
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 05 October 2022
Sec. Neonatology
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.1004750
 Yuanqiang Yu1,2
Yuanqiang Yu1,2 Qingyi Dong3
Qingyi Dong3 Suping Li4
Suping Li4 Huaxue Qi5
Huaxue Qi5 Xin Tan6
Xin Tan6 Hong Ouyang7
Hong Ouyang7 Jintao Hu3
Jintao Hu3 Wen Li3
Wen Li3 Tao Wang3
Tao Wang3 Yonghui Yang3
Yonghui Yang3 Xiaoyun Gong3
Xiaoyun Gong3 Xiaori He3*
Xiaori He3* Pingyang Chen1,2*
Pingyang Chen1,2*
Objective: General hospitals admit lower gestational age neonates than maternal and child health care centers, therefore associated with a higher morbidity and mortality. This study aimed to assess the etiology and clinical characteristics of neonatal sepsis in different medical setting models.
Methods: Neonates admitted to 5 tertiary medical centers, including one national general hospital, two maternal and child health care hospitals and two regional general hospitals, in central-south China with culture-proven sepsis between January 2010 and December 2019 were included in the study. We compared maternal and neonatal characteristics, pathogen distribution, treatment and neonatal outcomes among 3 different medical setting models in this retrospective cohort.
Results: We identified 757 episodes of culture-proven sepsis in 757 neonates. The predominant pathogens were coagulase-negative staphylococci, Klebsiella pneumoniae, Escherichia coli and Group B streptococci. A total of 683 neonates with detailed information were involved in further comparison; 54.6% were from the national general hospital, 35.9% were from the maternal and child health care hospital, and 9.5% were from the regional general hospital. Neonates in national and regional general hospitals had significantly lower gestational age and birthweight (P < 0.001). Patterns of pathogen distribution were different among these medical setting models. Early-onset sepsis was more common in maternal and child health care hospitals (61.4% vs. 42.1% vs. 46.7%, P < 0.001), while hospital-acquired late-onset sepsis was more common in national and regional general hospitals (32.7% vs. 33.3% vs. 11.4%, P < 0.001). The proportion of complications or comorbidities of neonates in maternal and child health care hospitals were significantly lower than neonates in national and regional general hospitals (P < 0.001). The case fatality rate was significantly higher in regional general hospitals (10.8% vs. 3.2% vs. 0.8%, P = 0.001).
Conclusion: We report distinct patterns of clinical characteristics, pathogens and outcomes in patient subgroups with neonatal sepsis from national general hospital, maternal and child health care hospital and regional general hospital. It might have some implications for improvement of prevention, management and empirical antibiotic use in neonatal sepsis in different setting models, especially in resource-limited settings from middle and low-income countries.
Sepsis remains a common and frequently fatal condition among neonates globally (1). In Asia, the incidence was higher than in resource-rich countries (2). The pattern of pathogen distribution in Asian settings was similar to that in industrialized countries, yet the important difference was the burden of non-fermenting Gram-negative bacteria, and high level of antibiotic resistance (3). The increasing number of multidrug-resistant Gram-negative micro-organisms is calling for thorough and efficient surveillance strategies and appropriate treatment regimens (4). Neonates in middle and low-income countries is being focused on with novel evidence-based treatment options that are available worldwide (5).
Maternal infection or colonization, total parenteral nutrition, and peripherally inserted central catheter placement were most relevant risk factors of neonatal sepsis (6, 7). Several guidelines had provided a framework for the development of evidence-based approaches to sepsis management (8–10). The application of such strategies like early-onset sepsis (EOS) risk calculator and quality improvement program in resource-limited settings has shown effectiveness in reducing neonatal infection and unnecessary antibiotic exposure (11, 12). However, surveillance of infections with population-level measures in the health facilities and community is still insufficient in middle and low-income countries (13). In China, most settings are still short of high-quality medical staffs, life-sustaining medical devices and sepsis management measures such as intrapartum antibiotic prophylaxis (IAP), application of EOS risk calculator or care bundles and multidisciplinary antimicrobial stewardship in neonatal units.
General hospitals are responsible for treatment of diseases and admit patients with severe conditions, while maternal and child health care hospitals are charged with basic labor services in China. We hypothesized that neonatal sepsis in resource-limited medical setting models including national general hospital, maternal and child health care hospital and regional general hospital represented distinct entities characterized by different pathogen distribution and clinical characteristics. Pathogen surveillance strategies and empirical antibiotic choices, as well as infection, prevention and control measures, may be different in these setting models. This study may provide novel perspective for better improvement in neonatal sepsis management in different medical setting models from middle and low-income countries.
This multi-center retrospective study included 5 tertiary medical centers with neonatal units from central-south China. Among these hospitals, one was the national general hospital (Second Xiangya Hospital, Central South University, 50 beds), two were maternal and child health care hospitals (Hunan Provincial Maternal and Child Health Care Hospital, 150 beds; Changsha Hospital for Maternal/Child Health Care, 70 beds) and two were regional general hospitals (The First Hospital of Changsha, 20 beds; Xiangtan Central Hospital, 50 beds). Four hospitals were from Changsha and one was from Xiangtan. All of them were perinatal centers with neonatal intensive care, providing labor service and neonatal health care for most population in central-south China.
Between January 1, 2010 and December 31, 2019, neonates admitted to these 5 neonatal units and diagnosed culture-proven sepsis were involved in this study. They were followed until death or cured from sepsis and discharge. Neonates were eligible if they developed culture-proven sepsis before 28 days of life (when gestational age ≥37 weeks) or 44 weeks corrected age (when gestational age <37 weeks). An episode of sepsis was defined as one or more positive culture gained from blood, cerebrospinal fluid (CSF) or other sterile sites when clinically indicated. A blood culture was often collected if patients had clinical signs and sepsis was suspected. The date of sepsis onset was defined by the date of first blood culture collection. The samples were taken onsite at each hospital, and a microbiologist was consulted to assist with interpretation of the culture results. Clinical Laboratory Standards Institute (CLSI) guidelines were followed in antimicrobial susceptibility testing (14). Repeatedly positive samples were considered to represent the same episode of infection unless they occurred more than 21 days after the last positive culture result.
Cases were excluded based on the following criteria: (1) cultures considered as contaminants with clinical indication [e.g., coagulase-negative staphylococci (CoNS) in the absence of clinical signs], and antimicrobial therapy was discontinued in less than 5 days; (2) neonates discharged before cured or transferred to other hospitals.
The study was approved by the Ethics Committee of the Second Xiangya Hospital of Central South University and recognized by all participating centers. Data on demographics, maternal and neonatal characteristics, pathogen distribution, antimicrobial resistance (AMR) of predominant isolates, treatment and neonatal outcomes were recorded retrospectively from medical information systems. Data were collected using a standardized questionnaire completed by clinicians for each episode of sepsis.
We defined early-onset sepsis (EOS) by onset in the first 72 h of life and late-onset sepsis (LOS) by onset more than 3 days. According to the time of sepsis onset, LOS was divided into hospital-acquired (onset during hospitalization and >48 h after admission) and community-acquired (onset ≤48 h after admission from home).
Premature rupture of membranes was defined as rupture of membranes >24 h prior to delivery. Neonatal bacterial meningitis was defined as positive CSF culture, Gram staining, or neutrophilic leukocytosis, with or without low sugar (less than 50% of plasma glucose level) and high protein content in CSF samples. Neonatal necrotizing enterocolitis (NEC) was defined as ≥ stage 2 according to Bell criteria (15). Intraventricular hemorrhage (IVH) was defined as ≥ grade 3 according to Papile's criteria (16). Bronchopulmonary dysplasia (BPD) was defined as mechanical ventilation or oxygen dependency at 36 weeks of postnatal age or discharge (17). Retinopathy of prematurity (ROP) was defined as ≥ stage 3 according to the International Classification of ROP (18).
We calculated the frequency for categorical variables, and differences in categorical variables were compared using Pearson χ2 test or Fisher's exact test, where appropriate. For continuous variables with normally distributed data, we calculated the mean ± standard deviation. Continuous variables were compared using one-way ANOVA test. The homogeneity test of variance was performed. We used Welch's ANOVA test for continuous variables with unequal variance. For continuous variables with data not normally distributed, we calculated the median and interquartile range. Accordingly, continuous variables were compared using the Kruskal-Wallis test. A 2-sided of P value of <0.05 was considered statistically significant. Statistical analysis was performed using SPSS 26.0 (SPSS Inc, Chicago, IL, USA).
During the 10-year study period, 757 neonates with 757 episodes of culture-proven sepsis met eligibility criteria and were involved in the study. A total of 788 isolates were identified in 5 settings. Therein, 469 isolates (59.5%) were identified in the national general hospital, 250 (31.7%) in maternal and child health care hospitals, and 69 (8.8%) in regional general hospitals. Nearly all isolates were identified from blood culture samples. 16 isolates were identified from CSF samples (including 3 isolates also identified from the blood culture samples) and 5 from other sterile site samples in the national general hospital; 1 isolate was identified both from the blood culture and CSF samples in maternal and child health care hospitals; and 4 isolates were identified from CSF samples (including 1 isolate also identified from the blood culture sample) in regional general hospitals. Multiple organisms were isolated from the same episode in 3.8% (n = 29) of neonates. The distribution of pathogens was listed in Table 1. Sepsis were mainly caused by Gram-positive bacteria (n = 457, 58.0%). CoNS (n = 318, 40.4%), K. pneumoniae (n = 124, 15.7%), E. coli (n = 114, 14.5%) and Group B streptococci (GBS) (n = 57, 7.2%) were the leading pathogens in all episodes. The most common pathogen isolated was CoNS in EOS episodes (n = 115, 35.8%) and in LOS episodes (n = 203, 43.5%). E. coli (n = 57, 17.8%) and GBS (n = 38, 11.8%) were also common in EOS. K. pneumoniae and E. coli were responsible for 20.8% (n = 97) and 12.2% (n = 57) of episodes in LOS.
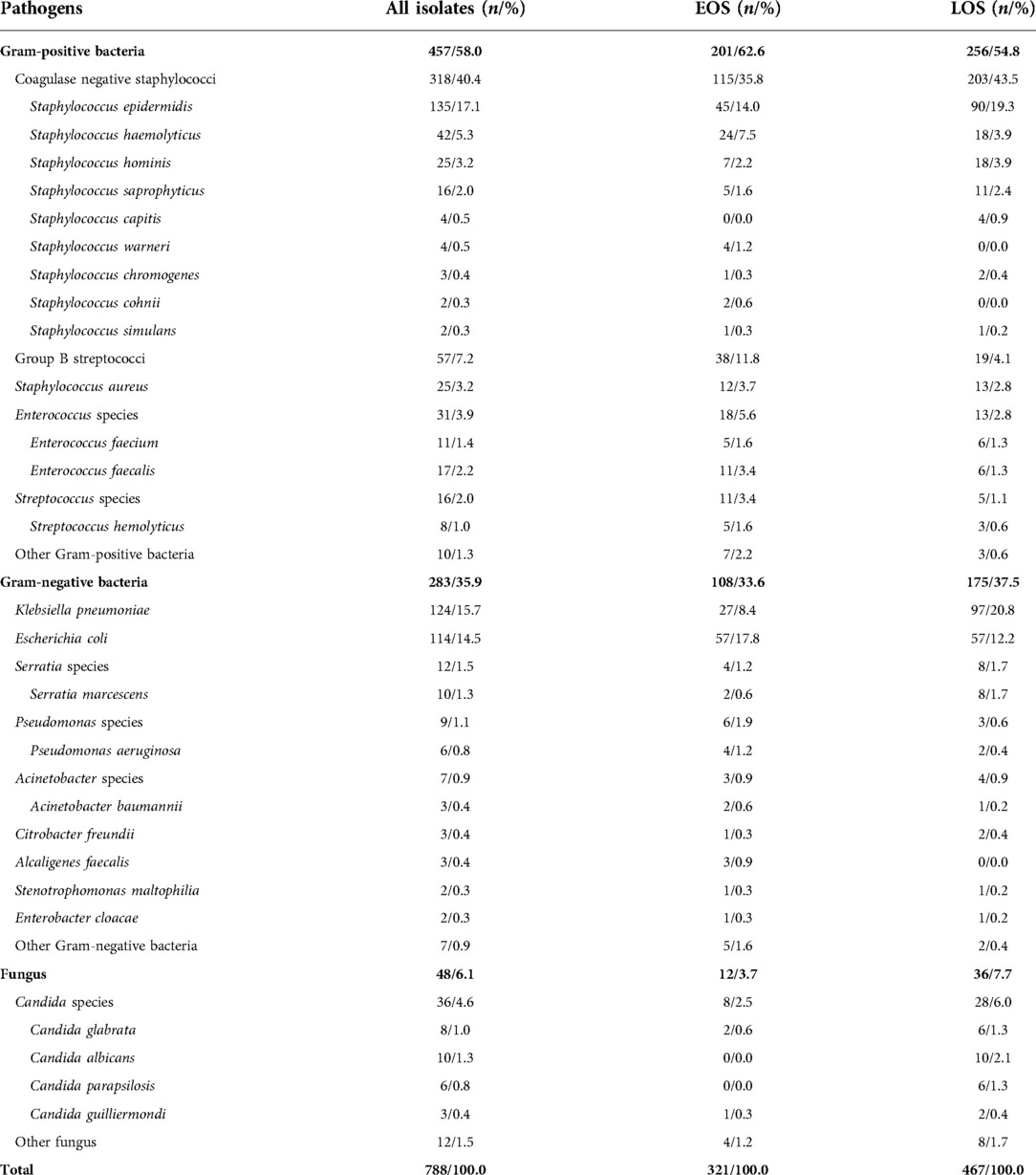
Table 1. Pathogen distribution of neonatal sepsis in 5 settings from central-south China, 2010–2019.
Tables 2, 3 showed the AMR of predominant pathogens in neonates. Most CoNS isolates were tested resistant to penicillins with or without β-lactamase inhibitors. Nearly all GBS isolates tested were sensitive to penicillin, ampicillin, and vancomycin. K. pneumoniae exhibited high resistance to penicillins and cephalosporins. The resistance of K. pneumoniae to meropenem was 23.4% and to imipenem was 28.4%. The resistance of E. coli to piperacillin/tazobactam was 2.9%, to ceftazidime 14.6% and to meropenem 1.2%. High resistance to ceftriaxone was found in K. pneumoniae (78.9%) and E. coli (51.4%).

Table 2. Antimicrobial resistance in predominant isolates of Gram-positive bacterial neonatal sepsis.
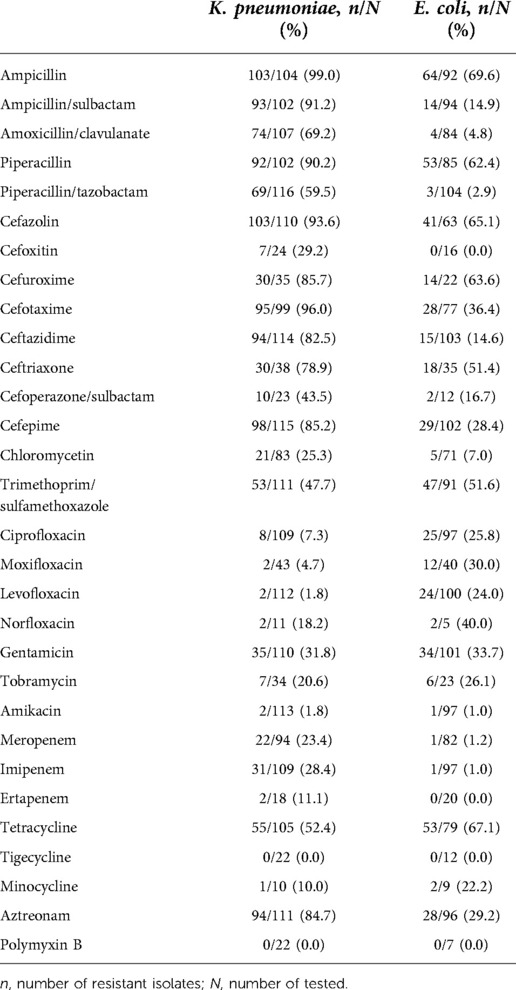
Table 3. Antimicrobial resistance in predominant isolates of Gram-negative bacterial neonatal sepsis.
In the 5 perinatal centers, a total of 683 neonates with detailed information of clinical characteristics were involved in further comparison. 384 (56.2%) of the neonates were boys. The mean gestational age was 36.5 ± 4.0 weeks, and the mean birthweight was 2704 ± 901 g. The median postnatal age at sepsis onset was 5 (0–16) days. The average hospital stay was 16 (13–27) days. Twenty-one (3.1%) patients died from sepsis before discharged, wherein 9 died from E. coli infection, 5 from CoNS infection and 5 from K. pneumoniae infection.
Of the 683 neonates with culture-proven sepsis, 373 (54.6%) were from the national general hospital, 245 (35.9%) were from the maternal and child health care hospital, and 65 (9.5%) were from the regional general hospital. Neonates in national and regional general hospitals had significantly lower gestational age and birthweight (P < 0.001). Patients transferred from other hospitals accounted for 18.2% and 12.3% in national and regional general hospitals, respectively. 41.2% of the patients in maternal and child health care hospitals were from community. Among the episodes of sepsis, early-onset sepsis was more common in maternal and child health care hospitals (61.4% vs. 42.1% vs. 46.7%, P < 0.001), while hospital-acquired late-onset sepsis was more common in national and regional general hospitals (32.7% vs. 33.3% vs. 11.4%, P < 0.001). Patterns of pathogen distribution were different in these medical setting models (Table 4). The distribution of GBS was significantly higher in maternal and child health care hospitals, while the distribution of K. pneumoniae and Candida spp were significantly higher in the national general hospital (P < 0.001). The case fatality rate was significantly higher in regional general hospitals (10.8% vs. 3.2% vs. 0.8%, P = 0.001).
The maternal characteristics of the neonates with culture-proven sepsis are shown in Table 5. Mothers in maternal and child health care hospitals had significantly higher proportion of chorioamnionitis (P = 0.002), higher proportion of premature rupture of membrane (PROM) (P = 0.013), and lower proportion of using intrapartum antibiotics (P < 0.001) than in national and regional general hospitals.
The proportion of complications or comorbidities of neonates in maternal and child health care hospitals were significantly lower than neonates in national and regional general hospitals (P < 0.001) (Table 6). Neonates in regional general hospitals showed significantly higher rates of pneumonia (P < 0.001), meningitis (P < 0.001), disseminated intravascular coagulation (DIC) (P = 0.045), and multiple organ failure (MOF) (P < 0.001).
Penicillins with β-lactamase inhibitors (61.0%), third-generation cephalosporins (33.9%) and carbapenems (31.8%) were widely used for directed therapy of neonates with culture-proven sepsis. Neonates in maternal and child health care hospitals had relatively higher proportion of using penicillins (P < 0.001) and lower proportion of using carbapenems (P = 0.002) than in national and regional general hospitals. Neonates in maternal and child health care hospitals had shorter duration of antibiotic treatment (P < 0.001). Neonates with suspected sepsis had higher proportion of adequate empiric antibiotic treatment in the national general hospital (P = 0.001).
Patients in national and regional general hospitals had significantly higher proportion of additional medical support, including blood transfusion, intravenous immune globulin (IVIG) and respiratory support than in maternal and child health care hospitals (P < 0.001) (Table 7).
This multi-center retrospective study described the pathogen distribution and AMR of predominant isolates in neonatal sepsis from central-couth China between January 1, 2010 and December 31, 2019. We also identified key differences among national general hospital, maternal and child health care hospital and regional general hospital in demographic characteristics, pathogens, maternal and neonatal characteristics, complications and comorbidities, and treatment. These findings provided novel perspective in management of neonatal sepsis in middle and low-income countries.
CoNS, GBS, K. pneumoniae and E. coli remained to be the predominant pathogens in neonatal sepsis in China (3, 7, 19). The pathogen distribution in EOS and LOS had a similar pattern with in developed countries, as well as in developing countries (20–22). High inter-regional heterogeneity was found in prevalence of these leading isolates, especially GBS (7). Our results showed a remarkable prevalence of CoNS both in EOS and LOS, which was different in pathogen distribution in south China (23). Furthermore, the AMR pattern amongst CoNS and K. pneumoniae isolates in our study has changed significantly when compared to previous data in Chinese neonates (24). AMR in K. pneumoniae and E. coli is growing and has become a serious threat to public health in China (25, 26). However, the presence of multidrug resistant micro-organisms was less valued and reported in neonatal units in China, including isolates of extended-spectrum β-lactamase producing or AmpC β-lactamase producing, and carbapenemase resistant Enterobacterales. The multidrug resistant nature of the predominant Gram negative bacteria will be explored in further studies. We also showed a high rate of antibiotic use in penicillins + β-lactamase inhibitors, third-generation cephalosporins and carbapenems as directed therapy. Empirical treatment choices in Chinese hospitals mostly followed the national guidelines, and were fewer reviewed and updated periodically. The pathogen distribution and antibiotic use showed significant difference in 3 medical setting models in our study. We raise the importance of ongoing surveillance of AMR infection (especially multidrug resistant micro-organisms) and individual empirical antibiotic treatment in different setting models. The detection of multidrug resistance forms is also important, and has implication in treatment and infection control that broader spectrum antimicrobial agents will be used.
National general hospitals represent the highest quality of medical services in different fields, including perinatology. Multiple disciplinary teamwork is becoming a prevalent model for clinical decision making in neonatal units in national general hospitals from China. Therefore, neonates with sepsis could be well managed and followed until discharge. In our study, we showed a relative better prognosis of neonatal sepsis in national general hospital than in regional general hospital. However, as a referral center, the gestational age and birthweight were relatively lower and hospital-acquired LOS was more common than in maternal and child health care hospital. The prevalence of K. pneumoniae and Candida spp were significantly higher in national general hospital, compared to maternal and child health care hospital and regional general hospital. Underlying conditions like catheter insertion and parenteral nutrition were more frequently seen in national general hospitals, thus increasing the incidence of neonatal LOS (27–29). Quality improvement initiatives using care bundles have proven to decrease infection rates in neonatal intensive care units (12, 30, 31). Other initiatives including implementation of the sepsis risk calculator showed good predictive value in EOS and were associated with the reduction in antibiotic use (11, 32, 33). Recently, efforts had been made in reducing unnecessary antibiotic use, and the relationship with neonatal outcomes and AMR was explored in developed countries (34–37). It is necessary to better prevent neonatal infection or sepsis and reduce AMR in national general hospital to improve neonatal outcomes.
In maternal and child health care hospitals, maternal and newborn care are much more paid attention to. Besides, the communication between the doctors and the parents is fluent and often. Any abnormality expressed from the neonates could be found timely and well treated. In our study, it was shown that neonates with sepsis in maternal and child health care hospital had better outcome than neonates from general hospital. However, it was noted that most cases in neonatal sepsis in maternal and child health care hospital were EOS, and GBS accounted 16.7% in pathogen distribution, significantly higher than in national and regional general hospital. GBS emerges to be the leading pathogen of maternal disease and neonatal sepsis in Chinese population since universal maternal GBS screening (38–40). It is known that maternal GBS colonization is the risk factor of neonatal sepsis, therefore, intrapartum antibiotic treatment has been an important strategy for management of neonatal EOS (8, 41). In the US, the application of IAP might lead to the decrease of infant GBS disease, but the burden of GBS and E. coli infection still remained (42–45). Maternal GBS screening and IAP strategies had only been applied in recent years in Chinese hospitals. Our study showed an insufficient application of IAP in maternal and child health care hospital, and the proportion of chorioamnionitis and PROM was higher, compared with neonates with sepsis in general hospital. This highlights the importance of administration of maternal GBS detection and IAP, and further management in newborn infants at risk for GBS disease in maternal and child health care hospital.
Regional general hospitals have perinatal centers responsible for treatment of diseases in pregnancy and infants in different cities. These are also referral centers for neonates with complex diseases or serious condition. It is noted that neonates with culture-proven sepsis in regional general hospital had the case fatality of 10.8%, much higher than neonates in national general hospital and maternal and child health care hospital. As reported, low gestational age and birthweight, as well as high requirements of mechanical ventilation might be the potential influence (46). Furthermore, in our study, E. coli was the leading pathogen (29.2%) in regional general hospital, and the proportion was significantly higher than in national general hospital and maternal and child health care hospital. Other studies showed that the prevalence of Gram-negative bacteria was associated with high mortality (47–49). The proportion of complications and comorbidities, especially pneumonia and meningitis, were higher in regional general hospital, which might independently lead to sepsis-attributable mortality (50, 51). It was shown that neonatal infection in very preterm infants was associated with an increased risk of poor postnatal weight growth and cerebral palsy at the age of 5 years (52, 53). Notably, compared with culture-proven sepsis, culture-negative LOS was associated with increased risks of adverse outcomes, including neurodevelopmental impairment (54, 55). Therefore, it has indication for more caution in sepsis management and treatment in regional general hospital, and more attention at follow up.
The strength of this study was the generalization of characteristics of neonatal sepsis in different medical setting models. We found that the etiology and clinical characteristics in sepsis had great differences among national general hospital, maternal and child health care hospital and regional general hospital. The variance in patient source and management in neonatal units might result in such differences. Therefore, we raise the focus on the exclusive management of neonatal sepsis in medical settings when interhospital transfers are frequent in resource-limited countries.
There are still some limitations should be considered. First, specialized children's hospitals without labor services were not involved in the study. Patients in these hospitals are transferred from other hospitals or from community. It is a necessity to generalize clinical characteristics of neonatal sepsis in these hospitals in further studies. Second, it should be mentioned that neonates in general hospital were smaller and more prone to morbidity and mortality, which may result in a risk of bias. Finally, we involved cases between January, 2010 and December, 2019 in 5 settings in central-south China. The sample size was still limited. We would further explore the patterns as a multi-center prospective study in different regions.
In conclusion, we described the pathogen distribution and AMR of predominant isolates in neonatal sepsis from central-south China between January, 2010 and December, 2019. The predominant pathogens were CoNS, K. pneumoniae, E. coli and GBS. We also provided a detailed comparison of neonatal sepsis in 3 different medical setting models. GBS had a higher proportion in maternal and child health care hospital, while the proportion of K. pneumoniae and Candida spp were higher in national general hospital. The proportion of complications and comorbidities, as well as the mortality rate, in regional general hospital was higher, compared to national general hospital and maternal and child health care hospital. Therefore, we highlight the distinct strategies for improvement of sepsis management in these setting models (quality improvement initiatives for prevention in national general hospital, maternal GBS screening and IAP strategies in maternal and child health care hospital, and staff training in treatment and follow up in regional general hospital). Also, neonatal units in different setting models should have ongoing pathogen surveillance and individual empirical antibiotic treatment choices. These findings may have implications for better management of neonatal sepsis in resource-limited settings from middle and low-income countries.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by The Ethics Committee of the Second Xiangya Hospital of Central South University. Written informed consent from the participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
YuY and QD conceptualized and designed the study, coordinated and acquired the data, carried out the initial analyses, drafted the initial manuscript, and critically revised the manuscript. SL, HQ, XT and HO coordinated and contributed to the acquisition of data, and critically revised the manuscript. JH, WL, TW, YoY and XG was responsible for the statistical analysis, data interpretation, and critical revision of the manuscript. XH and PC contributed to the analysis, interpretation of data, and critical revision of the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by The Clinical Big Data System of Central South University: Xiangya Clinical Big Data System for Premature Infants (056).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Fleischmann-Struzek C, Goldfarb DM, Schlattmann P, Schlapbach LJ, Reinhart K, Kissoon N. The global burden of paediatric and neonatal sepsis: a systematic review. Lancet Respir Med. (2018) 6(3):223–30. doi: 10.1016/S2213-2600(18)30063-8
2. Tiskumara R, Fakharee SH, Liu CQ, Nuntnarumit P, Lui KM, Hammoud M, et al. Neonatal infections in Asia. Arch Dis Child Fetal Neonatal Ed. (2009) 94(2):F144–8. doi: 10.1136/adc.2008.139865
3. Al-Taiar A, Hammoud MS, Cuiqing L, Lee JK, Lui KM, Nakwan N, et al. Neonatal infections in China, Malaysia, Hong Kong and Thailand. Arch Dis Child Fetal Neonatal Ed. (2013) 98(3):F249–55. doi: 10.1136/archdischild-2012-301767
4. Dong Y, Speer CP. Late-onset neonatal sepsis: recent developments. Arch Dis Child Fetal Neonatal Ed. (2015) 100(3):F257–63. doi: 10.1136/archdischild-2014-306213
5. Folgori L, Ellis SJ, Bielicki JA, Heath PT, Sharland M, Balasegaram M. Tackling antimicrobial resistance in neonatal sepsis. Lancet Glob Health. (2017) 5(11):e1066–8. doi: 10.1016/S2214-109X(17)30362-5
6. Chan GJ, Lee AC, Baqui AH, Tan J, Black RE. Risk of early-onset neonatal infection with maternal infection or colonization: a global systematic review and meta-analysis. PLoS Med. (2013) 10(8):e1001502. doi: 10.1371/journal.pmed.1001502
7. Yu YQ, He XR, Wan LJ, Yang YH, Chen PY. Etiology, antimicrobial resistance, and risk factors of neonatal sepsis in China: a systematic review and meta-analysis from data of 30 years. J Matern Fetal Neonatal Med. (2021):1–10. doi: 10.1080/14767058.2021.1951217. [Epub ahead of print]
8. Puopolo KM, Lynfield R, Cummings JJ, COMMITTEE ON FETUS AND NEWBORN, COMMITTEE ON INFECTIOUS DISEASES. Management of infants at risk for group B streptococcal disease. Pediatrics. (2019) 144(2):e20191881. doi: 10.1542/peds.2019-1881
9. Puopolo KM, Benitz WE, Zaoutis TE, COMMITTEE ON FETUS AND NEWBORN, COMMITTEE ON INFECTIOUS DISEASES. Management of neonates born at ≤34 6/7 weeks’ gestation with suspected or proven early-onset bacterial sepsis. Pediatrics. (2018) 142(6):e20182896. doi: 10.1542/peds.2018-2896
10. Puopolo KM, Benitz WE, Zaoutis TE, COMMITTEE ON FETUS AND NEWBORN, COMMITTEE ON INFECTIOUS DISEASES. Management of neonates born at ≥35 0/7 weeks’ gestation with suspected or proven early-onset bacterial sepsis. Pediatrics. (2018) 142(6):e20182894. doi: 10.1542/peds.2018-2894
11. He Y, Chen J, Liu Z, Yu J. Efficacy and safety of applying a neonatal early-onset sepsis risk calculator in China. J Paediatr Child Health. (2020) 56(2):237–43. doi: 10.1111/jpc.14572
12. Zhou Q, Lee SK, Hu XJ, Jiang SY, Chen C, Wang CQ, et al. Successful reduction in central line-associated bloodstream infections in a Chinese neonatal intensive care unit. Am J Infect Control. (2015) 43(3):275–9. doi: 10.1016/j.ajic.2014.12.001
13. Huynh BT, Padget M, Garin B, Delarocque-Astagneau E. Guillemot D, BIRDY study group. Bacterial neonatal sepsis and antibiotic resistance in low-income countries. Lancet. (2016) 387(10018):533–4. doi: 10.1016/S0140-6736(16)00220-8
14. CLSI. M100-S30 performance standards for antimicrobial susceptibility testing. Thirtieth informational supplement (2020).
15. Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. (1978) 187(1):1–7. doi: 10.1097/00000658-197801000-00001
16. Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. (1978) 92(4):529–34. doi: 10.1016/s0022-3476(78)80282-0
17. Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. (2001) 163(7):1723–9. doi: 10.1164/ajrccm.163.7.2011060
18. International Committee for the Classification of Retinopathy of Prematurity. The international classification of retinopathy of prematurity revisited. Arch Ophthalmol. (2005) 123(7):991–9. doi: 10.1001/archopht.123.7.991
19. Li JY, Chen SQ, Yan YY, Hu YY, Wei J, Wu QP, et al. Identification and antimicrobial resistance of pathogens in neonatal septicemia in China: a meta-analysis. Int J Infect Dis. (2018) 71:89–93. doi: 10.1016/j.ijid.2018.04.794
20. Cailes B, Kortsalioudaki C, Buttery J, Pattnayak S, Greenough A, Matthes J, et al. Epidemiology of UK neonatal infections: the neonIN infection surveillance network. Arch Dis Child Fetal Neonatal Ed. (2018) 103(6):F547–53. doi: 10.1136/archdischild-2017-313203
21. Investigators of the Delhi Neonatal Infection Study (DeNIS) collaboration. Characterisation and antimicrobial resistance of sepsis pathogens in neonates born in tertiary care centres in Delhi, India: a cohort study. Lancet Glob Health. (2016) 4(10):e752–60. doi: 10.1016/S2214-109X(16)30148-6
22. Yusef D, Shalakhti T, Awad S, Algharaibeh H, Khasawneh W. Clinical characteristics and epidemiology of sepsis in the neonatal intensive care unit in the era of multi-drug resistant organisms: a retrospective review. Pediatr Neonatol. (2018) 59(1):35–41. doi: 10.1016/j.pedneo.2017.06.001
23. Gao K, Fu J, Guan X, Zhu S, Zeng L, Xu X, et al. Incidence, bacterial profiles, and antimicrobial resistance of culture-proven neonatal sepsis in south China. Infect Drug Resist. (2019) 12:3797–805. doi: 10.2147/IDR.S223597
24. Zhang J, Folgori L, Hsia Y, Sharland M, Yang Y. Pattern of antimicrobial resistance in bloodstream isolates from Chinese neonates. Pediatr Infect Dis J. (2019) 38(6):600–4. doi: 10.1097/INF.0000000000002246
25. Lu Q, Zhou M, Tu Y, Yao Y, Yu J, Cheng S. Pathogen and antimicrobial resistance profiles of culture-proven neonatal sepsis in southwest China, 1990-2014. J Paediatr Child Health. (2016) 52(10):939–43. doi: 10.1111/jpc.13278
26. Zhu M, Jin Y, Duan Y, He M, Lin Z, Lin J. Multi-drug resistant Escherichia coli causing early-onset neonatal sepsis - a single center experience from China. Infect Drug Resist. (2019) 12:3695–702. doi: 10.2147/IDR.S229799
27. Chen IL, Chiu NC, Chi H, Hsu CH, Chang JH, Huang DT, et al. Changing of bloodstream infections in a medical center neonatal intensive care unit. J Microbiol Immunol Infect. (2017) 50(4):514–20. doi: 10.1016/j.jmii.2015.08.023
28. Kung YH, Hsieh YF, Weng YH, Lien RI, Luo J, Wang Y, et al. Risk factors of late-onset neonatal sepsis in Taiwan: a matched case-control study. J Microbiol Immunol Infect. (2016) 49(3):430–5. doi: 10.1016/j.jmii.2013.10.001
29. Hei MY, Zhang XC, Gao XY, Zhao LL, Wu ZX, Tian L, et al. Catheter-related infection and pathogens of umbilical venous catheterization in a neonatal intensive care unit in China. Am J Perinatol. (2012) 29(2):107–14. doi: 10.1055/s-0031-1295650
30. Bowen JR, Callander I, Richards R, Lindrea KB, Sepsis Prevention in NICUs Group. Decreasing infection in neonatal intensive care units through quality improvement. Arch Dis Child Fetal Neonatal Ed. (2017) 102(1):F51–7. doi: 10.1136/archdischild-2015-310165
31. Payne V, Hall M, Prieto J, Johnson M. Care bundles to reduce central line-associated bloodstream infections in the neonatal unit: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. (2018) 103(5):F422–9. doi: 10.1136/archdischild-2017-313362
32. Meyers JM, Tulloch J, Brown K, Caserta MT, D'Angio CT, GOLISANO CHILDREN’S HOSPITAL NICU ANTIBIOTIC STEWARDSHIP TEAM. A quality improvement initiative to optimize antibiotic use in a level 4 NICU. Pediatrics. (2020) 146(5):e20193956. doi: 10.1542/peds.2019-3956
33. Achten NB, Klingenberg C, Benitz WE, Stocker M, Schlapbach LJ, Giannoni E, et al. Association of use of the neonatal early-onset sepsis calculator with reduction in antibiotic therapy and safety: a systematic review and meta-analysis. JAMA Pediatr. (2019) 173(11):1032–40. doi: 10.1001/jamapediatrics.2019.2825
34. Ting JY, Roberts A, Sherlock R, Ojah C, Cieslak Z, Dunn M, et al. Duration of initial empirical antibiotic therapy and outcomes in very low birth weight infants. Pediatrics. (2019) 143(3):e20182286. doi: 10.1542/peds.2018-2286
35. Ting JY, Synnes A, Roberts A, Deshpandey A, Dow K, Yoon EW, et al. Association between antibiotic use and neonatal mortality and morbidities in very low-birth-weight infants without culture-proven sepsis or necrotizing enterocolitis. JAMA Pediatr. (2016) 170(12):1181–7. doi: 10.1001/jamapediatrics.2016.2132
36. Carr D, Barnes EH, Gordon A, Isaacs D. Effect of antibiotic use on antimicrobial antibiotic resistance and late-onset neonatal infections over 25 years in an Australian tertiary neonatal unit. Arch Dis Child Fetal Neonatal Ed. (2017) 102(3):F244–50. doi: 10.1136/archdischild-2016-310905
37. Cantey JB, Wozniak PS, Pruszynski JE, Sánchez PJ. Reducing unnecessary antibiotic use in the neonatal intensive care unit (SCOUT): a prospective interrupted time-series study. Lancet Infect Dis. (2016) 16(10):1178–84. doi: 10.1016/S1473-3099(16)30205-5
38. Huang J, Lin XZ, Zhu Y, Chen C. Epidemiology of group B streptococcal infection in pregnant women and diseased infants in mainland China. Pediatr Neonatol. (2019) 60(5):487–95. doi: 10.1016/j.pedneo.2019.07.001
39. Ying Q, Wang S, Lou X, Ding J, Ding J. Burden and risk factors of invasive group B Streptococcus disease among neonates in a Chinese maternity hospital. BMC Infect Dis. (2019) 19(1):123. doi: 10.1186/s12879-018-3660-1
40. Dong Y, Jiang SY, Zhou Q, Cao Y. Group B Streptococcus causes severe sepsis in term neonates: 8 years experience of a major Chinese neonatal unit. World J Pediatr. (2017) 13(4):314–20. doi: 10.1007/s12519-017-0034-5
41. Puopolo KM, Draper D, Wi S, Newman TB, Zupancic J, Lieberman E, et al. Estimating the probability of neonatal early-onset infection on the basis of maternal risk factors. Pediatrics. (2011) 128(5):e1155–63. doi: 10.1542/peds.2010-3464
42. Schrag SJ, Farley MM, Petit S, Reingold A, Weston EJ, Pondo T, et al. Epidemiology of invasive early-onset neonatal sepsis, 2005 to 2014. Pediatrics. (2016) 138(6):e20162013. doi: 10.1542/peds.2016-2013
43. Stoll BJ, Hansen NI, Sanchez PJ, Faix RG, Poindexter BB, Van Meurs KP, et al. Early onset neonatal sepsis: the burden of group B streptococcal and E. coli disease continues. Pediatrics. (2011) 127(5):817–26. doi: 10.1542/peds.2010-2217
44. Stoll BJ, Puopolo KM, Hansen NI, Sanchez PJ, Bell EF, Carlo WA, et al. Early-onset neonatal sepsis 2015 to 2017, the rise of Escherichia coli, and the need for novel prevention strategies. JAMA Pediatr. (2020) 174(7):e200593. doi: 10.1001/jamapediatrics.2020.0593
45. Nanduri SA, Petit S, Smelser C, Apostol M, Alden NB, Harrison LH, et al. Epidemiology of invasive early-onset and late-onset group B streptococcal disease in the United States, 2006 to 2015: multistate laboratory and population-based surveillance. JAMA Pediatr. (2019) 173(3):224–33. doi: 10.1001/jamapediatrics.2018.4826
46. Giannoni E, Agyeman PKA, Stocker M, Posfay-Barbe KM, Heininger U, Spycher BD, et al. Neonatal sepsis of early onset, and hospital-acquired and community-acquired late onset: a prospective population-based cohort study. J Pediatr. (2018) 201:106–114.e4. doi: 10.1016/j.jpeds.2018.05.048
47. Jiang S, Yang C, Yang C, Yan W, Shah V, Shah PS, et al. Epidemiology and microbiology of late-onset sepsis among preterm infants in China, 2015-2018: a cohort study. Int J Infect Dis. (2020) 96:1–9. doi: 10.1016/j.ijid.2020.03.034
48. Johnson J, Robinson ML, Rajput UC, Valvi C, Kinikar A, Parikh TB, et al. High burden of bloodstream infections associated with antimicrobial resistance and mortality in the neonatal intensive care unit in pune, India. Clin Infect Dis. (2021) 73(2):271–80. doi: 10.1093/cid/ciaa554
49. Goh GL, Lim CSE, Sultana R, De La Puerta R, Rajadurai VS, Yeo KT. Risk factors for mortality from late-onset sepsis among preterm very-low-birthweight infants: a single-center cohort study from Singapore. Front Pediatr. (2022) 9:801955. doi: 10.3389/fped.2021.801955
50. Wu IH, Tsai MH, Lai MY, Hsu LF, Chiang MC, Lien R, et al. Incidence, clinical features, and implications on outcomes of neonatal late-onset sepsis with concurrent infectious focus. BMC Infect Dis. (2017) 17(1):465. doi: 10.1186/s12879-017-2574-7
51. Tsai MH, Lee CW, Chu SM, Lee IT, Lien R, Huang HR, et al. Infectious complications and morbidities after neonatal bloodstream infections: an observational cohort study. Medicine. (2016) 95(11):e3078. doi: 10.1097/MD.0000000000003078
52. Mitha A, Foix-L'Hélias L, Arnaud C, Marret S, Vieux R, Aujard Y, et al. Neonatal infection and 5-year neurodevelopmental outcome of very preterm infants. Pediatrics. (2013) 132(2):e372–80. doi: 10.1542/peds.2012-3979
53. Flannery DD, Jensen EA, Tomlinson LA, Yu Y, Ying GS, Binenbaum G, et al. Poor postnatal weight growth is a late finding after sepsis in very preterm infants. Arch Dis Child Fetal Neonatal Ed. (2021) 106(3):298–304. doi: 10.1136/archdischild-2020-320221
54. Jiang S, Yang Z, Shan R, Zhang Y, Yan W, Yang Y, et al. Neonatal outcomes following culture-negative late-onset sepsis among preterm infants. Pediatr Infect Dis J. (2020) 39(3):232–8. doi: 10.1097/INF.0000000000002558
Keywords: pathogens, antimicrobial resistance, clinical characteristics, neonatal sepsis, medical setting
Citation: Yu Y, Dong Q, Li S, Qi H, Tan X, Ouyang H, Hu J, Li W, Wang T, Yang Y, Gong X, He X and Chen P (2022) Etiology and clinical characteristics of neonatal sepsis in different medical setting models: A retrospective multi-center study. Front. Pediatr. 10:1004750. doi: 10.3389/fped.2022.1004750
Received: 27 July 2022; Accepted: 20 September 2022;
Published: 5 October 2022.
Edited by:
Sithembiso Velaphi, University of the Witwatersrand, South AfricaReviewed by:
Prenika Jaglal, University of the Witwatersrand, South Africa© 2022 Yu, Dong, Li, Qi, Tan, Ouyang, Hu, Li, Wang, Yang, Gong, He and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pingyang Chen Y2hlbnBpbmd5YW5nQGNzdS5lZHUuY24= Xiaori He aGV4aWFvcmlAY3N1LmVkdS5jbg==
Specialty Section: This article was submitted to Neonatology, a section of the journal Frontiers in Pediatrics
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.