
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 09 January 2023
Sec. Pediatric Hematology and Hematological Malignancies
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.1006817
Background: Hemophagocytic lymphohistiocytosis (HLH) is a rapidly progressive and potentially life-threatening disorder. Identifying risk factors and timely adjustment of the given treatment regimens is critical to reducing the early mortality in HLH patients. Hypocholesterolemia has been reported to be associated with poor prognosis in a variety of critical illnesses. However, serum cholesterol is rarely studied in HLH patients, and its prognostic value is unclear.
Methods: We conducted a retrospective cohort study in National Clinical Research Center for Child Health and Disorders (Chongqing), identifying pediatric HLH patients (including genetically confirmed pHLH and not genetically confirmed pHLH) diagnosed with the HLH-2004 protocol and treated with immunochemotherapy between January 2008 and December 2020. The patients' blood lipid levels at initial diagnosis of HLH, including triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein-cholesterol (LDL-C), were reviewed based on electronic medical records. Both Cox and logistic regression models were used to estimate the effects of blood lipid indicators on early death (within 30 days after diagnosis).
Results: A total of 353 patients were enrolled in the study, with a median age at diagnosis of 45 months. The observed 30-day mortality rate was 19.05% (64/336, 17 were lost to follow-up) and Kaplan-Meier-estimated 3-year survival rate was 61.67% (95% CI, 56.27%–67.59%). DNA-targeted sequencing of HLH-related genes was performed in 173 (49.0%, 173/353) patients (not all patients with suspected pHLH underwent genetic testing), and 29 patients were diagnosed with genetically confirmed pHLH. Lipid panel was performed in 349 patients: 91.98% (321/349) had TG ≥ 1.80 mmol/L, 62.75%(219/349) had TG ≥ 3.00 mmol/L, 92.84% (324/349) had HDL-C ≤ 1.04 mmol/L, 58.74% (205/349) had LDL-C ≤ 1.30 mmol/L and 24.64% (86/349) had TC ≤ 3.11 mmol/L. TC ≤ 3.11 mmol/L and BUN ≥ 7.14 mmol/L were the independent risk factors for 30-day mortality [HR(95%CI): 2.85(1.46, 5.57) and 2.90(1.48, 5.68), respectively]. The presence of one of these risk factors increased the 30-day mortality rate by 6-fold [HR = 6.24, 95%CI: (3.18, 12.22)] and the presence of two risk factors by nearly 10-fold [HR = 9.98, 95%CI: (4.23, 23.56)] compared with the patients with no risk factors.
Conclusion: Severe derangement of lipoproteins is common in children with HLH, and decreased TC is an independent risk factor for early death. Hypocholesterolemia should be included as a biomarker during the diagnosis and management of HLH patients.
Hemophagocytic lymphohistiocytosis (HLH) is a rare and severe hyperinflammatory disease characterized by prolonged fever, pancytopenia, hepatosplenomegaly, hemophagocytosis, and excessive immune activation (1, 2). Traditionally, HLH has been classified into primary hemophagocytic lymphohistiocytosis (pHLH) and secondary hemophagocytic lymphohistiocytosis (sHLH) (3). pHLH occurs mainly in the presence of an underlying predisposing genetic defect in immune function, and sHLH may be triggered by infections (4, 5), malignancies (6), rheumatic diseases (7, 8), transplants (9), and metabolic diseases (10). HLH is primarily seen in children, accounting for approximately 60% of all cases (11). The incidence of suspected pHLH in the pediatric population varies widely by region, ranging from 1 to 225 per 300,000 live births (12).
HLH is a clinical syndrome with a poor prognosis, which results in uncontrolled activation of T-lymphocytes and macrophages and life-threatening cytokine storm (3). The long-term survival rate was less than 5% without appropriate treatment (13). In the last few decades, the International Society of Histocytes proposed diagnostic and therapeutic guidelines for HLH, which have greatly improved the understanding and treatment of HLH (2, 14). Survival of HLH patients has also improved considerably (estimated 5-year survival rates of 54%–61% according to HLH-94 and HLH-04 protocols), whereas early pre-transplant mortality remains the most frequent cause of treatment failure (15–17). In most patients with HLH, early imumnochemotherapy treatment has shown improved survival amongst affected patients (16–18). Therefore, identifying risk factors for early death is critical for assessing the progression and severity of HLH and intervening with timely measures to improve the survival of HLH patients. However, HLH is a rare clinical syndrome with a complex underlying etiology, lacking specificity in clinical presentation and laboratory tests, and the risk factors affecting early mortality in HLH have not been fully elucidated (19–21).
Cholesterol is involved in the progression of various critical illnesses by modulating inflammatory immune responses and neutralizing endotoxins in pathological situations (22–24), and hypocholesterolemia has been reported to be associated with poor prognosis in sepsis (25, 26) heart failure (27), ischemic stroke (28), and malignancy (29, 30). However, studies on serum cholesterol levels in HLH patients are scarce. One recent study reported the presence of acquired hypolipoproteinemia in 18 adults with sHLH (31), and another study found that decreased HDL-C was common in a cohort of 227 pediatric HLH patients (32). The association between serum cholesterol levels and the prognosis of HLH remains unclear. The aim of this study was to investigate the lipid levels in children with HLH (excluding patients with underlying etiology of rheumatic diseases and malignancies) and to evaluate the relationship between clinical indicators and early death. These findings may be incorporated into the assessment of clinical severity and risk management in patients with HLH in the future.
This was an observational retrospective cohort study conducted at the Children's Hospital of Chongqing Medical University. This institution is one of the two National Clinical Research Center for Child Health and Disorders in China, which has carried out a long-term clinical studies of HLH in pediatric patients (33–36). From January 2008 to December 2020, a total of 441 children with HLH were diagnosed and treated with immunochemotherapy using the HLH-2004 protocol (2). To minimize the complexity of etiology and the variability of treatment regimens, we excluded patients with clear evidence of rheumatologic diseases (n = 80) and malignancies (n = 8). Finally, a total of 353 cases were included in the analysis. The patient selection flowchart is shown in Figure 1. All included patients were aged ≤18 years, and were followed up by telephone or outpatient clinic, with the last follow-up on August 5, 2021. The study procedures were conducted in accordance with the declaration of Helsinki, and approved by the institutional ethical review board (File No. 2020, 299), with a waiver of the requirement for informed consent.
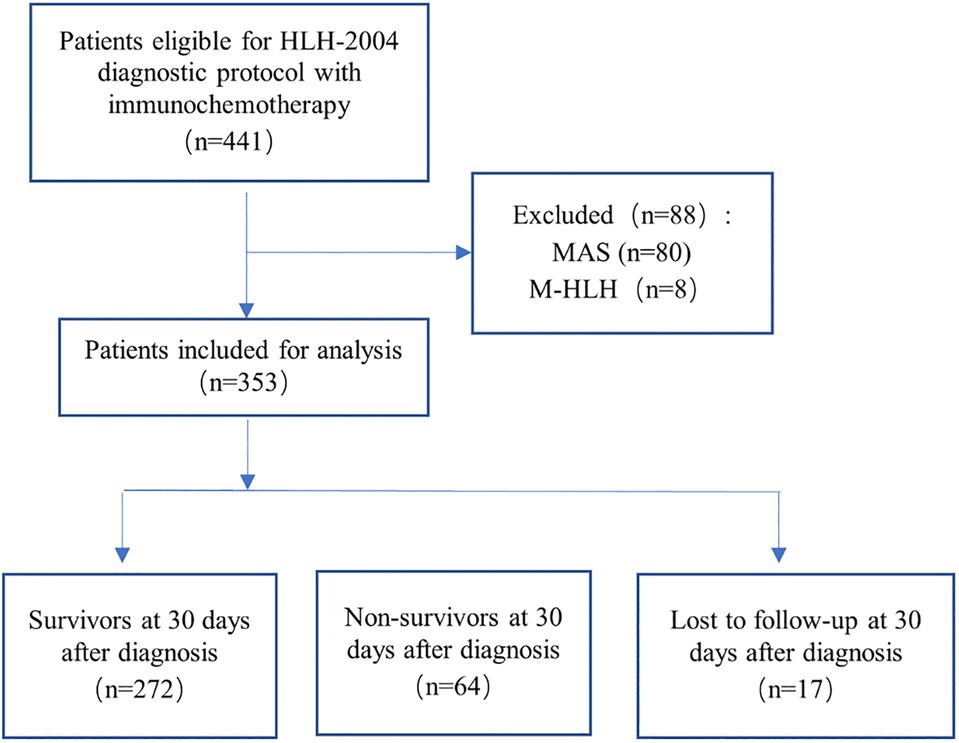
Figure 1. Flowchart of inclusion and exclusion. Abbreviations: MAS, macrophage activation syndrome; M-HLH, malignancy-associated HLH.
The patient characteristics studied included sex, age, primary diseases, laboratory results, treatment protocol, and outcomes. Identification of underlying etiology was based on the assessments by attending physicians or evidence from genetic testing. Approximately half of patients did not undergo HLH-related genetic testing due to the high cost of genetic testing. Lipid evaluation was performed when HLH was suspected, and patients were fasting for a minimum of six hours prior to evaluation. Categorizations of laboratory results were based on the institutional laboratory reference ranges. We defined early death as death within 30 days of diagnosis. Patients were divided into survival and non-survival groups according to their survival status within 30 days. Survival times were calculated from the date of diagnosis.
All statistical analyses were performed using R statistical language (version 4.1.0, https://www.r-project.org). Continuous variables were presented as median and interquartile range (IQR), and categorical variables were presented as frequencies and percentages (n, %). Continuous variables between survivors and non-survivors were compared using Mann–Whitney U test, and categorical variables were compared using Pearson's Chi-square test. Survival probability was estimated by Kaplan–Meier method, with the log-rank tests used to assess group differences. Univariable and multivariable analyses were performed according to Cox and logistic regression models. The ROC curve graph shows the performance of a classification model. To address the issue of missing data, numerous interpolations were performed using the Multivariate Imputation by Chained Equations (MICE) package (37). In particular, fifty imputed, complete datasets were generated by logistic regression or multinomial logit models, and the estimated results from each dataset were pooled together by Rubin's rules (38). The logistic and Cox regression models were also analyzed using only the complete cases. Pearson correlation analysis was applied to the lipid indicators. A p-value less than .05 (2-sided) was considered statistically significant.
The clinical and laboratory test characteristics of patients at diagnosis are summarized in Table 1, with 189 males and 164 females and with a median age of 45 months (range: 1–213 months).
DNA-targeted sequencing of the HLH related genes was performed in 173 (49.0%, 173/353) patients. The HLH related genes including PRF1, UNC13D, STX11, STXBP2, AP3B1, LYST, RAB27A, SH2D1A, XIAP, ITK, MAGT1 and CD27. Among the 173 patients, 29 were with genetically confirmed pHLH, including 15 family HLH (FHL) cases (3 FHL-2, 11 FHL-3, 1 FHL-4), 14 other pHLH (4 Chediak-Higashi syndrome, 3 X-Linked Lymphoproliferative syndrome-1, 3 X-Linked Lymphoproliferative syndrome-2, 2 combined immunodeficiency, 1 X-linked chronic granulomatous disease, and 1 X-linked immunodeficiency with magnesium defect, Epstein–Barr virus infection, and neoplasia syndrome); no HLH-causing genes or related gene mutations were found in the remaining 144 patients.
Three hundred thirty-four patients (94.62%, 334/353) had a reported infection at diagnosis, and two or more pathogenic infections may have been present in the same patient: viruses are the most common pathogens, including Epstein-Barr virus (EBV) (90.2%, 314/348), cytomegalovirus (CMV) (6.9%, 24/348), with 19 patients including EBV/CMV co-infection, and 34 patients were not found to be infected with either virus. Of the 314 EBV-infected patients, 13 were genetically confirmed pHLH based genetic testing.
The median TG, TC, HDL-C, and LDL-C were 3.61 (2.53–5.10) mmol/L, 4.65 (3.16–6.09) mmol/L, 0.38 (0.21–0.62) mmol/L, and 1.13 (0.53–1.74) mmol/L, respectively. The distributions and correlations of blood lipid indicators were shown in Figure 2. The distributions of lipid indicators in different subgroups were further investigated. There were no statistically significant differences of the four lipid indicators were identified between the EBV-infected and non-EBV-infected groups (Figure 3). The patients with genetically confirmed pHLH (n = 28) had higher TG than those without genetically confirmed pHLH (n = 144) (5.00 mmol/L vs. 5.18 mmol/L, p = 0.023), while no statistically significant differences were found between the two groups for other lipid marker (Figure 4).
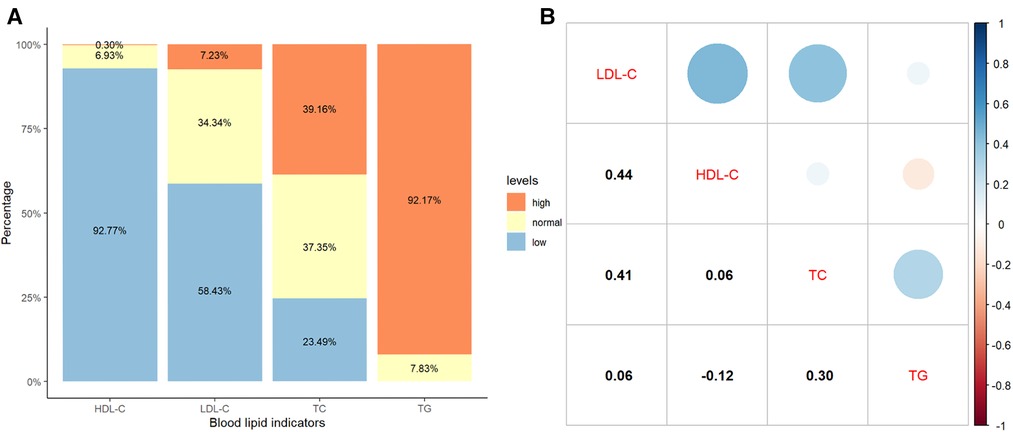
Figure 2. Distribution and correlation of blood lipid indicators in 349 patients. (A) Percentage bar-chart of blood lipid indicators; (B) Pearson correlations between blood lipid indicators. Note: Normal thresholds for TG, TC, HDL-C and LDL-C are defined as <1.80 mmol/L, 3.11–5.18 mmol/L, 1.04–2.27 mmol/L and 1.30–3.40 mmol/L, respectively, according to the standard reference ranges used in the clinical laboratory of the Children's Hospital of Chongqing Medical University.
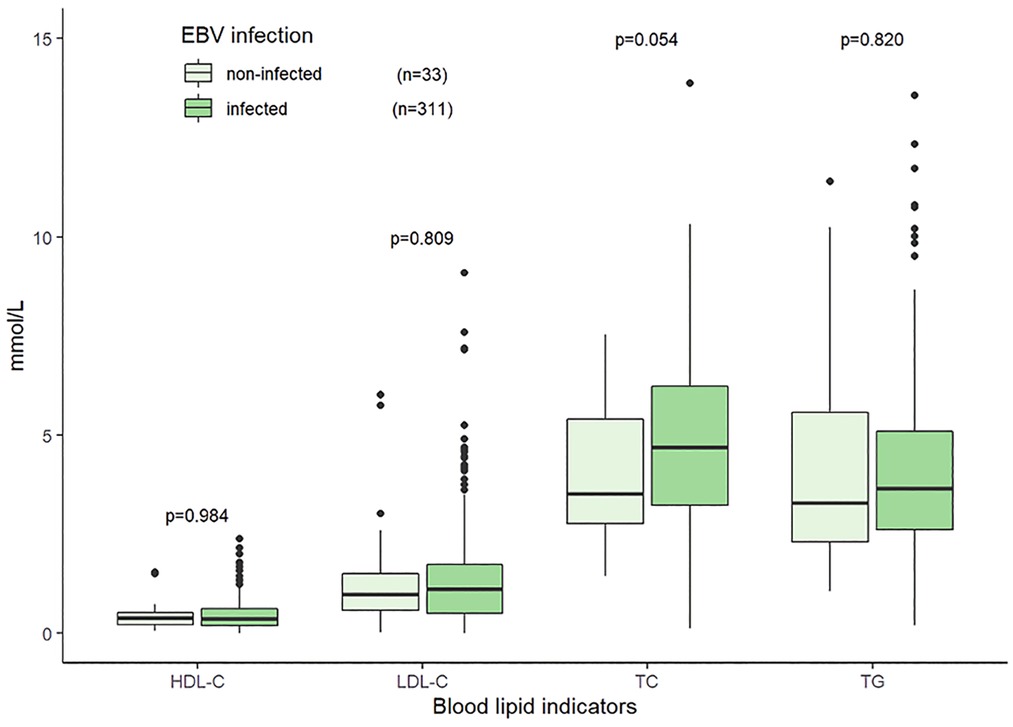
Figure 3. Distributions of blood lipid indicators in the patients with EBV infection and without EBV infection. Note: 344 patients tested for both EBV and lipid indicators.
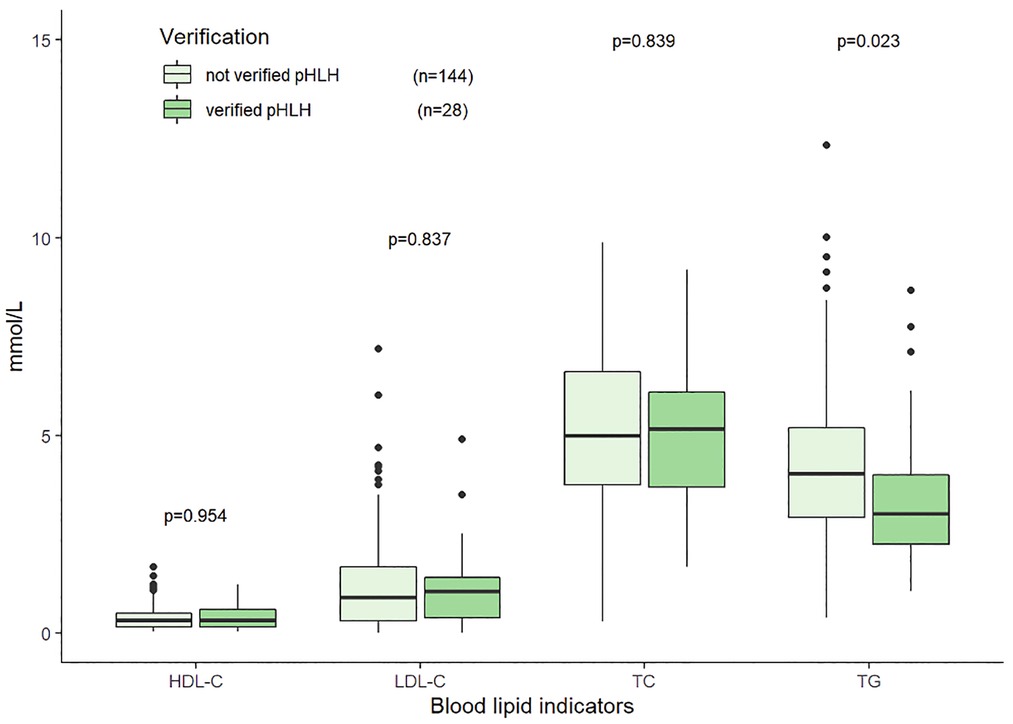
Figure 4. Distributions of blood lipid indicators in the patients with genetically verified pHLH and without verified pHLH. Note: 172 patients tested for both HLH related gene testing and lipid indicators.
The median follow-up time for 353 pediatric patients was 23.9 months (95% CI: 19.4–28.5), with follow-up ending on August 5, 2021; 97 patients were lost to follow-up, 17 of whom were lost within 30 days of diagnosis, none of whom were in a critical condition at discharge. A total of 124 patients died, and 132 patients survived at the last follow-up. By Kaplan-Meier analysis, the estimated survival rates at 1, 2, and 3 years were 64.09% (95% CI: 58.97%–69.66%), 62.42% (95% CI: 57.14%–68.20%), and 61.67% (95% CI: 56.27%–67.59%), respectively (Figure 5).
Sixty-four patients died within 30 days, accounting for more than half of the deaths. The observed 30-day mortality rate was 19.05% (64/336, 17 were lost to follow-up). Compared with the survivors (n = 272) at 30-day, the non-survivors at (n = 64) had lower TC (2.64 mmol/L vs. 3.72 mmol/L, p < 0.001) and HDL-C (0.31 mmol/L vs. 0.38 mmol/L, p = 0.043), while the differences in TG and LDL-C between the two groups were not statistically significant (p = 0.935, p = 0.156, respectively) (Figure 6). Detailed comparison of clinical and laboratory results between the two groups was presented in Table 2.
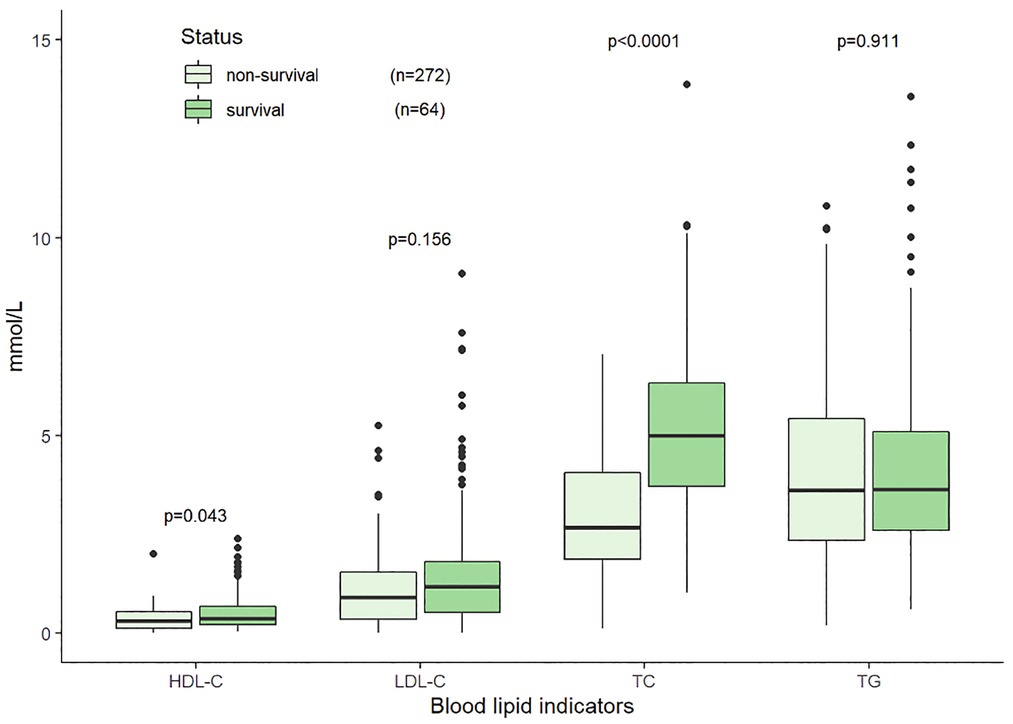
Figure 6. Distributions of blood lipid indicators at initial diagnosis in the non-survival and survival groups within 30 days in 336 HLH patients.
Table 3 shows the results of univariable and multivariable Cox regression analyses of early mortality in children with HLH. The multivariable analysis showed that BUN ≥7.14 mmol/L [HR = 2.90, 95% CI: (1.48–5.68), p = 0.003] and TC ≤ 3.11 mmol/L [HR = 2.85, 95% CI: (1.46–5.57), p = 0.003] were significantly associated with the survival within 30 days after diagnosis. To validate the robustness of results, a multivariable logistic regression was also performed and identified the same risk factors (Table 4). In addition, similar results were obtained from the Cox and logistic regression models using only complete cases (Figures 7, 8).
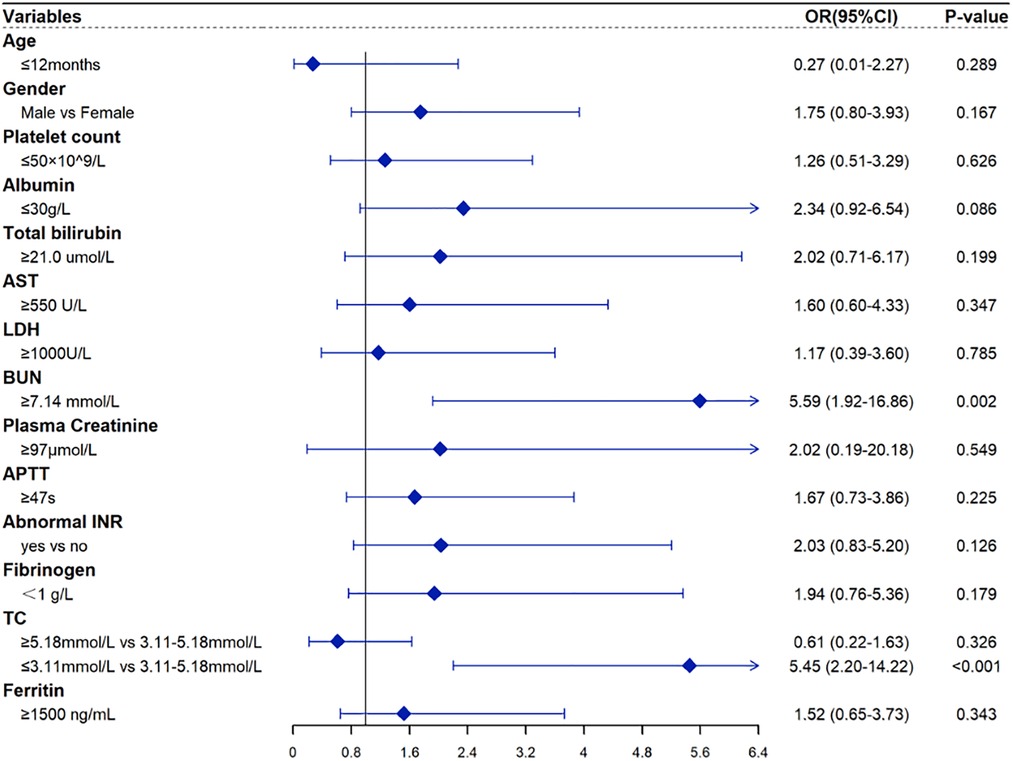
Figure 7. Forest plots for the multivariate logistic regression analysis with only complete HLH cases.
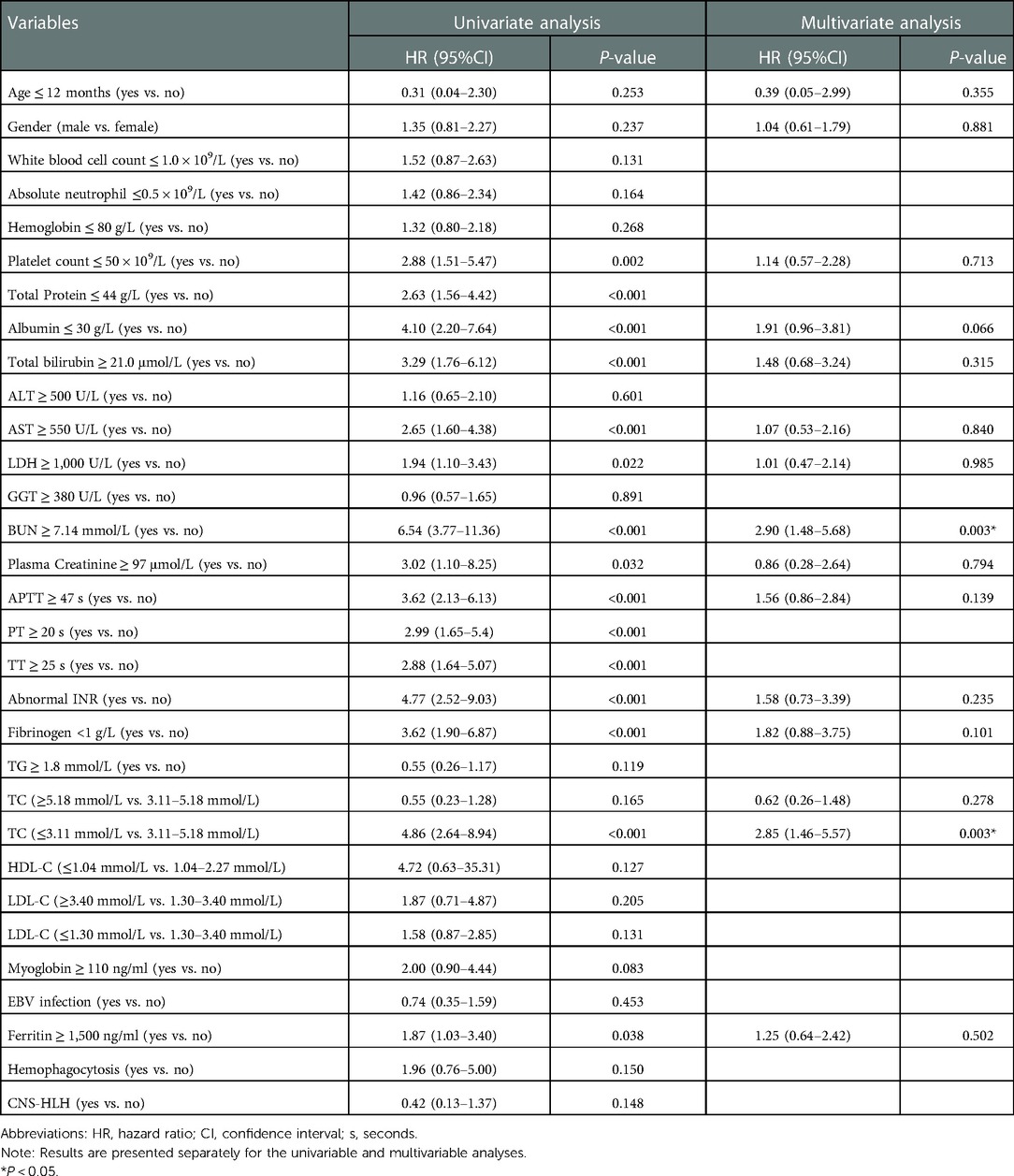
Table 3. Risk factors for 30-day mortality by univariable and multivariable Cox regression analyses in 353 HLH patients.
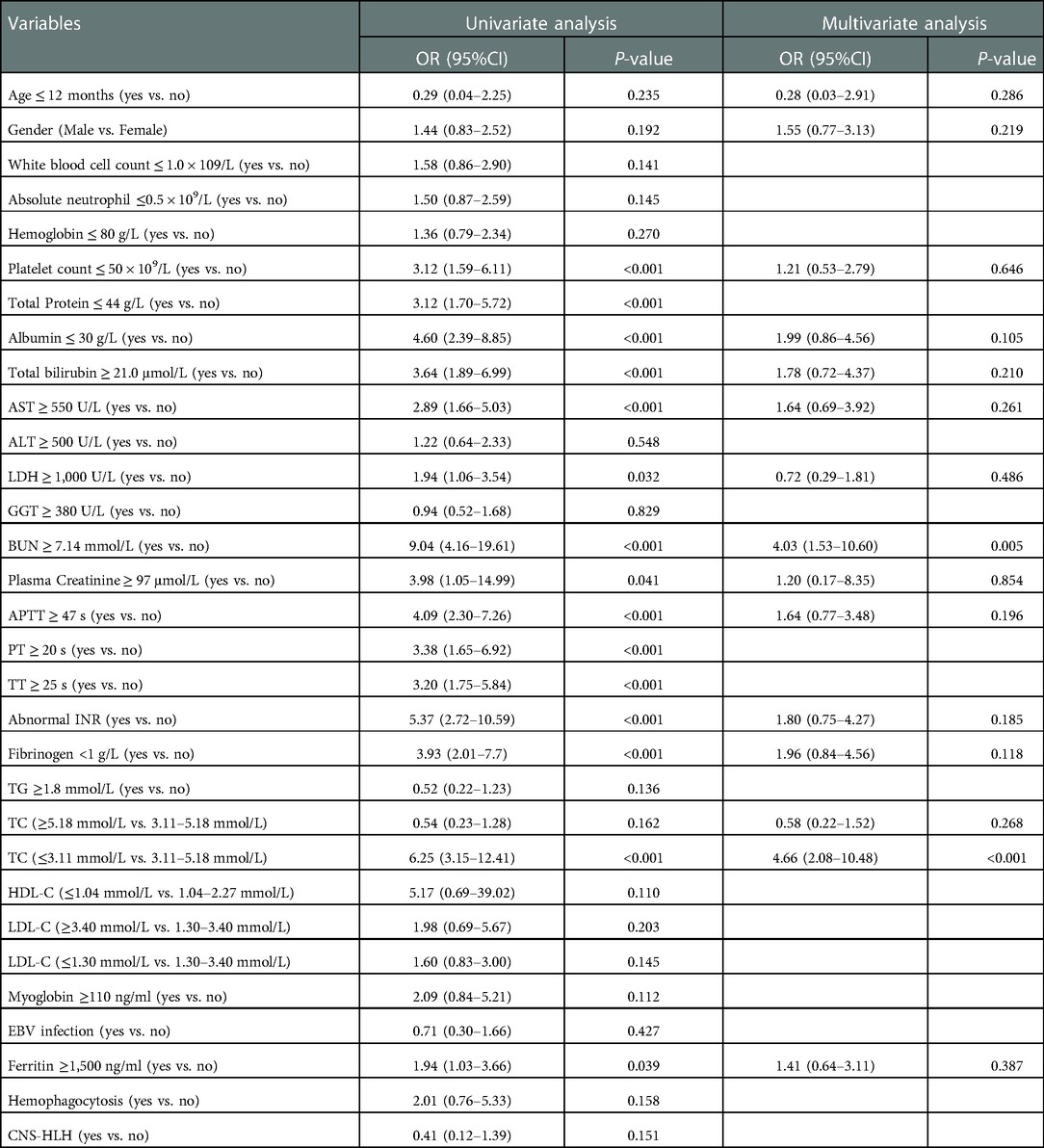
Table 4. Risk factors for 30-day survival by univariate and multivariate logistic regression analysis in 336 HLH patients.
From ROC curve analysis, the AUCs (area under the curve) of TC and BUN were 75.2% (95%CI: 68.5%–81.8%) and 63.0% (95%CI: 57.1%–69.0%), respectively (Figures 9A,B). Compared with the normal TC group (3.11 mmol/L–5.18 mmol/L), the 30-day survival rate in the low TC group (≤3.11 mmol/L) was significantly lower (52.29% vs. 88.22%, p < 0.0001) (Figure 9C), while in the high TC group (≥5.18 mmol/L), there was no significant difference (93.33% vs. 88.22%, p = 0.157) (Figure 9C). Compared with the normal BUN group (<7.14 mmol/L), the 30-day survival rate in the high BUN group (≥7.14 mmol/L) was significantly lower (40.18% vs. 86.13%, p < 0.0001) (Figure 9D). Then, we considered the two risk factors simultaneously and classified the patients into three risk groups: low-risk (no risk factors present), intermediate-risk (one risk factor was present), and high-risk (two risk factors were present,), with the 30-day survival rate of 90.61% (95% CI: 85.48%–96.05%), 52.69% (95% CI: 42.76%–64.91%)% and 39.71% (95% CI: 21.77%–72.40%), respectively (Figure 10). Compared with the low-risk group, the patients had a nearly 10-fold increased risk of early death [HR = 9.98, 95%CI: (4.23, 23.56)] in the high-risk group and a 6-fold increased risk of early death [HR = 6.27, 95%CI: (3.18, 12.22)] in the intermediate-risk group.
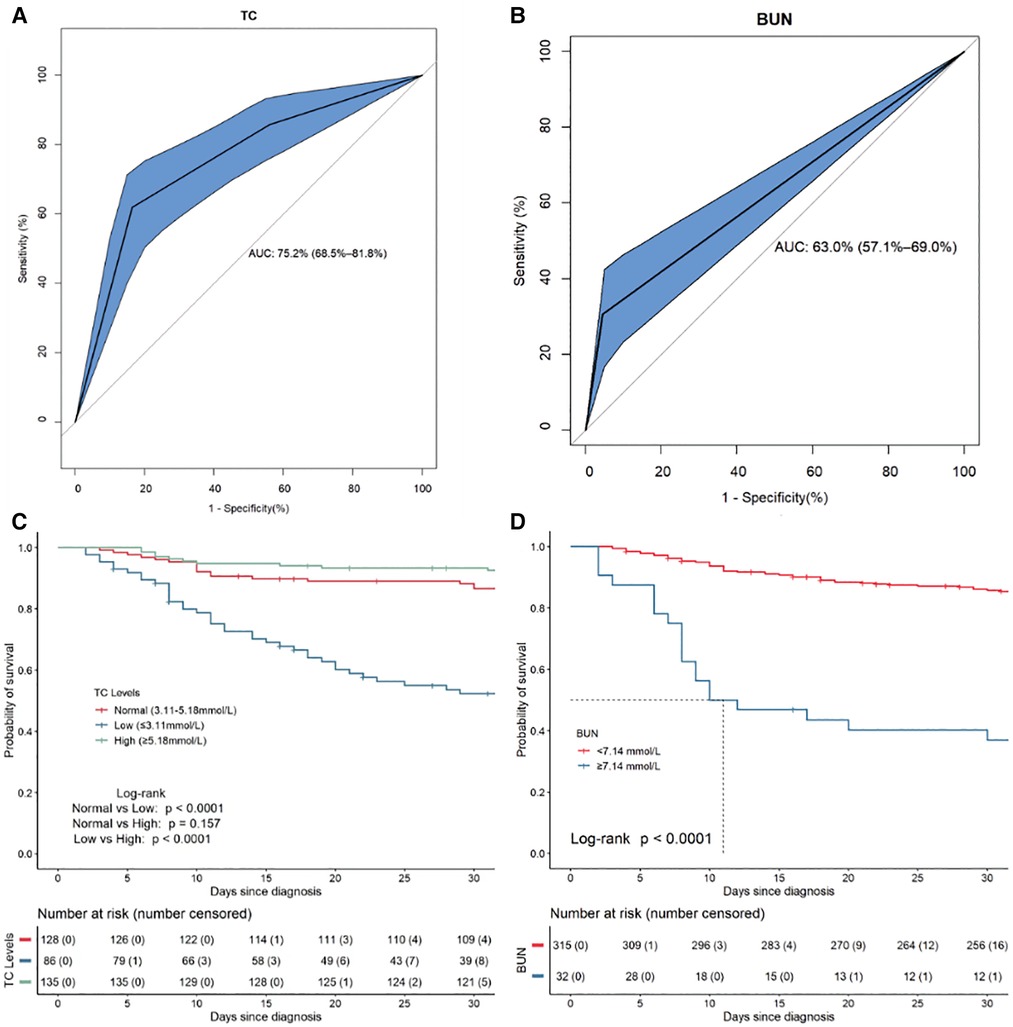
Figure 9. The ROC curves and Kaplan–Meier survival curves for the independent risk factors for 30-day mortality in 353 HLH patients. (A) The ROC plots for TC. (B) The ROC plots for BUN. (C) The Kaplan–Meier survival curves for subgroups of patients according to TC. (D) The Kaplan–Meier survival curves for subgroups of patients according to BUN.
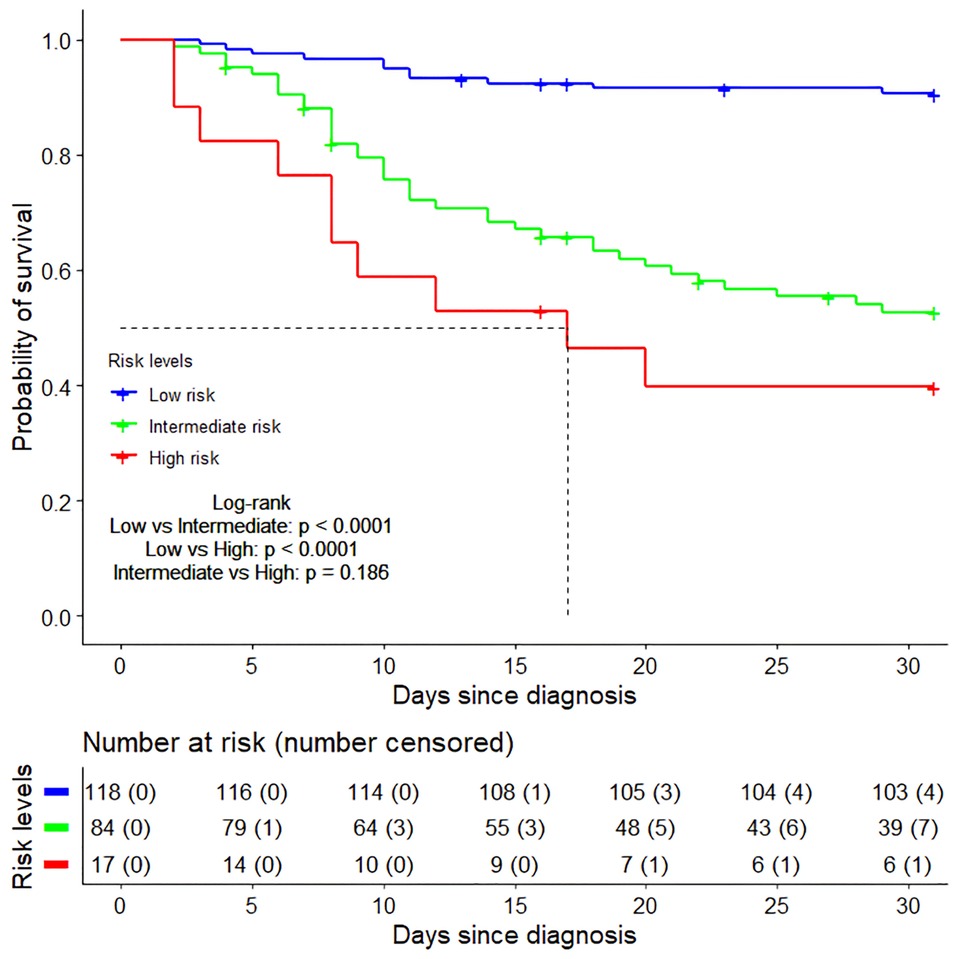
Figure 10. The Kaplan–Meier survival curves of three risk groups within 30 days after diagnosis. Low risk group (no risk factors present), Intermediate risk group (one risk factor was present), High risk group (two risk factors were present).
In addition, we also used the receiver operating characteristic (ROC) analysis to determine the optimal prognostic cut-off value for TC and BUN, and the cut-off values were 2.82 mmol/L and 6.80 mmol/L. The area under the ROC curve for TC and BUN were 79.4% (72.9%–85.8%) and 68.0% (60.1%–75.8%), respectively. Compared with the high TC group (>2.82 mmol/L), the 30-day survival rate in the low TC group (≤2.82 mmol/L) was significantly lower (45.45% vs. 90.46%, p < 0.0001). Compared with the low BUN group (≦6.80 mmol/L), the 30-day survival rate in the high BUN group (>6.80 mmol/L) was significantly lower (44.74% vs. 86.73%, p < 0.0001). The ROC curves and Kaplan–Meier survival curves for the independent risk factors by cut-off value for 30-day mortality are shown in Supplementary Figure S1.
This study evaluated the lipid levels at the initial diagnosis in pediatric HLH patients and analyzed the risk factors for 30-day mortality. To our knowledge, this is the largest retrospective cohort study of pediatric HLH patients reported to date. The findings in this study revealed that almost all patients exhibited different types of dyslipidemia at the time of initial diagnosis and low TC was associated with early death. In the comparative analysis of survivors and non-survivors, patients who died within 30 days of diagnosis had significantly lower TC. After adjusting for other potential prognostic factors, low TC remained an independent risk factor for early mortality in HLH.
It is well known that increased TG is prevalent in patients with HLH and is used as one of the eight diagnostic criteria by the International Society of Histiocytes (2, 15). However, lipoprotein cholesterol levels have been rarely studied in HLH. We found only two recent studies that mentioned cholesterol levels of HLH patients at the time of diagnosis: one based on a cohort of 18 adults with sHLH, reporting that 100% of the patients had HDL-C less than 30 mg/dl (0.78 mmol/L), 74% had LDL-C < 100 mg/dl (2.59 mmol/L), 47% had LDL-C < 50 mg/dl (1.26 mmol/L), and 33% had undetectable LDL-C levels (31); similar results were obtained in our study, 84. 2% of the patients had HDL-C less than 30 mg/dl (0.78 mmol/L), 87.1% had LDL-C < 100 mg/dl (2.59 mmol/L), 86.0% had LDL-C < 50 mg/dl (1.26 mmol/L), 9.6% had LDL-C < 3.87 mg/dl (0.1 mmol/L); the other study reported a cohort of 227 pediatric HLH patients in which a low HDL-C level <1.04 mmol/L was common (99.1%) (32), and in our study the outcome was similar in patients with HDL-C levels <1.04 mmol/L (92.8%). In our study, all 349 pediatric HLH patients who underwent lipid testing exhibited different types of dyslipidemia at initial diagnosis. In particular, 92.8% (324/349) had decreased HDL-C, which is consistent with the finding in Zhou et al. (32), and a decrease in LDL-C was observed in 58.7% of the 349 patients. In the present study, Pearson correlation analysis showed that TG was weakly correlated with HDL-C (r = −0.12, p = 0.028) and LDL-C (r = 0.06, p = 0.232), suggesting the potential added value of HDL-C and LDL-C in the diagnosis of HLH. Further studies can be designed to identify optimal thresholds for HDL-C and LDL-C as diagnostic criteria for HLH.
There are various possible interpretations for the aberrant lipid levels in HLH patients. First, patients may suffer from liver dysfunction, resulting in impaired lipid biosynthesis. Our data showed that serum levels of AST, LDH, and TB were statistically significantly elevated in the non-survival group compared with those in the survival group. The liver is the main organ for the synthesis, conversion and excretion of blood lipids, and damage to hepatocytes may cause dyslipidemia by affecting the process of lipid metabolism. Second, inflammatory cytokine storms may also affect lipid metabolism. HLH is a clinical syndrome caused by a dysregulated hyperinflammatory and cytokine storm, usually accompanied by markedly elevated levels of cytokines such as IFN-γ, IL-10, IL-6, and soluble IL-2 in the early stages of the disease (39). The elevated cytokine concentrations that occur during inflammation may lead to the rapid decrease in cholesterol concentrations (40, 41). Previous studies have also revealed reduced lipoprotein synthesis in hepatocyte cell lines exposed to TNF-α and IL-6 (42); decrease in HDL-C may be also associated with high levels of secreted phospholipase A2 (43) or with the downregulation of phospholipid and cholesterol influx into HDL mediated by ATP-binding cassette transporter-1 in inflammation (44).
Low cholesterol has been shown to be associated with the severity of disease progression and poor prognosis in various critical illnesses such as sepsis, heart failure, ischemic stroke, and malignancy (25–29, 45–50). A recent study found that patients with severe COVID-19 also had dyslipidemia, characterized by lower HDL-C and TC levels related to disease progression (51). HLH shares overlapping symptoms with sepsis, such as excessive inflammatory response and multi-organ failure. However, studies on the relationship between cholesterol and disease severity and the prognosis in HLH patients are limited. In the current study, we found for the first time that TC is a risk factor for early death in HLH. Patients with low TC (≤3.11 mmol/L) had a nearly 2.8-fold increased risk of early death compared to patients with normal TC (3.11–5.18 mmol/L) (p = 0.003). Previous studies have shown that cholesterol and lipoprotein levels were negatively correlated with concentrations of interleukin-6, soluble interleukin-2 receptor and interleukin-10, and reduced cholesterol levels may impair the ability to modulate inflammatory immune responses and neutralize endotoxins in pathological situations (22, 23). Cholesterol has also been shown to play an essential role in reducing cytokine responses and decreasing mortality in animal models of sepsis (52). Cholesterol levels are correlated with disease severity, and changes in cholesterol may also be useful for monitoring treatment response in HLH. The present study is a retrospective study that failed to monitor cholesterol levels in patients who obtained a treatment response and could be further validated and evaluated in the future by designing appropriate prospective studies.
In addition to TC, both the Cox and logistic regression analysis showed that BUN ≥ 7.14 mmol/L was an independent risk factor associated with early death in HLH. Elevated BUN may be associated with infiltration of activated macrophages or cytotoxic T cells into the renal parenchyma, ischemic renal lesions, or abnormal coagulation (51, 52). Of the 353 pediatric patients included in this study, 32 had elevated BUN, and 19 of them died within 30 days of diagnosis. Three of the four patients with elevated BUN combined with elevated plasma creatinine died within 30 days of diagnosis, considering the possibility of concomitant acute kidney injury in patients with elevated BUN. Previous studies have also shown that acute kidney injury is also a common clinical manifestation and prognostic risk factor for HLH (53, 54). Elevated BUN implies that patients with HLH may have more severe circulatory perfusion deficit, infection, or acute kidney injury, thereby increasing the risk of death (53–56). In this study, a stratified survival analysis based on the number of risk factors showed that patients in the high-risk group had an almost 10-fold increased risk of early death compared to those in the low-risk group, while those in the intermediate-risk group had a 6-fold increased risk of early death. By attending on lipid levels and renal function in the early stages of diagnosis, clinicians may be able to better predict the risk of patients with HLH, identify high-risk children early and take more aggressive treatment measures to reducing early mortality in children with HLH.
In the multivariable analysis, in addition to lipids and BUN, we also considered the clinical indicators that have been previously reported to have prognostic value for early death in HLH. Trottestam et al. found hyperbilirubinemia, methemoglobinemia, and cerebrospinal fluid pleocytosis associated with early death in 232 children with HLH (20); Bin Q et al. found that neutrophils <0.5 × 109/L, total bilirubin over the two-fold upper limit of standard value, and albumin ≤20 g/L at diagnosis were independent risk factors for 30-day mortality in 116 pediatric HLH patients (57); Shaar RA et al. found that changes in ferritin levels are the most significant prognostic factor of 30-day mortality in 123 adult HLH patients (58). In a study on 162 adults with HLH, older age, underlying lymphoma, lower platelet count, elevated aspartate aminotransferase and lactate dehydrogenase, and absence of etoposide were found to be associated with poorer prognosis (19). In this study, hyperbilirubinemia, methemoglobinemia, hypoalbuminemia, low platelet count, and elevated aspartate aminotransferase and lactate dehydrogenase were statistically significant from univariable analysis but not multivariable analysis. The inconsistency of these findings may be due to the differences of included covariates and the variations in study population in terms of age structure and underlying etiology, among others.
There were several limitations in this study. First, being a single-center study may limit the generalizability and representativity of the findings. A multicenter study with more participants is warranted to establish and validate the risk stratification model in future studies. Second, there were missing data in the clinical variables included in this retrospective study. To address this issue, we considered two approaches, namely multiple imputation, and complete-case analysis, and obtained robust results in identifying the risk factors associated with early death. We were also unable to complete 30-day follow-up after diagnosis in 17 patients (4.8%), which led to censoring bias. We used a Cox model to account for censoring bias. Third, many patients were not tested for various cytokines such as IFN-γ, IL-10, IL-6, and soluble CD25, and cholesterol levels were not dynamically monitored, therefore we were not able to explore the relationship between hypocholesterolemia and these known markers of HLH disease activity, as well as possible underlying mechanisms. Fourth, although we collected fairly rich data on demographic characteristics, primary disease, laboratory results, and central nervous system involvement to adjust for confounding effects, other uncollected potential confounders (e.g., treatment response, cytokine levels) may have biased our results.
Dyslipidemia is common in children with HLH, and decreased TC is a risk factor associated with early mortality. Monitoring biochemical indicators, such as lipid levels and renal function, at the early stage of diagnosis may allow for better risk stratification and management of patients with HLH.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by The ethics committee of the Children's Hospital of Chongqing Medical University. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin. Written informed consent was obtained from the minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
JY contributed to the design of the study. XMX helped with the study design and data analyses. ZLZ conducted the data analysis, YD, XMG and YXG performed the clinical aspects of the study. LX conducted the data analysis and wrote the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the 2022 Research Projects of Chongqing Municipal Health and Family Planning Commission (2022WSJK005) and Chongqing Innovation Program for Returned Overseas Chinese Scholars (ex2021112).
The authors are grateful to all colleagues in the Department of Hematology and Oncology of Children's Hospital of Chongqing Medical University for their excellent assistance. Two earlier versions of this manuscript were deposited as preprints at Research Square (57, 58).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.1006817/full#supplementary-material.
1. Janka G, Lehmberg K. Hemophagocytic syndromes — an update. Blood Rev. (2014) 28:135–42. doi: 10.1016/j.blre.2014.03.002
2. Henter J-I, Horne A, Aricò M, Egeler RM, Filipovich AH, Imashuku S, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. (2007) 48:124–31. doi: 10.1002/pbc.21039
3. Janka G. Familial and acquired hemophagocytic lymphohistiocytosis. Annu Rev Med. (2012) 63:233–46. doi: 10.1146/annurev-med-041610-134208
4. Rouphael N, Talati NJ, Vaughan CP, Cunningham K, Moreira RK, Gould CV. Infections associated with haemophagocytic syndrome. Lancet Infect Dis. (2007) 7:814–22. doi: 10.1016/s1473-3099(07)70290-6
5. Kayaaslan BU, Asilturk D, Eser F, Korkmaz M, Kucuksahin O, Pamukcuoglu M, et al. A case of hemophagocytic lymphohistiocytosis induced by COVID-19, and review of all cases reported in the literature. J Infect Dev Ctries. (2021) 15:1607–14. doi: 10.3855/jidc.14829
6. Daver N, McClain KL, Allen CE, Parikh SA, Otrock ZK, Rojas-Hernandez CM, et al. A consensus review on malignancy-associated hemophagocytic lymphohistiocytosis in adults. Cancer. (2017) 123:3229–40. doi: 10.1002/cncr.30826
7. Arlet J-B, Arlet JB, Le Thi Huong D, Marinho A, Amoura Z, Wechsler B, et al. Reactive haemophagocytic syndrome in adult-onset still’s disease: a report of six patients and a review of the literature. Ann Rheum Dis. (2006) 65:1596–601. doi: 10.1136/ard.2005.046904
8. Fukaya S, Yasuda S, Hashimoto T, Oku K, Kataoka H, Horita T, et al. Clinical features of haemophagocytic syndrome in patients with systemic autoimmune diseases: analysis of 30 cases. Rheumatology. (2008) 47:1686–91. doi: 10.1093/rheumatology/ken342
9. Karras A, Thervet E, Legendre C. Hemophagocytic syndrome in renal transplant recipients: report of 17 cases and review of literature. Transplantation. (2004) 77:238–43. doi: 10.1097/01.tp.0000107285.86939.37
10. Saneifard H, Shamsian B, Shakiba M, Karizi Zarea S, Sheikhy A. A rare case of glycogen storage disease type 1a presenting with hemophagocytic lymphohistiocytosis (HLH). Case Rep Pediatr. (2020) 2020:1–4. doi: 10.1155/2020/8818617
11. Ramos-Casals M, Brito-Zerón P, López-Guillermo A, Khamashta MA, Bosch X. Adult haemophagocytic syndrome. Lancet. (2014) 383:1503–16. doi: 10.1016/s0140-6736(13)61048-x
12. Ponnatt TS, Lilley CM, Mirza KM. Hemophagocytic lymphohistiocytosis. Arch Pathol Lab Med. (2022) 146:507–19. doi: 10.5858/arpa.2020-0802-RA
13. Janka G. Familial hemophagocytic lymphohistiocytosis. Eur J Pediatr. (1983) 140:221–30. doi: 10.1007/bf00443367
14. Henter J-I, Aricò M, Egeler RM, Elinder G, Favara BE, Filipovich AH, et al. HLH-94: a treatment protocol for hemophagocytic lymphohistiocytosis. Med Pediatr Oncol. (1997) 28:342–7. doi: 10.1002/(sici)1096-911x(199705)28:5%3C342::aid-mpo3%3E3.0.co;2-h
15. Henter J-I, Samuelsson-Horne A, Aricò M, Egeler RM, Elinder G, Filipovich AH, et al. Treatment of hemophagocytic lymphohistiocytosis with HLH-94 immunochemotherapy and bone marrow transplantation. Blood. (2002) 100:2367–73. doi: 10.1182/blood-2002-01-0172
16. Trottestam H, Horne A, Aricò M, Egeler RM, Filipovich AH, Gadner H, et al. Chemoimmunotherapy for hemophagocytic lymphohistiocytosis: long-term results of the HLH-94 treatment protocol. Blood. (2011) 118:4577–84. doi: 10.1182/blood-2011-06-356261
17. Bergsten E, Horne A, Aricò M, Astigarraga I, Egeler RM, Filipovich AH, et al. Confirmed efficacy of etoposide and dexamethasone in HLH treatment: long-term results of the cooperative HLH-2004 study. Blood. (2017) 130:2728–38. doi: 10.1182/blood-2017-06-788349
18. Machowicz R, Basak G. How can an internal medicine specialist save a patient with hemophagocytic lymphohistiocytosis (HLH)? Pol Arch Intern Med. (2020) 130(5):431–37. doi: 10.20452/pamw.15226
19. Arca M, Fardet L, Galicier L, Rivière S, Marzac C, Aumont C, et al. Prognostic factors of early death in a cohort of 162 adult haemophagocytic syndrome: impact of triggering disease and early treatment with etoposide. Br J Haematol. (2015) 168:63–8. doi: 10.1111/bjh.13102
20. Trottestam H, Berglöf E, Horne A, Onelöv E, Beutel K, Lehmberg K, et al. Risk factors for early death in children with haemophagocytic lymphohistiocytosis. Acta Paediatr. (2012) 101:313–8. doi: 10.1111/j.1651-2227.2011.02501.x
21. Yoon J-H, Park S-S, Jeon Y-W, Lee S-E, Cho BS, Eom K-S, et al. Treatment outcomes and prognostic factors in adult patients with secondary hemophagocytic lymphohistiocytosis not associated with malignancy. Haematologica. (2019) 104:269–76. doi: 10.3324/haematol.2018.198655
22. Rauchhaus M, Coats AJS, Anker SD, Anker SD. The endotoxin-lipoprotein hypothesis. Lancet. (2000) 356:930–3. doi: 10.1016/s0140-6736(00)02690-8
23. Gordon BR, Parker TS, Levine DM, Saal SD, Wang J, Sloan B-J, et al. Relationship of hypolipidemia to cytokine concentrations and outcomes in critically ill surgical patients. Crit Care Med. (2001) 29:1563–8. doi: 10.1097/00003246-200108000-00011
24. Goldfarb RD, Parker TS, Levine DM, Glock D, Akhter I, Alkhudari A, et al. Protein-free phospholipid emulsion treatment improved cardiopulmonary function and survival in porcine sepsis. Am J Physiol Regul Integr Comp Physiol. (2003) 284(2):R550–7. doi: 10.1152/ajpregu.00285.2002
25. Memiş D, Gursoy O, Tasdogan M, Sut N, Kurt I, Türe M, et al. High C-reactive protein and low cholesterol levels are prognostic markers of survival in severe sepsis. J Clin Anesth. (2007) 19:186–91. doi: 10.1016/j.jclinane.2006.10.008
26. Yamano S, Shimizu K, Ogura H, Hirose T, Hamasaki T, Shimazu T, et al. Low total cholesterol and high total bilirubin are associated with prognosis in patients with prolonged sepsis. J Crit Care. (2016) 31:36–40. doi: 10.1016/j.jcrc.2015.09.033
27. Horwich TB, Hamilton MA, MacLellan WR, Fonarow GC. Low serum total cholesterol is associated with marked increase in mortality in advanced heart failure. J Card Fail. (2002) 8:216–24. doi: 10.1054/jcaf.2002.0804216
28. Zhao W, An Z, Hong Y, Zhou G, Guo J, Zhang Y, et al. Low total cholesterol level is the independent predictor of poor outcomes in patients with acute ischemic stroke: a hospital-based prospective study. BMC Neurol. (2016) 16:36. doi: 10.1186/s12883-016-0561-z
29. Kitahara CM, de Gonzalez AB, Freedman ND, Huxley RR, Mok Y, Jee SH, et al. Total cholesterol and cancer risk in a large prospective study in Korea. J Clin Oncol. (2011) 29:1592–8. doi: 10.1200/jco.2010.31.5200
30. Öztürk E. The relationship between hematological malignancy and lipid profile. Medeni Med J. (2021) 36:146–51. doi: 10.5222/mmj.2021.91145
31. Reap L, Ma VT, Takiar R, Forman A. Acquired hypolipoproteinemia and hemophagocytic lymphohistiocytosis: a previously unexplored association? J Clin Oncol. (2021) 39:15. doi: 10.1200/jco.2021.39.15_suppl.e19041
32. Zhou Y-H, Han X-R, Xia F-Q, Poonit N-D, Liu L. Clinical features and prognostic factors of early outcome in pediatric hemophagocytic lymphohistiocytosis: a retrospective analysis of 227 cases. J Pediatr Hematol Oncol. (2021) 44(1):e217–e222. doi: 10.1097/mph.0000000000002283
33. Xiao L, Xian Y, Bt D, Dai B, Su Y, Xiao J, et al. Clinical features and outcome analysis of 83 childhood epstein-barr virus-associated hemophagocytic lymphohistiocytosis with HLH-2004 protocol. Chin J Hematol. (2011) 32:668–72.
34. Xiao L, Guan X, Meng Y, Su Y, Xian Y, Xiao J, et al. Analysis of clinical and laboratory features of 217 pediatric hemophagocytic lymphohistiocytosis. Chin J Hematol. (2014) 35:628–32. doi: 10.3760/cma.j.issn.0253-2727.2014.07.013
35. An YF, Luo XB, Yang X, Wang J, Li L, Zhao XD. Clinical and molecular characteristics of Chinese patients with X-linked lymphoproliferative syndrome type 1. Pediatr Blood Cancer. (2014) 61:2043–7. doi: 10.1002/pbc.25126
36. Wen FY, Xiao L, Xian Y, Wen XH, Guan XM, Liao ML, et al. Prognosis of the central nervous system involvement in patients with hemophagocytic lymphohistiocytosis. Chin J Hematol. (2017) 38:848–52. doi: 10.3760/cma.j.issn.0253-2727.2017.10.005
37. van Buuren S, Groothuis-Oudshoorn K. Multivariate imputation by chained equations r package mice version 3 11 0. (2020).
38. Sugden RA. Multiple imputation for nonresponse in surveys. J R Stat Soc Ser A Stat Soc. (1988) 151:567. doi: 10.2307/2983027
39. Klop B, Elte J, Cabezas M. Dyslipidemia in obesity: mechanisms and potential targets. Nutrients. (2013) 5:1218–40. doi: 10.3390/nu5041218
40. Akgün S, Ertel NH, Mosenthal A, Oser W. Postsurgical reduction of serum lipoproteins: interleukin-6 and the acute-phase response. J Lab Clin Med. (1998) 131:103–8. doi: 10.1016/S0022-2143(98)90083-X
41. Fraunberger P, Pilz G, Cremer P, Werdan K, Walli AK. Association of serum tumor necrosis factor levels with decrease of cholesterol during septic shock. Shock. (1998) 10:359–63. doi: 10.1097/00024382-199811000-00009
42. Ettinger WH, Varma VK, Sorci-Thomas MG, Parks JS, Sigmon RC, Smith TK, et al. Cytokines decrease apolipoprotein accumulation in medium from hep G2 cells. Arterioscler, Thromb, Vasc Biol. (1994) 14:8–13. doi: 10.1161/01.atv.14.1.8
43. Tietge UJF, Maugeais C, Cain W, Grass DS, Glick JM, de Beer FC, et al. Overexpression of secretory phospholipase A2 causes rapid catabolism and altered tissue uptake of high density lipoprotein cholesteryl ester and apolipoprotein A-I. J Biol Chem. (2000) 275:10077–84. doi: 10.1074/jbc.275.14.10077
44. Panousis CG, Zuckerman SH. Interferon-γ induces downregulation of tangier disease gene (ATP-binding-cassette transporter 1) in macrophage-derived foam cells. Arterioscler, Thromb, Vasc Biol. (2000) 20:1565–71. doi: 10.1161/01.atv.20.6.1565
45. Lekkou A, Mouzaki A, Siagris D, Ravani I, Gogos C. Serum lipid profile, cytokine production, and clinical outcome in patients with severe sepsis. J Crit Care. (2014) 29:723–7. doi: 10.1016/j.jcrc.2014.04.018
46. Windler E, Windler EET, Ewers-Grabowl U, Thiery J, Walli AK, Seidel D, et al. The prognostic value of hypocholesterolemia in hospitalized patients. J Mol Med. (1994) 72:939–43. doi: 10.1007/bf00577732
47. Vyroubal P, Vyroubal P, Chiarla C, Giovannini I, Hyspler R, Ticha A, et al. Hypocholesterolemia in clinically serious conditions–review. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. (2008) 152:181–9. doi: 10.5507/bp.2008.029
48. Huang GS, Dunham CM, Chance EA, Hileman BM, DelloStritto DJ. Body mass index interaction effects with hyperglycemia and hypocholesterolemia modify blunt traumatic brain injury outcomes: a retrospective study. Int J Burns Trauma. (2020) 10:314–23.33500843
49. Nguyen VK, Colacino JA, Chung MK, Le Goallec A, Jolliet O, Patel CJ. Characterising the relationships between physiological indicators and all-cause mortality (NHANES): a population-based cohort study. Lancet Healthy Longev. (2021) 2(10):e651–e662. doi: 10.1016/s2666-7568(21)00212-9
50. Chmielewski M, Verduijn M, Drechsler C, Lindholm B, Stenvinkel P, Rutkowski B, et al. Low cholesterol in dialysis patients–causal factor for mortality or an effect of confounding? Nephrol Dial Transplant. (2011) 26:3325–31. doi: 10.1093/ndt/gfr008
51. Yue J, Xu H, Zhou Y, Liu W, Han X, Mao Q, et al. Dyslipidemia is related to mortality in critical patients with coronavirus disease 2019: a retrospective study. Front Endocrinol. (2021) 12:611526. doi: 10.3389/fendo.2021.611526
52. Netea MG, Demacker PNM, Kullberg BJ, Boerman OC, Boerman OC, Verschueren I, et al. Low-density lipoprotein receptor-deficient mice are protected against lethal endotoxemia and severe gram-negative infections. J Clin Invest. (1996) 97:1366–72. doi: 10.1172/jci118556
53. Malaga-Dieguez L, Ming W, Trachtman H. Direct reversible kidney injury in familial hemophagocytic lymphohistiocytosis type 3. J Am Soc Nephrol. (2015) 26:1777–80. doi: 10.1681/asn.2014111090
54. Thaunat O, Delahousse M, Fakhouri F, Martinez F, Stephan J-L, Noël L-H, et al. Nephrotic syndrome associated with hemophagocytic syndrome. Kidney Int. (2006) 69(10):1892–8. doi: 10.1038/sj.ki.5000352
55. Aulagnon F, Lapidus N, Canet E, Galicier L, Boutboul D, Peraldi M-N, et al. Acute kidney injury in adults with hemophagocytic lymphohistiocytosis. Am J Kidney Dis. (2015) 65:851–9. doi: 10.1053/j.ajkd.2014.10.012
56. Wang S, Zhou J, Yang J, Wang X, Chen X, Ji L, et al. Clinical features and prognostic factors of acute kidney injury caused by adult secondary hemophagocytic lymphohistiocytosis. J Nephrol. (2022) 35(4):1223–33. doi: 10.1007/s40620-021-01147-2
57. Bin Q, Gao J-H, Luo J-M. Prognostic factors of early outcome in pediatric hemophagocytic lymphohistiocytosis: an analysis of 116 cases. Ann Hematol. (2016) 95:1411–8. doi: 10.1007/s00277-016-2727-6
Keywords: hemophagocytic lymphohistiocytosis, total cholesterol, early mortality, pediatric, risk factor, lipids
Citation: Xiao L, Xu X, Zhang Z, Dou Y, Guan X, Guo Y and Yu J (2023) Low total cholesterol predicts early death in children with hemophagocytic lymphohistiocytosis. Front. Pediatr. 10:1006817. doi: 10.3389/fped.2022.1006817
Received: 29 July 2022; Accepted: 9 December 2022;
Published: 9 January 2023.
Edited by:
Seth Corey, Cleveland Clinic, United StatesReviewed by:
Zhigang LI, Capital Medical University, China© 2023 Xiao, Xu, Zhang, Dou, Guan, Guo and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Yu eXVqaWVAaG9zcGl0YWwuY3FtdS5lZHUuY24=
Specialty Section: This article was submitted to Pediatric Hematology and Hematological Malignancies, a section of the journal Frontiers in Pediatrics
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.