the admission outcomes, the partial pressure of carbon dioxide on admission was lower in the PPV group (49.8 ± 11.3) than in the dynPEEP group (56.4 ± 13.2, p = 0.004), and the lactate levels were higher in the PPV group (3.6 (2.1, 5.9)) than in the dynPEEP group (2.3 (1.4, 3.3), p = 0.002).
No significant differences in the other DR, transfer, or admission outcomes were noted.
3.3.2. Respiratory and other outcomes
Table 4 displays respiratory and other outcomes. The percentage of the less invasive surfactant administration (LISA) method was lower in the PPV group (25.5%) than in the dynPEEP group (50%, p = 0.006). No differences in other secondary respiratory or other outcomes were noted between the two groups, including mortality within 48 h after birth, mortality rate, composite BPD and/or mortality rates.
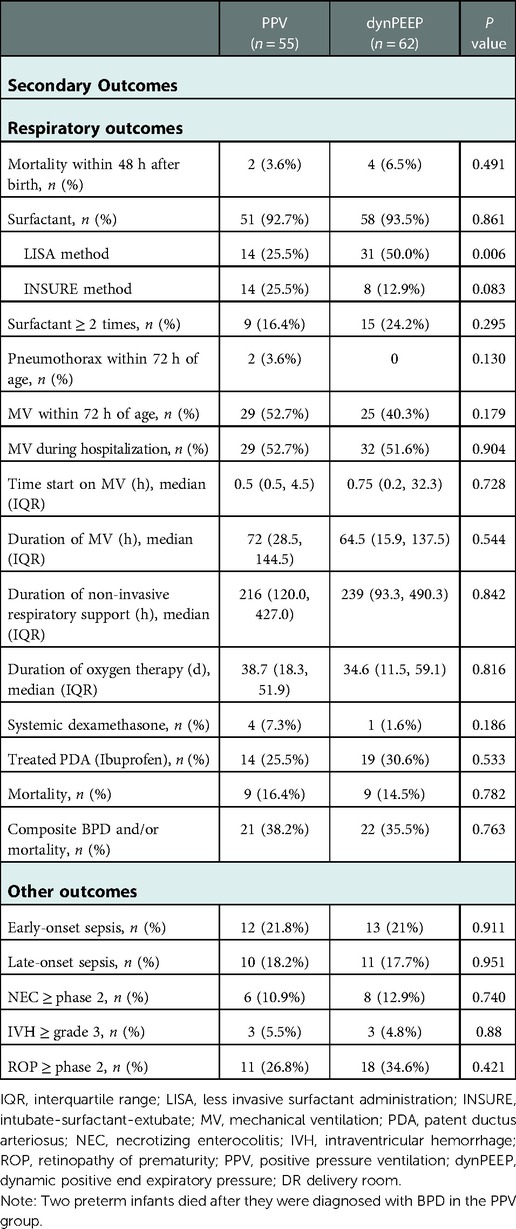
Table 4. The secondary outcomes (respiratory and other) of preterm infants with gestational Age less than 30 weeks who received PPV or DynPEEP in the DR.
The HFO mode was used for 14 infants in the PPV group and for 16 infants in the dynPEEP group.
3.4. Multivariate logistic regression
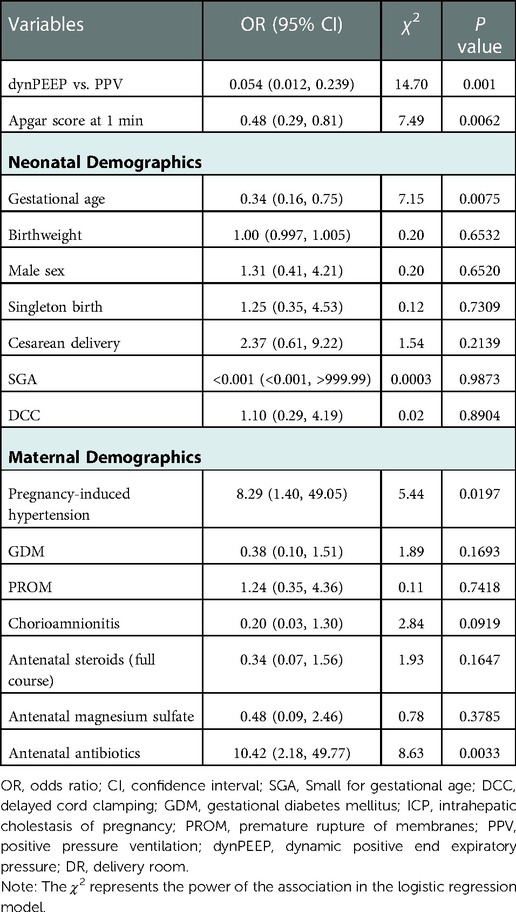
We applied the demographic characteristics, dynPEEP vs. PPV and Apgar score at 1 min (variables with P value < 0.05) in the univariate analysis to the multivariate analysis based on logistic regression (Table 5).
Logistic regression showed that dynPEEP was a protective factor compared with PPV. Moreover, the Apgar score at 1 min, GA, pregnancy-induced hypertension, and antenatal antibiotics were associated with DR intubation.
3.5. Subgroup analysis among infants with a ga less than 28 weeks
We further performed a subgroup analysis among infants with a GA less than 28 weeks. The maternal and neonatal demographics are shown in Supplementary Table S1. The percentage of singleton infants in the PPV group (57.7%) was significantly higher than that in the dynPEEP group (20.0%, p = 0.001). No significant differences in the other demographics were noted between the two groups.
Regarding the primary outcomes, the DR intubation rate was higher in the PPV group (84.6%) than in the dynPEEP group (53.3%, p = 0.008) (Table 2). No significant differences in the BPD rate were noted between the two groups.
With regard to the secondary outcomes (Supplementary Table S2), the DR chest compression rate was higher in the PPV group (23.1%) than in the dynPEEP group (0.0%, p = 0.000), and the lactate levels were higher in the PPV group (3.8 (2.0, 6.0)) than in the dynPEEP group (2.3 (1.4, 3.3), p = 0.013). No significant differences in the other secondary outcomes were noted.
4. Discussion
Clinical data regarding the effect of dynPEEP on both DR and NICU outcomes are still lacking. We conducted this retrospective cohort study to compare dynPEEP vs. PPV. We showed in this study that the dynPEEP strategy with a PEEP level of 8–15 cmH2O decreased the DR intubation rate. However, the dynPEEP strategy showed no impact on the BPD and/or mortality rates. In addition, the dynPEEP strategy is feasible and might represent an alternative respiratory strategy to PPV in the DR.
Endotracheal intubation is an emergency treatment for some preterm infants. Intubation is associated with increased BPD and mortality rates (27). Therefore, the dynPEEP strategy could theoretically decrease the BPD rate. In contrast, the dynPEEP strategy showed no impact on the BPD and mortality rates. We hypothesize that BPD is a multifactorial condition with antenatal genetic and environmental factors; thus, respiratory support in the DR is not the only risk factor. In addition, approximately half of the included infants had a GA > 28 weeks, among whom the BPD and mortality rates were relatively lower. This finding might also explain the lack of significant differences in the outcomes. However, when we performed a subgroup analysis among infants with a GA less than 28 weeks, we still found no differences between the two groups. The twofold higher rate of multiple births in the dynPEEP group could also partially explain the lack of difference in the BPD rate between the two groups. Moreover, as the sample size was small, the statistical analysis may not have been sufficiently statistically powered to draw a conclusion. Notably, although there was no significant difference, the rate of systemic dexamethasone decreased from 15.4% in the PPV group to 2.2% in the dynPEEP group among infants with a GA less than 28 weeks (Supplementary Table S2), which was a sevenfold decrease. We suspected that this phenomenon might explain the lessened pulmonary morbidity during the second epoch. Nevertheless, a larger, randomized control trial (RCT) is needed to evaluate the effect of the dynPEEP strategy on BPD prevalence.
Although the dynPEEP strategy could decrease the DR intubation rate, the DR intubation rate in the PPV group was 80%, which was considerably greater than those among VPIs (17%) and EPIs (36%), as described in the Background section. These data were obtained from all the VPIs and EPIs in our hospital. Nevertheless, we excluded infants supported only with PEEP levels of 6–8 cmH2O (n = 240), as shown in Figure 1. We presumed that this group of infants might have more mature lungs than those who needed dynPEEP or PPV. Therefore, the DR intubation rate in the PPV group was much higher. Consistent with the lower DR intubation rate, the chest compression rate and the percentage of 5 min Apgar scores < 5 were also lower in the dynPEEP group. Consequently, the rate of the LISA method and the percentage of infants with noninvasive respiratory support in the dynPEEP group during the transfer from the DR to the NICU were higher. Due to higher PEEP levels in the dynPEEP group, the PIP levels were lower than those in the PPV group. Given that a ΔP was noted in the PPV group, which could lower the CO2 levels, the PaCO2 values were higher in the dynPEEP group. Interestingly, lower lactate levels were noted in the dynPEEP group, which might be explained by the enhanced alveolar recruitment and oxygenation in the dynPEEP group. However, the PaO2 and P/F values were not significantly different between the two groups. This phenomenon should be considered in future studies.
There are concerns that higher PEEP levels could overexpand the lungs, thereby increasing the risk of pneumothorax and causing lung injury (17). The SAIL trial showed that death at less than 48 h of age occurred in 16 infants (7.4%) in the SLI group vs. 3 infants (1.4%) in the PPV group. We found no significant adverse events in the dynPEEP group in our study, but the rates of deaths at less than 48 h of age were 6.5% in the dynPEEP group and 3.6% in the PPV group. As seen from the above data, there was a higher mortality rate in the PEEP group than that in the PPV group both in our study and in the SAIL trial. Given that this was a single-center retrospective study with a small sample size, it is difficult to draw a conclusion regarding the safety of the dynPEEP strategy. A well-designed prospective study is needed to evaluate the safety of the dynPEEP strategy with SLI or PPV.
The European Guidelines on RDS management suggest that routine use of positive pressure breaths should be discouraged (9, 28). However, for babies who remain apneic or bradycardic, gentle PPV may be needed. Herein, we demonstrated that the dynPEEP strategy decreased the DR intubation rate compared to the PPV strategy. Therefore, the dynPEEP strategy might represent an alternative respiratory strategy to PPV in the DR.
A randomized controlled, multinational, multicenter trial has been ongoing since May 2021 (29). This is a two-arm study comparing the dynPEEP strategy of 8–12 cmH2O with the standard PEEP strategy of 5–6 cmH2O in the DR. The aim of the POLAR trial and our study is to compare the optimal amount or level of PEEP to give at birth and its consequential outcomes. Although the POLAR trial also focuses on the effect of dynPEEP, the PEEP range in the dynPEEP group is between 8 and 12 cmH2O, as mentioned above, which is different from that used in our study. One of the inclusion criteria in the POLAR trial is that infants receive respiratory intervention at birth with CPAP and/or PPV in the DR. Thus, infants in the dynPEEP group might also receive PPV, which differs from our study design. In addition, the primary outcome of the POLAR trial is the prevalence of the composite outcome of either death or BPD, as assessed by a standard oxygen reduction test, which is different from the BPD definition in our study. To date, the POLAR trial has not posted results on ClinicalTrials.gov and is currently recruiting participants. We believe that the results from the POLAR trial would be more convincing than our study because it is a prospective and multicenter RCT.
Given that this was a retrospective cohort study, some limitations should be noted. First, given the retrospective design, there may be confounding factors (twin differences, gestation, unknown, etc.) and bias, and a prospective RCT is needed to make categorical statements regarding safety or benefit. Clinicians might have been more likely to intubate the infants in the PPV group once PPV had started, which might be a potential bias. However, PPV was not allowed in the dynPEEP group because we were concerned that PPV would mask the lung protection of the dynPEEP method (13, 15, 30). Second, half of the included infants had a GA > 28 weeks; thus, the requirement for early respiratory support and the BPD and mortality rates were relatively low. This might explain the lack of significant differences in the outcomes. Although a stratified analysis among infants less than 28 weeks gestation still showed no significant difference, the small sample size might not have been sufficiently statistically powered. Furthermore, the BPD assessment had limitations due to missing data (infants were transferred to another hospital). Third, the lack of systematic assessment of compliance with dynPEEP in the DR was another limitation. Finally, the sample size was relatively small, as this was a time-based feasibility sample, and the multiple secondary statistical analyses made in this study limited the value of significant results.
5. Conclusions
Our study showed that the dynPEEP strategy was feasible for preterm infants with a GA < 30 weeks in the DR. Although the dynPEEP strategy with a PEEP level of 8–15 cmH2O could decrease the need for DR intubation, decreased BPD and/or mortality rates were not observed, and the corresponding measurable clinical outcomes were not approved. The dynPEEP strategy might be an alternative respiratory strategy in the DR instead of PPV. Because another RCT for preterm infants of less than 32 weeks GA in the DR was conducted in our hospital, we could not perform a dynPEEP RCT. A larger, multicenter RCT including more preterm infants with GAs less than 30 or 28 weeks is needed to evaluate the complete effects of the dynPEEP strategy. Above all, the dynPEEP strategy in the DR is worth further exploration.
Data availability statement
The original raw data (individual de-identified subject data) can be obtained and reviewed by email correspeondence to [YW, e-mail address: bird8227@163.com] upon reasonable request.
Ethics statement
The studies involving human participants were reviewed and approved by The Ethics Committee of Chongqing Health Center for Women and Children.. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
SS performed the data analyses and wrote the manuscript; YZ contributed significantly to analysis and manuscript preparation JL helped perform the analysis with constructive discussions. HG contributed to data collection XZ participated in designing the study initiated and managed the study YW contributed to the conception of the study, writing and revision. All authors contributed to the article and approved the submitted version.
Funding
The work was supported by the Natural Science Foundation of Chongqing (cstc2020jcyj-msxmX0483), Chongqing Science and Health Joint Medical Research Project (No. 2022ZDXM035) and Chongqing Maternal and Child Scientific Research Project (No. 2021FY109).
Acknowledgments
The authors thank Professor GJ and HM for guiding the writing of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.1007632/full#supplementary-material.
References
1. O’Donnell CP, Kamlin CO, Davis PG, Morley CJ. Crying and breathing by extremely preterm infants immediately after birth. J Pediatr. (2010) 156:846–7. doi: 10.1016/j.jpeds.2010.01.007
2. te Pas AB, Davis PG, Hooper SB, Morley CJ. Fromliquid to air: breathing after birth. J Pediatr. (2008) 152:607–11. doi: 10.1016/j.jpeds.2007.10.041
3. Poulton DA, Schmolzer GM, Morley CJ, Davis PG. Assessment of chest rise during mask ventilation of preterm infants in the delivery room. Resuscitation. (2011) 82:175–9. doi: 10.1016/j.resuscitation.2010.10.012
4. Huberts TJP, Foglia EE, Narayen IC, van Vonderen JJ, Hooper SB, Te Pas AB. The breathing effort of very preterm infants at birth. J Pediatric. (2018) 194:54–9. doi: 10.1016/j.jpeds.2017.11.008
5. Schilleman K, van der Pot CJ, Hooper SB, Lopriore E, Walther FJ, te Pas AB. Evaluating manual inflations and breathing during mask ventilation in preterm infants at birth. J Pediatr. (2013) 162:457–63. doi: 10.1016/j.jpeds.2012.09.036
6. Gagliardi L, Bellu R, Lista G, Zanini R. Network neonatale lombardo study group. Do differences in delivery room intubation explain different rates of bronchopulmonary dysplasia between hospitals? Arch Dis Child Fetal Neonatal Ed. (2011) 96:F30–35. doi: 10.1136/adc.2010.183905
7. May C, Patel S, Kennedy C, Pollina E, Rafferty GF. Prediction of bronchopulmonary dysplasia. Arch Dis Child Fetal Neonatal Ed. (2011) 96:F410–416. doi: 10.1136/adc.2010.189597
8. Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, Te PA, et al. European Consensus guidelines on the management of respiratory distress syndrome - 2019 update. Neonatol. (2019) 115(4):432–50. doi: 10.1159/000499361
9. Wyckoff MH, Aziz K, Escobedo MB, Kapadia VS, Kattwinkel J, Perlman JM, et al. Part 13: neonatal resuscitation: 2015 American heart association guidelines update for cardiopulmonary resuscitation and emergency ardiovascular care. Circulation. (2015) 132(18 Suppl. 2):S543–560. doi: 10.1161/CIR.0000000000000267
10. Ou JF, Zhong XY, Wu Y, Zhang XT, Gong H, Chen W. Resuscitation quality improvement and its outcomes in very low birth weight infants from 2017 to 2019. Chin J Perinat Med. (2020) 23(9):600–7. (in Chinese). doi: 10.3760/cma.j.cn113903-20200228-00173
11. Fischer HS, Schmölzer GM, Cheung PY, Bührer C. Sustained inflations and avoiding mechanical ventilation to prevent death or bronchopulmonary dysplasia: a meta-analysis. EurRespir Rev. (2018) 27(150):180083. doi: 10.1183/16000617.0083-2018
12. Kirpalani H, Ratcliffe SJ, Keszler M, Davis PG, Foglia EE, Te PA, et al. Effect of sustained inflations vs intermittent positive pressure ventilation on bronchopulmonary dysplasia or death among extremely preterm infants: the sail randomized clinical trial. JAMA. (2019) 321(12):1165–75. doi: 10.1001/jama.2019.1660
13. Tingay DG, Pereira-Fantini PM, Oakley R, McCall KE, Perkins EJ, Miedema M, et al. Gradual aeration at birth is more lung protective than a sustained inflation in preterm lambs. Am J Respir Crit Care Med. (2019) 200(5):608–16. doi: 10.1164/rccm.201807-1397OC
14. Hillman NH, Kemp MW, Noble PB, Kallapur SG, Jobe AH. Sustained inflation at birth did not protect preterm fetal sheep from lung injury. Am J Physiol Lung Cell Mol Physiol. (2013) 305(6):L446–53. doi: 10.1152/ajplung.00162.2013
15. Tingay DG, Togo A, Pereira-Fantini PM, Miedema MA, McCall KE, Perkins EJ, et al. Aeration strategy at birth influences the physiological response to surfactant in preterm lambs. Arch Dis Child Fetal Neonatal Ed. (2019) 104(6):F587–93. doi: 10.1136/archdischild-2018-316240
16. Te Pas AB, Kitchen MJ, Lee K, Wallace MJ, Fouras A, Lewis RA, et al. Optimizing lung aeration at birth using a sustained inflation and positive pressure ventilation in preterm rabbits. Pediatr Res. (2016) 80(1):85–91. doi: 10.1038/pr.2016.59
17. Martherus T, Oberthuer A, Dekker J, Hooper SB, McGillick EV, Kribs A, et al. Supporting breathing of preterm infants at birth: a narrative review. Arch Dis Child Fetal Neonatal Ed. (2019) 104(1):F102–7. doi: 10.1136/archdischild-2018-314898
18. Martherus T, Oberthuer A, Dekker J, Kirchgaessner C, van Geloven N, Hooper SB, et al. Comparison of two respiratory support strategies for stabilization of very preterm infants at birth: a matched-pairs analysis. Front Pediatr. (2019) 7(29):3. doi: 10.3389/fped.2019.00003
19. Mehler K, Grimme J, Abele J, Huenseler C, Roth B, Kribs A. Outcome of extremely low gestational age newborns after introduction of a revised protocol to assist preterm infants in their transition to extrauterine life. Acta Paediatr. (2012) 101(12):1232–9. doi: 10.1111/apa.12015
20. Kanaan Z, Bloch-Queyrat C, Boubaya M, Lévy V, Bolot P, Waszak P. Feasibility of combining two individualized lung recruitment maneuvers at birth for very low gestational age infants: a retrospective cohort study. BMC Pediatr. (2020) 20(1):144. doi: 10.1186/s12887-020-02055-3
21. Dawson JA, Kamlin CO, Vento M, Wong C, Cole TJ, Donath SM, et al. Defining the reference range for oxygen saturation for infants after birth. Pediatr. (2010) 125(6):e1340–7. doi: 10.1542/peds.2009-1510
22. Higgins RD, Jobe AH, Koso-Thomas M, Bancalari E, Viscardi RM, Hartert TV, et al. Bronchopulmonary dysplasia: executive summary of a workshop. J Pediatr. (2018) 197:300–8. doi: 10.1016/j.jpeds.2018.01.043
23. The subspecialty group of neonatology, the society of pediatric, Chinese medical association; professional committee of infectious diseases, neonatology society, Chinese medical doctor association. Expert consensus on the diagnosis and management of neonatal sepsis (version 2019). Chin J Pediatr. (2019) 57(4):252–7. (in Chinese) doi: 10.3760/cma.j.issn.0578-1310.2019.04.005
24. Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. (1978) 187:1–7. doi: 10.1097/00000658-197801000-00001
25. Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. (1978) 92:529–34. doi: 10.1016/S0022-3476(78)80282-0
26. Alice L. Retinopathy of prematurity. N Carol Med J March. (2017) 78:124–8. doi: 10.18043/ncm.78.2.124
27. Hutchison AA, Bignall S. Non-invasive positive pressure ventilation in the preterm neonate: reducing endotrauma and the incidence of bronchopulmonary dysplasia. Arch Dis Child Fetal Neonatal Ed. (2008) 93:F64–8. doi: 10.1136/adc.2006.103770
28. Jobe AH, Ikegami M. Mechanisms initiating lung injury in the preterm. Early Hum Dev. (1998) 53(1):81–94. doi: 10.1016/s0378-3782(98)00045-0
29. ClinicalTrials.gov. Positive End-Expiratory Pressure (PEEP) Levels During Resuscitation of Preterm Infants at Birth (The POLAR Trial). (POLAR). ClinicalTrials.gov Identifier: NCT04372953. https://clinicaltrials.gov/ct2/show/record/NCT04372953?term=04372953&draw=2&rank=1&view=record
Keywords: dynamic PEEP, positive pressure ventilation, bronchopulmonary dysplasia, intubation, delivery room management
Citation: Song S, Zhu Y, Li J, Wang Q, Gong H, Zhong X and Wu Y (2023) Individualized dynamic PEEP (dynPEEP) vs. positive pressure ventilation in delivery room management: A retrospective cohort study. Front. Pediatr. 10:1007632. doi: 10.3389/fped.2022.1007632
Received: 30 July 2022; Accepted: 12 December 2022;
Published: 11 January 2023.
Edited by:
Robin McKinney, Brown University, United StatesReviewed by:
David Tingay, Murdoch Childrens Research Institute, AustraliaPaul Waszak, Centre Hospitalier Saint-Denis, France
© 2023 Song, Zhu, Li, Wang, Gong, Zhong and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Wu YmlyZDgyMjdAMTYzLmNvbQ==
Specialty Section: This article was submitted to Neonatology, a section of the journal Frontiers in Pediatrics
 Sijie Song
Sijie Song Yefang Zhu1
Yefang Zhu1 Xiaoyun Zhong
Xiaoyun Zhong Yan Wu
Yan Wu