- 1Department of Medical Microbiology, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran
- 2Department of Medical Mycology, Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran
- 3Social Development & Health Promotion Research Center, Health Institute, Kermanshah University of Medical Sciences, Kermanshah, Iran
- 4Department of Toxicology, Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran
- 5Cellular and Molecular Research Center, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran
- 6Department of Pharmacology, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran
In HIV-infected pediatrics, oral candidiasis (OC) is a global issue of concern due to its association with dysphagia, malnutrition, and mortality. The present systematic review and meta-analysis are the first to determine the prevalence of OC in HIV-infected pediatrics worldwide. We searched international (PubMed, Web of Science, Scopus, and Embase) databases for studies published between January 2000 to May 2020 reporting the epidemiologic features of OC in HIV-infected pediatrics. Inclusion and exclusion criteria were defined to select eligible studies. Data were extracted and presented according to PRISMA guidelines. The results of the meta-analysis were visualized as a forest plot. Heterogeneity was also analyzed using the I2, and τ2 statistics. The publication bias was evaluated using Egger test. The literature search revealed 1926 studies, of which 34 studies met the eligibility criteria, consisting of 4,474 HIV-infected pediatrics from 12 different countries. The overall prevalence of OC among HIV-infected pediatrics was 23.9% (95% CI 17.3–32.0%), and Candida albicans was the most prevalent etiologic agent. Pseudomembranous candidiasis was the predominant clinical manifestation in HIV-infected pediatrics suffering from OC. Thirty articles involving 4,051 individuals provided data on HIV treatment status. Among the 4,051 individuals, 468 (11.53%) did not receive HIV treatment. The data from 11 articles demonstrated that HIV treatment was significantly associated with a reduction in oral Candida colonization or infection. In contrast, others showed the opposite relationship or did not report any statistical data. A high level of I2 (I2 = 96%, P < 0.01) and τ2 (τ2 = 1.36, P < 0.01) was obtained among studies, which provides evidence of notable heterogeneity between studies. OC is approximately frequent in HIV-positive children. Therefore, efforts should be made to teach dental and non-dental clinicians who care for HIV-infected pediatrics to diagnose and treat this infection.
Introduction
Candida species reside as part of the normal flora of the oral cavity in about 66% of the pediatric population known as carriers (1). In the event of immunosuppression, especially in acquired immunodeficiency syndrome (AIDS), there is a shift from commensalisms to an exponential increase in colonization, which eventually leads to clinical signs and symptoms of oral candidiasis (OC) (2, 3). CD4 lymphocytes have a major role in the suppression of OC, and as a result, AIDS patients with low CD4 lymphocyte counts are especially susceptible to Candida infections (4). OC occurs in up to 95% of human immunodeficiency virus (HIV)-infected individuals during their illness and often represents the first form in which immunosuppression shows itself (5). According to the Joint United Nations Programme on HIV/AIDS (UNAIDS)-2020, there were 38.0 million people living with HIV in 2019, and 1.8 million of whom were pediatrics (6). The majority of studies conducted worldwide agree that OC is the most common HIV-related oral lesion; both in patients undergoing highly active antiretroviral therapy (HAART) and in untreated ones (7). There is some evidence that candidal infection may induce immunosuppression. Thus, candidiasis may enhance the progression to more severe disease and AIDS (8).
In recent years, the number of HIV-infected infants and children has grown remarkably, with more than 230% increase from 2010 (3). In 2017, an estimated 180,000 children ages 0–14 years were newly infected with HIV (9). The development of HIV infection in children has different characteristics to those noted in adults, due mainly to the immaturity of the immunologic system, and other body structures (10). In children, diseases usually progress faster, and outcomes are more serious than adults, resulting in a high mortality rate due to opportunistic infections like candidiasis (11).
The majority of oral Candida infections in HIV-infected patients are caused by Candida albicans, although, recently, an increasing number of infections have been associated with other Candida species which are more resistant to azoles and had created a public health concern (12–14). Some of the changes in Candida species prevalence have been associated with the increased use of antifungal agents for both prophylactic and treatment purposes (15). The common non-Candida albicans species associated with OC include Candida glabrata, Candida parapsilosis, Candida tropicalis, and Candida krusei (Issatchenkia orientalis) (15, 16).
OC is an important part of the clinical picture of HIV infection. Children with OC manifest discomfort during eating; this situation may reduce their food intake. Malnutrition is an important mortality risk factor in HIV-infected children because it reduces the capacity of the body to fight this infection by compromising various immune parameters (17).
Furthermore, OC has been attributed with important diagnostic and prognostic values to HIV infection and as a marker of antiretroviral therapy failure and, therefore can be used as a criterion to make important therapeutic decision-making (18).
Several articles have investigated the prevalence of OC in HIV-infected pediatric patients in different countries, but until now there is no comprehensive estimation of the infection among HIV-infected pediatric patients across the world. Therefore, performing a systematic review and meta-analysis was necessary to provide a more in-depth understanding and summarized evidence relating to OC in HIV-infected children and assist in its control.
Materials and Methods
Research Question
This study aims to determine the prevalence, the main etiologic agent, the predominant clinical manifestation, and the demographic characteristics of OC (colonization and/or infection) among HIV-infected pediatrics across the world.
Research Strategy
This systematic review and meta-analysis was conducted based on PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (19). We searched English databases (PubMed, Web of Science, Scopus, and Embase) for articles regarding the prevalence of oral Candida colonization or infection in HIV-infected pediatric patients published from January 2000 to May 2020. We also used references of included primary articles for search.
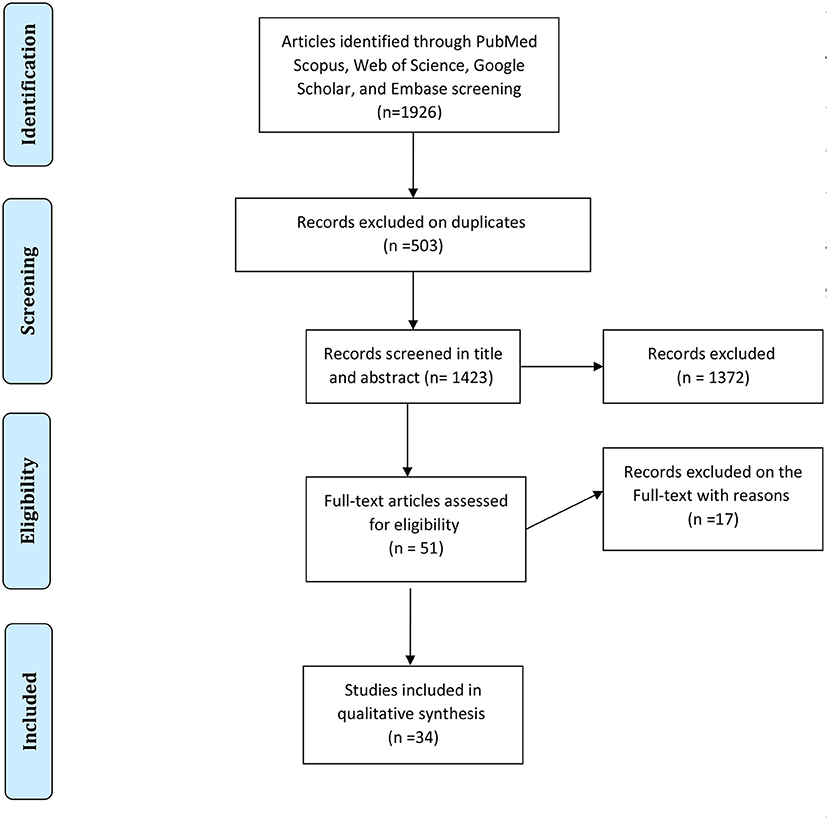
In each electronic database, various combinations of the following search terms were used: “Candidiasis, Oral,” and “HIV,” or “human immunodeficiency virus,” and “epidemiology.” Searching and collecting the relevant papers were done by two authors. The disagreements between the reviewers were solved with discussions between the two authors in a joint session, and, whether or not an agreement was not reached, a decision was made by a third author. At the end of the search, the collected articles were managed with EndNote X7.1 software (Thomson Reuters). A PRISMA flowchart showing the search and study selection strategy is presented in Figure 1.
Inclusion and Exclusion Criteria
The inclusion criteria of the present research consisted of all original articles in the English language reporting the prevalence of oral Candida colonization or infection in HIV-infected children from the age of 0–18 years. Oral candidiasis can include both forms of oral Candida colonization and oral Candida infection. Candida colonization was defined as the isolation of ≥50 Candida colonies from the oral cavity without oral lesions (20). Candida infection included several lesions such as pseudomembranous, erythematous, and angularchelitis that most are caused in immunosuppressive patients. The most discrete lesion represents a conversion from benign colonization to pathological overgrowth (21).
In all included studies the diagnosis of HIV should be performed according to the World Health Organization (WHO) 1997's criteria. Exclusion criteria were: review articles, case reports, randomized controlled trials, letters, workshop reports, and case series without original data. We also excluded studies if they were not contained details of oral Candida species, done in HIV-infected patients older than 18 years of age, do not provide any information about the participant's age, published in a language other than English, investigated only a single pathogen of OC, discussed oral Candida carriage (Candida oral flora/oral Candida occurrence), reported sporadic OC, and were not contained patterns of clinical presentations associated with oral Candida colonization or infection.
Data Extraction and Quality Assessment
The primary citations obtained during the database survey were recorded in a text file according to their topics and abstracts. Following an initial screening, especially eligible records were selected for full-text download. The final eligibility and inclusion criteria for the downloaded full texts were appraised by 2 separate investigators at three levels: title, abstract, and full text. Afterward, 2 authors extracted the requisite data. Authors have independently assessed the risk of bias in the included studies, according to the criteria from the modified STROBE checklist, as a validated method for assessing the quality of observational and case-control studies (22). The instrument used a system to evaluate cross-sectional studies based on some criteria: title and abstract, background, objectives, study design, setting, participants, variables, data sources, and measurements, bias, study size, quantitative variables, statistical methods, participants' result, descriptive data result, outcome data and main results, other analyses, key results, limitations, interpretation, and generalizability (22).
For each domain, the following description was used for management of the risk of bias: “Yes,” “No,” and “Unclear.” We graded the quality of included studies and risk of bias, using grading 1 for “yes” and 0 for “no and unclear,” and disagreement was resolved by discussion.
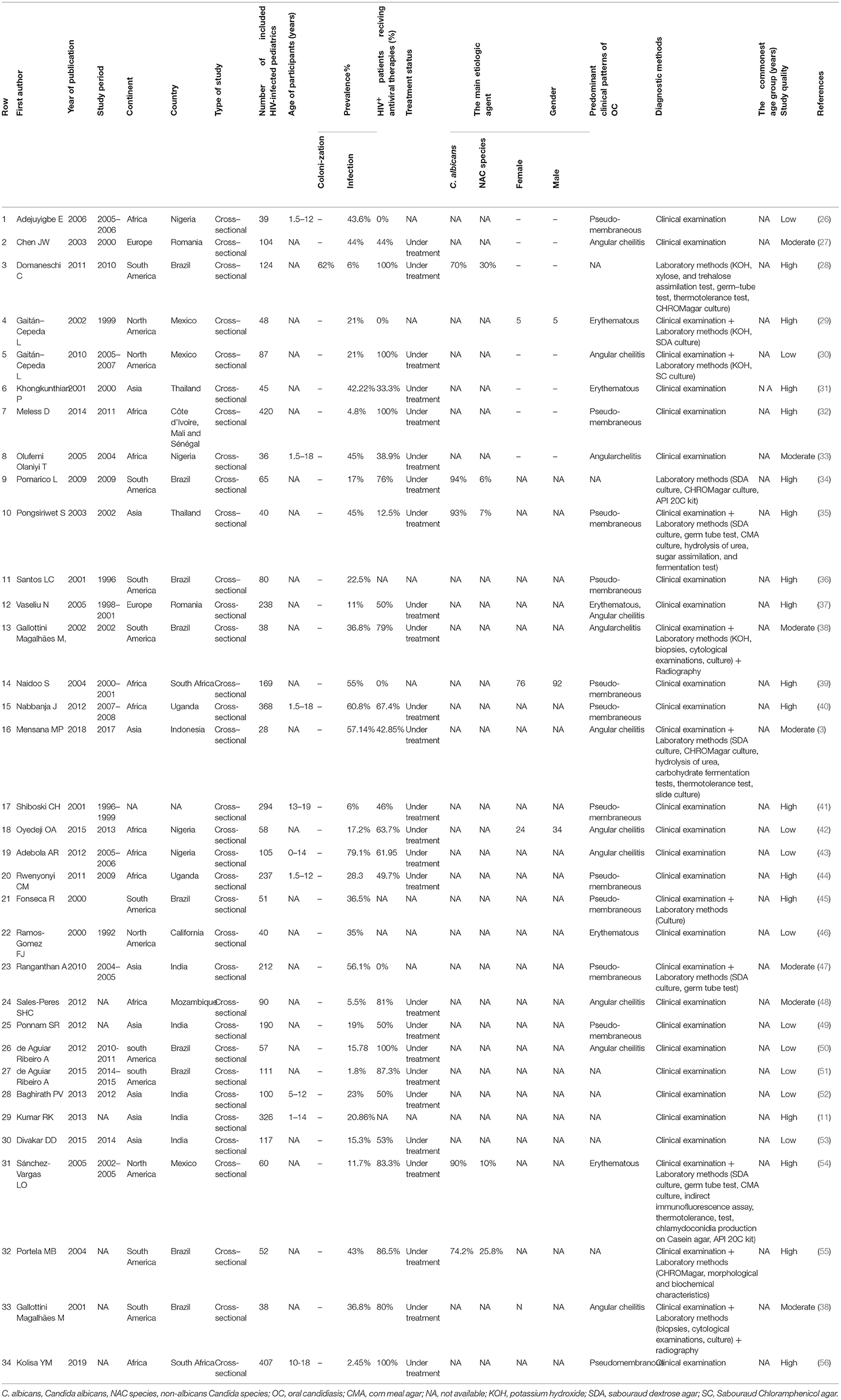
All included articles were prepared for data extraction by a pre-prepared checklist in Microsoft Excel spreadsheets. The checklist included the first author last name, year of publication, study period, the location of the study (country and continent), type of study, the number of HIV-infected pediatrics (sample size), age of participants, the prevalence percentage of oral Candida colonization and/or infection, gender distribution among patients with oral Candida colonization and/or infection, method for Candida species identification, the number of patients undergoing antiretroviral therapy (ART) or HAART, treatment status, the main etiologic agent of oral Candida colonization/infection, the predominant manifestations, and the commonest age group related to oral Candida colonization and/or infection. Regarding oral manifestations of Candida colonization and/or infection, we focused on the most significant ones including pseudomembranous, erythematous, and angular cheilitis, which were reported in the updated classification of HIV-related oral diseases (23). When the reported data were insufficient or in the case of articles whose full text was not available, we contacted the corresponding by email to request additional information or full text. After three emails with a week's interval, these studies were excluded.
Statistical Analyses
All analyses were performed using SPSS software (IBM SPSS Statistics for Windows, Version 21.0, IBM Corp, Armonk, NY, USA). We used Random Effect Model for combining the effect size measures. The tau-squared (τ2 or Tau2) and I2 indexes were used to measure the heterogeneity in the results of studies. Studies with an I2 index 0–40%, 30–60%, 50–90%, 75–100%, categorized as might not be important, moderate heterogeneity, substantial heterogeneity, and considerable heterogeneity respectively (24). The publication bias was evaluated using Egger test (25). The results of the meta-analysis were visualized as a forest plot representing the prevalence estimates of each study. Statistical significance was defined as a P < 5%.
Results
The first search identified a total of 1,926 records (212 in PubMed, 902 in Scopus, 193 in Embase, and 619 in Web of Science). After the removal of duplicates, 1,423 papers remained. Totally, 1,372 articles were not considered relevant after the title and abstract screening. Finally, 51 studies that met the inclusion criteria were reviewed in-depth and 34 study papers (3, 11, 26–56) were found to be eligible for meta-analysis. The detailed information of the 34 included studies is summarized in Table 1.
All included papers were published between 2000 and 2019. The majority of the studies were conducted in Africa (n = 10), South America (n = 9), and Asia (n = 8) (Figure 2).
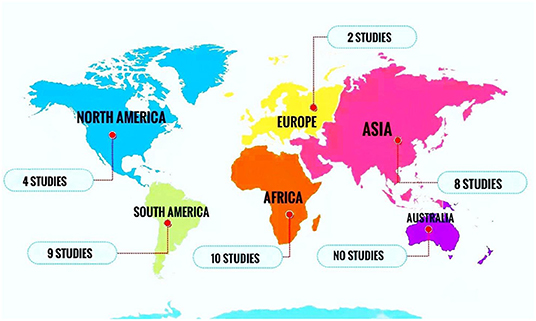
Figure 2. Number of studies reporting the prevalence of oral Candida colonization or infection in HIV-infected pediatrics in different continents.
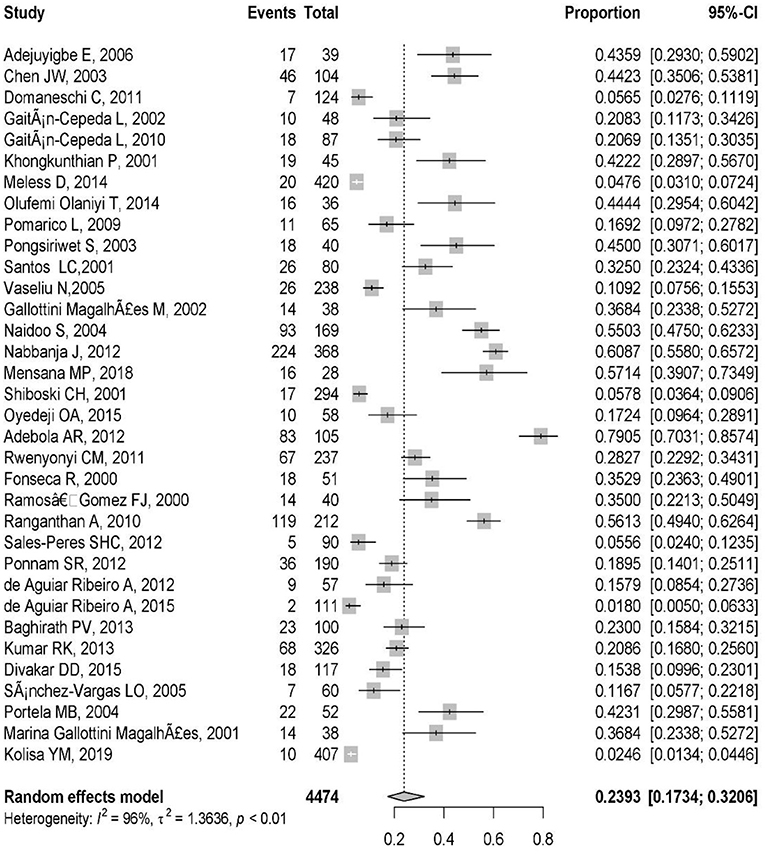
These included studies consisted of a total of 4,474 HIV-infected pediatric patients from 12 different countries. The country with the highest number of studies was Brazil (9/34). The study with the smallest population sample was the one conducted by Mensana et al. (3) (28 patients), while the study by Meless et al. (32), with 420 patients, was the one with the largest sample. The majority of the articles (n = 33; 97%) reported OC, and one (3%) presented both OC and oral Candida colonization (Table 1). The prevalence of OC among HIV-infected pediatrics was ranged from 1.8% (95% CI: 0.5–6.3%) in Brazil to 79.1% (95% CI: 70.3–85.7%) in Nigeria. The pooled prevalence of OC among HIV-infected pediatrics was 23.9% (95% CI 17.3–32.0%) (Figure 3).
The high obtained percentage of I2 (I2 = 96%, P < 0.01) and τ2 (τ2 = 1.36, P < 0.01) represent the high variation and notable heterogeneity among the extracted studies (Figure 3). The Egger test results was statistically significant (p = 0.01) that shows there is publication bias.
In the majority of the included articles (n = 12; 35.29%) pseudomembranous candidiasis was reported as the most prevalent form of OC in HIV-infected pediatrics followed by angular cheilitis (n = 10, 29.41%), and erythematous candidiasis (n = 4, 11.77%). Also, a single article (n = 1, 2.94%) in Romania reported both erythematous candidiasis and angular cheilitis as the most prevalent form of OC (Table 1).
The prevalence of pseudomembranous candidiasis ranged from 1.7% in South Africa (56) to 72.2% in and Brazil (36). Also, the prevalence of angular cheilitis was highest in Indonesia, at 46.6% (3). Furthermore, the prevalence of erythematous candidiasis was highest in Thailand (35) at 55.5%.
Five articles reported the etiologic agents of OC among HIV-infected pediatric, and in all of them (n = 5, 100%) Candida albicans was the predominant etiologic agent (Table 1).
In the 34 reviewed studies clinical examination and/or laboratory techniques were used for the diagnosis of OC. In studies in which the diagnosis of OC carried out according to clinical examination, the evaluation of the oral health status of patients had been performed by an oral physician. The laboratory techniques used in these studies included: morphological identification (10% KOH, biopsies, cytological smears, and chlamydoconidia production on corn meal agar and Casein agar, slide culture, and immunofluorescence assay), culture method [sabouraud dextrose agar (SDA), sabouraud chloramphenicol agar (SC)], methods based on chromogenic substrates (CHROMagar), physiological methods (germ tube, thermotolerance test at 42–45°C, sugar assimilation and fermentation, hydrolysis of urea), and commercial identification kits (API 20C) (Table 1).
No clear data of the age and gender distribution regarding OC among HIV-infected pediatrics were given in the studies included in the meta-analysis (Table 1).
Thirty articles involving 4,057 individuals provided data on HIV treatment status. Among the 4,057 individuals, 468 (11.53%) did not receive HAART or ART. The data from 11 articles (28, 33, 43, 44, 48, 49, 51–54, 56) demonstrated that HAART or ART were significantly associated with reduction in oral Candida colonization or infection, while others showed the opposite relationship (12, 16, 32, 34, 41, 42, 55), or did not report any statistical data (11, 14, 26, 35–40, 47, 50).
Discussion
Our results showed that most of the examined children came from African and South American countries. This confirmed the fact that these countries are those in which HIV is more widespread, especially among the pediatric population. Actually, Africa continent countries are home to about 15.2 percent of the world's population and more than two-thirds of the total HIV-infected patients worldwide are Africans (56). On the other hand, vertical transmission of HIV continues to be a largely uncontrolled problem in African countries where 95% of HIV-infected pregnant women live (57). Also, the results of four surveys in South America showed that although several countries in this continent have shown impressive declines in HIV incidence, the number of new HIV infections in the region increased and the main reason was that about 25% of 15–19-year-olds did not know how to protect themselves against HIV (58). Also, the country with the highest number of studies was Brazil. This finding could be explained by the tradition of dentistry research in this country.
The pooled prevalence of OC among HIV-infected pediatrics was 23.9% (95% CI 17.3–32.0%) (Figure 2) which is similar to the prevalence of OC in HIV-infected adult patients reported in Brazil (59, 60), Nepal (61), and United States (62). It was different from that reported in Uganda (63) and Indonesia (64) as they reported a prevalence of 70 and 66.7% for OC among HIV-infected adult patients. It should be noted, until now there is no comprehensive meta-analysis discussing the prevalence of OC in HIV-infected adults in the world, so the pooled prevalence related to it is unavailable. Our results showed that the prevalence of OC among HIV-infected pediatrics was ranged from 1.8% (95% CI: 0.5–6.3%) in Brazil (43) to 79.1% (95% CI: 70.3–85.7%) in Nigeria (51). Universal access to antiretroviral treatment in Brazil may explain the low incidence of OC among HIV-infected Brazilian pediatrics and difficult access to treatment may be the main reason for the high prevalence of OC observed in Nigerian HIV-infected pediatrics, as well as in the study from Nigeria 61.9% of investigated HIV-infected pediatrics were under treatment with antiretroviral therapy (43), while in the study from Brazil 87.3% of included HIV-infected pediatrics were under treatment (51).
Also, the prevalence of OC among HIV-infected pediatrics represented a high variation and notable heterogeneity. This difference in the prevalence of the infection in different studies can be due to the differences in the methodology used for the collection and analysis of data (in fact, the procedures that were used for the diagnosis of oral lesions and for the execution of statistical analysis in most of the studies have been not reported in a detailed and precise way).
In the studies included in the meta-analysis, we could also observe that access to treatment for HIV-positive pediatrics is still difficult: Only in 5 studies (27, 29, 31, 38, 49), 100% of children underwent treatment (ART or HAART), while results of other studies included in the meta-analysis (3, 26, 30, 32–34, 36, 37, 39–43, 47, 48, 50–55) showed that some but not all participating pediatrics were on antiretroviral therapy at the beginning of the study. Also, different studies on the effect of antiretroviral therapy on OC among HIV-positive pediatrics showed conflicting results. While some authors reported a significant reduction in the prevalence of OC (27, 32, 38, 42, 43, 47, 48, 50–53), others did not observe any changes in the occurrence of HIV-related OC in children receiving antiretroviral therapy (12, 16, 31, 33, 40, 41, 54). The reasons for such different results are not fully understood. Some authors have associated these variations with differences in oral care habits, social and demographic factors, HIV-transmission mode, and types of co-infections, disease stage, and immune reconstitution (64–66). Nevertheless, one should emphasize that oral lesions may develop again due to HAART failure and multi-drug resistance (67).
The following list of medications was used in the treatment of HIV in studies included in the present investigation: Zidovudine (26, 48, 52, 53), Lamivudine (26, 42, 48, 52), Nevirapine (42, 48, 52), Stavudine (42, 48), and co-trimoxazole (43).
Pseudomembranous candidiasis was the predominant clinical pattern of OC among HIV-infected pediatrics. In accordance, the majority of studies conducted around the world agree that pseudomembranous candidiasis is the most common clinical pattern of OC among HIV-infected patients (7). A second form erythematous candidiasis and a third form, angular cheilitis, were also reported as the most common clinical pattern of OC among HIV-infected patients (7). These data are in agreement with what emerged from this meta-analysis.
According to the results of the present study, Candida albicans is still the most prevalent species causing OC in HIV-infected pediatric patients. The ability to grow in both yeast and hyphal forms, the production of secreted proteinase activity, and the ability to biofilm formation are important virulence factors related to this microorganism (68–70). On the other hand, clinical manifestations of oral candidiasis vary depending on the type of causative agent. Pseudomembranous candidiasis is common in oral infections due to Candida albicans (71).
The present study shows that the epidemiology of HIV-associated oral candidiasis in pediatrics has not been studied in many countries worldwide. Also, it indicates in many countries the diagnosis of oral candidiasis in HIV-infected pediatrics is made based on clinical signs and symptoms, and without laboratory testing. Due to the fact that different Candida species have various degrees of susceptibility to common antifungal agents and the increasing prevalence of infections caused by non-C. albicans Candida, there is a need for investigating the distribution of Candida species causing oral candidiasis in each country, and clinicians should be aware of the importance of laboratory testing in diagnostic decision making and know that the identification of the causative agents at the species level is essential for appropriate treatment (14, 15). In addition, the opportunistic Candida species existing as part of commensal microflora in humans are usually the etiological agents causing infections. Hence, isolates from various sources and patients of different climate regions may have different characters.
Among limitations to be noted in this study is that we did not attempt to identify studies from the “gray” literature, such as conference proceedings or research published in master's or PhD theses. Another limitation is that we did not include any articles published in languages other than English. Also, because the search of this study has been done worldwide and the number of preliminary studies for selection was high, we used date restriction filter to limit primary studies. Furthermore, there was evidence of statistical heterogeneity when calculating a pooled estimate of the prevalence of OC among HIV-infected pediatrics. As discussed above, we feel the most likely source of heterogeneity was the differences in the methodology used for the collection and analysis of data, because the procedures that were used for the diagnosis of oral lesions and for the execution of statistical analysis in most of the studies have been not reported in a detailed and precise way.
Conclusion
The present systematic review and meta-analysis gives an insight into the scenario of OC related to HIV-infection among pediatrics in the two last decades and helps to provide a comprehensive overview on the most common oral manifestations in HIV-positive pediatrics. Given that this infection can lead to fatal complications such as candidemia, meningitis, endocarditis, esophagitis, and endophthalmitis in HIV-positive pediatrics whose immune systems are very weak taking preventive measures such as awareness of parents as well as monitoring and controlling of symptoms are essential. On the other hand, it is very important that physicians and dentists know the main clinical manifestations and associated factors of oral candidiasis among HIV-infected pediatrics, since it may be the first manifestation of a local or systemic disorder.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
ZR, MS, and ES: conceptualization, supervision, and project administration. ZR: writing original draft. ES, YS, SH, and DR: data curation, methodology, and validation. YS: data analysis. ZR, ES, and YS: writing-review and editing. All authors discussed the results, contributed to the final manuscript, and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Jabra-Rizk MA, Torres S, Rambob I, Meiller T, Grossman L, Minah G. Prevalence of oral Candida species in a North American pediatric population. J Clin Pediatr Dent. (2007) 31:260–3. doi: 10.17796/jcpd.31.4.820968206675v577
2. Rafat Z, Hashemi SJ, Ashrafi K, Nikokar I, Jafari A, Foroushani AR, et al. Fungal isolates of the respiratory tract in symptomatic patients hospitalized in pulmonary units: a mycological and molecular epidemiologic study. J Multidiscip Healthc. (2020) 13:661–9. doi: 10.2147/JMDH.S252371
3. Mensana MP, Ernawati DS, Nugraha AP, Soebadi B, Triyono EA, Husada D, et al. Oral candidiasis profile of the Indonesian HIV-infected pediatric patients at UPIPI Dr. Soetomo General Hospital, Surabaya, Indonesia. HIV AIDS Rev. (2018) 17:272–7. doi: 10.5114/hivar.2018.80259
4. Ratnam MV, Nayyar AS, Reddy DS, Ruparani B, Chalapathi KV, Azmi SM. CD4 cell counts and oral manifestations in HIV infected and AIDS patients. J Oral Maxillofac Pathol. (2018) 22:282–92. doi: 10.4103/jomfp.JOMFP_191_17
5. Mushi MF, Bader O, Taverne-Ghadwal L, Bii C, Groß U, Mshana SE. Oral candidiasis among African human immunodeficiency virus-infected individuals: 10 years of systematic review and meta-analysis from sub-Saharan Africa. J Oral Microbiol. (2017) 9:1317579. doi: 10.1080/20002297.2017.1317579
6. The Joint United Nations Programme on HIV/AIDS (UNAIDS) (2018). UNAIDS Data. (2018). Available online at: https://www.unaids.org/en/resources/fact-sheet (accessed December 16, 2021).
7. Gaitán-Cepeda LA, Sánchez-Vergas O, Castillo N. Prevalence of oral candidiasis in HIV/AIDS children in highly active antiretroviral therapy era. a literature analysis. Int J STD AIDS. (2015) 26:625–32. doi: 10.1177/0956462414548906
8. McCarthy GM. Host factors associated with HIV-related OC: a review. Oral Surg Oral Med Oral Pathol. (1992) 73:181–6. doi: 10.1016/0030-4220(92)90192-S
9. UNAIDS. Fact Sheet–World AIDS Day 2018. Geneva: UNAID (2017). Available online at: www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf (accessed December 16, 2021).
10. Soares LF, Castro GF, Souza IP, Pinheiro M. Pediatric HIV-related oral manifestations: a five-year retrospective study. Braz Oral Res. (2004) 18:6–11. doi: 10.1590/S1806-83242004000100002
11. Kumar RK, Mohan G, Reddy NV, Rao VA, Shameer M, Christopher A. Associated oral lesions inhuman immunodefeciency virus infected children of age 1 to 14 years in antiretroviral therapy centers in Tamil Nadu. Contemp Clin Dent. (2013) 4:467–71. doi: 10.4103/0976-237X.123043
12. Redding SW, Kirkpatrick WR, Dib O, Fothergill AW, Rinaldi MG, Patterson TF. The epidemiology of non-albicans Candida in oropharyngeal candidiasis in HIV patients. Spec Care Dentist. (2000) 20:178–81. doi: 10.1111/j.1754-4505.2000.tb00015.x
13. Anwar KP, Malik A, Subhan KH. Profile of candidiasis in HIV infected patients. Iran J Microbiol. (2012) 4:204–9.
14. Nadagir SD, Chunchanur SK, Halesh LH, Yasmeen K, Chandrasekhar MR, Patil BS. Significance of isolation and drug susceptibility testing of non-Candida albicans species causing oropharyngeal candidiasis in HIV patients. Southeast Asian J Trop Med Public Health. (2008) 39:492–5.
15. Belazi M, Velegraki A, Koussidou-Eremondi T, Andreadis D, Hini S, Arsenis G, et al. Oral Candida isolates in patients undergoing radiotherapy for head and neck cancer: prevalence, azole susceptibility profiles and response to antifungal treatment. Oral Microbiol Immunol. (2004) 19:347–51. doi: 10.1111/j.1399-302x.2004.00165.x
16. Mushi MF, Mtemisika CI, Bader O, Bii C, Mirambo MM, Groß U, et al. High oral carriage of non-albicans Candida spp. among HIV-infected individuals. Int J Infect Dis. (2016) 49:185–8. doi: 10.1016/j.ijid.2016.07.001
17. Jesson J, Masson D, Adonon A, Tran C, Habarugira C, Zio R, et al. Prevalence of malnutrition among HIV-infected children in Central and West-African HIV-care programmes supported by the Growing Up Programme in 2011: a cross-sectional study. BMC Infect Dis. (2015) 15:1–12. doi: 10.1186/s12879-015-0952-6
18. Gaitan-Cepeda LA, Marti'nez-Gonza'lez M, CeballosSalobren~ a A. Oral candidosis as a clinical marker of immune failure in patients with HIV/AIDS on HAART. AIDS Patient Care STDS. (2005) 19:70–7. doi: 10.1089/apc.2005.19.70
19. Moher D, Shamseer L, Clarke M, Ghersi D, Liebrati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. (2015) 4:1. doi: 10.1186/2046-4053-4-1
20. Berberi A, Noujeim Z, Aoun G. Epidemiology of oropharyngeal candidiasis in human immunodeficiency virus/acquired immune deficiency syndrome patients and CD4+ counts. J Int Oral Health. (2015) 7:20–3.
21. Dangi YS, Soni ML, Namdeo KP. Oral candidiasis: a review. Int J Pharm Pharm Sci. (2010) 2:36–41.
22. Vandenbroucke J P, von Elm E, Altman D G, Gøtzsche P C, Mulrow C D, Pocock S, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Int J Surg. (2014) 12:1500–24. doi: 10.1016/j.ijsu.2014.07.014
23. Shiboski CH, Patton LL, Webster-Cyriaque JY, Greenspan D, Traboulsi RS, Ghannoum M, et al. The Oral HIV/AIDS research alliance: updated casedefinitions of oral disease endpoints. J Oral Pathol Med. (2009) 38:481–8. doi: 10.1111/j.1600-0714.2009.00749.x
24. Deeks JJ, Higgins JP, Altman DG, Cochrane Statistical Methods Group. Analyzing data and undertaking meta-analyses. Cochrane Handbook Syst Rev Intervent. (2019) 23:241–84. doi: 10.1002/9781119536604.ch10
25. Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
26. Adejuyigbe E, Owotade F, Folayan M, Adedigba M, Anyabolu H. Oro-facial lesions in human immunodeficiency virus infected children in Ile-Ife, Nigeria. J Pediatr Infect Dis. (2007) 2:83–8.
27. Chen JW, Flaitz CM, Wullbrandt B, Sexton J. Association of dental health parameters with oral lesion prevalence in human immunodeficency virus-infected Romanian children. Pediatr Dent. (2003) 25:479–84.
28. Domaneschi C, Massarente DB, De Freitas RS, de Sousa Marques HH, Paula CR, Migliari DA, et al. Oral colonization by Candida species in AIDS pediatric patients. Oral Dis. (2011) 17:393–8. doi: 10.1111/j.1601-0825.2010.01765.x
29. Gaitán-Cepeda L, Morales J, Sánchez-Vargas L, Aquino-Garcia S, Fragoso-Ríos R, Cuairán-Ruidiaz V, et al. Prevalence of oral lesions in Mexican children with perinatally acquired HIV: association with immunologic status, viral load, and gender. AIDS Patient Care STDS. (2002) 16:151–6. doi: 10.1089/10872910252930858
30. Gaitán-Cepeda LA, Domínguez-Sánchez A, Pavía-Ruz N, Muñoz-Hernández R, Verdugo-Díaz R, Valles-Medina AM, et al. Oral lesions in HIV+/AIDS adolescents perinatally infected undergoing HAART. Med Oral Patol Oral Cir Bucal. (2010) 15:e545–50. doi: 10.4317/medoral.15.e545
31. Khongkunthian P, Grote M, Isaratanan W, Piyaworawong S, Reichart PA. Oral manifestations in 45 HIV-positive children from Northern Thailand. J Oral Pathol Med. (2001) 30:549–52. doi: 10.1034/j.1600-0714.2001.300907.x
32. Meless D, Ba B, Faye M, Diby JS, N'zoré S, Datté S, et al. Oral lesions among HIV-infected children on antiretroviral treatment in West Africa. Trop Med Int Health. (2014) 19:246–55. doi: 10.1111/tmi.12253
33. Olufemi Olaniyi T, Sunday P. Oral manifestations of HIV infection in 36 Nigerian children. J Clin Pediatr Dent. (2006) 30:89–92. doi: 10.17796/jcpd.30.1.a75w1602n0x6577r
34. Pomarico L, Cerqueira DF, de Araujo Soares RM, de Souza IP, de Araujo Castro GF, Socransky S, et al. Associations among the use of highly active antiretroviral therapy, oral candidiasis, oral Candida species and salivary immunoglobulin A in HIV-infected children. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. (2009) 108:203–10. doi: 10.1016/j.tripleo.2009.05.008
35. Pongsiriwet S, Iamaroon A, Sriburee P, Pattanaporn K, Krisanaprakornkit S. Oral colonization of Candida species in perinatally HIV-infected children in northern Thailand. J Oral Sci. (2004) 46:101–5. doi: 10.2334/josnusd.46.101
36. dos SANTOS LD, Castro GF, de SOUZA IP, Oliveira RH. Oral manifestations related to immunosuppression degree in HIV-positive children. Braz Dent J. (2001) 12:135–8.
37. Vaseliu N, Carter AB, Kline NE, Kozinetz C, Cron SG, Matusa R, et al. Longitudinal study of the prevalence and prognostic implications of oral manifestations in Romanian children infected with human immunodeficiency virus type 1. Pediatr infect Dis J. (2005) 24:1067–71. doi: 10.1097/01.inf.0000190024.76795.bc
38. Gallottini Magalhães M, Franco Bueno D, Serra E, Gonçalves R. Oral manifestations of HIV positive children. J Clin Pediatr Dent. (2002) 25:103–6. doi: 10.17796/jcpd.25.2.f01k062j7315660v
39. Naidoo S, Chikte U. Oro-facial manifestations in paediatric HIV: a comparative study of institutionalized and hospital outpatients. Oral Dis. (2004) 10:13–8. doi: 10.1046/j.1354-523X.2003.00973.x
40. Nabbanja J, Gitta S, Peterson S, Rwenyonyi CM. Orofacial manifestations in HIV positive children attending Mildmay Clinic in Uganda. Odontol. (2013) 101:116–20. doi: 10.1007/s10266-012-0060-7
41. Shiboski CH, Wilson CM, Greenspan D, Hilton J, Greenspan JS, Moscicki AB. Adolescent Medicine HIV/AIDS Research Network. HIV-related oral manifestations among adolescents in a multicenter cohort study. J Adolesc Health. (2001) 29(3 Suppl):109–14. doi: 10.1016/S1054-139X(01)00280-4
42. Oyedeji OA, Gbolahan OO, Abe EO, Agelebe E. Oral and dental lesions in HIV infected Nigerian children. Pan Afr Med J. (2015) 20:287. doi: 10.11604/pamj.2015.20.287.5273
43. Adebola AR, Adeleke SI, Mukhtar M, Osunde OD, Akhiwu BI, Ladeinde A. Oral manifestation of HIV/AIDS infections in paediatric Nigerian patients. Niger Med J. (2012) 53:150–4. doi: 10.4103/0300-1652.104385
44. Rwenyonyi CM, Kutesa A, Muwazi L, Okullo I, Kasangaki A, Kekitinwa A. Oral manifestations in HIV/AIDS-infected children. Eur J Dent. (2011) 5:291–8. doi: 10.1055/s-0039-1698894
45. Fonseca R, Cardoso AS, Pomarico I. Frequency of oral manifestations in children infected with human immunodeficiency virus. Quintessence Int. (2000) 31:419–22.
46. Ramos-Gomez FJ, Petru A, Hilton JF, Canchola AJ, Wara D, Greenspan JS. Oral manifestations and dental status in paediatric HIV infection. Int J Paediatr Dent. (2000) 10:3–11. doi: 10.1046/j.1365-263x.2000.00170.x
47. Ranganathan K, Geethalakshmi E, Krishna mohan rao UM, Vidya KM, Kumarasamy N, Solomon S. Orofacial and systemic manifestations in 212 pediatric HIV patients from Chennai, South India. Int J Paediatr Dent. (2010) 20:276–82. doi: 10.1111/j.1365-263X.2010.01050.x
48. Sales-Peres SH, Mapengo MA, Moura-Grec PG, Marsicano JA, Sales-Peres AD, Sales-Peres A. Oral manifestations in HIV+ children in Mozambique. Ciênc saúde colet. (2012) 17:55–60. doi: 10.1590/S1413-81232012000100008
49. Ponnam SR, Srivastava G, Theruru K. Oral manifestations of human immunodeficiency virus in children: An institutional study at highly active antiretroviral therapy centre in India. J Oral Maxillofac Pathol. (2012) 16:195–202. doi: 10.4103/0973-029X.98499
50. de Aguiar Ribeiro A, Portela MB, de Souza IP. The oral health of HIV-infected Brazilian children. Int J Paediatr Dent. (2013) 23:359–65. doi: 10.1111/ipd.12008
51. Oliscovicz NF, Pomarico L, de Araújo Castro GF, Souza IP. Effect of highly active antiretroviral therapy use on oral manifestations in pediatric patients infected with HIV. Indian J Dent Res. (2015) 26:200–4. doi: 10.4103/0970-9290.159169
52. Baghirath PV, Krishna AB, Gannepalli A, Ali MM. Oral manifestations of HIV in children receiving anti-retroviral therapy in Hyderabad, India. Eur Arch Paediatr Dent. (2013) 14:14:389–95. doi: 10.1007/s40368-013-0028-5
53. Divakar DD, Al Kheraif AA, Ramakrishnaiah R, Khan AA, Sandeepa NC, Alshahrani OA, et al. Oral manifestations in human immunodeficiency virus infected pediatric patients receiving and not receiving antiretroviral therapy: a cross sectional study. Methods. (2015) 59:152–8. doi: 10.13112/PC.2015.23
54. Sánchez-Vargas LO, Ortiz-López NG, Villar M, Moragues MD, Aguirre JM, Cashat-Cruz M, et al. Prevalencia, microbiología y patrones de sensibilidad a los antifúngicos de los aislamientos orales de Candida que colonizaban o infectaban a pacientes mexicanos con infección por VIH o sida ya personas sanas. Rev Iberoam Micol. (2005) 22:83–92. doi: 10.1016/S1130-1406(05)70014-0
55. Portela MB, Souza IP, Costa EM, Hagler AN, Soares RM, Santos AL. Differential recovery of Candida species from subgingival sites in human immunodeficiency virus-positive and healthy children from Rio de Janeiro, Brazil. J Clin Microbil. (2004) 42:5925–7. doi: 10.1128/JCM.42.12.5925-5927.2004
56. Malele Kolisa Y, Yengopal V, Shumba K, Igumbor J. The burden of oral conditions among adolescents living with HIV at a clinic in Johannesburg, South Africa. PLoS One. (2019) 14:e0222568. doi: 10.1371/journal.pone.0222568
57. Appiah A, Gates HL. Encyclopedia of Africa. Oxford: Oxford University Press. (2010). p. 8. doi: 10.1093/acref/9780195337709.001.0001
58. Hampanda K. Vertical transmission of HIV in Sub-Saharan Africa: applying theoretical frameworks to understand social barriers to PMTCT. Int Scholarly Res Notices. (2013) 2013:420361. doi: 10.5402/2013/420361
59. Evans CA, Friedland JS, Gotuzzo E. HIV in South America. Medicine. (2005) 33:44–5. doi: 10.1383/medc.33.6.44.66003
60. Lourenço AG, Figueiredo LT. Oral lesions in HIV infected individuals from Ribeirão Preto, Brazil. Med Oral Patol Oral Cir Bucal. (2008) 13:E281–6.
61. Noce CW, Ferreira SM, Silva Júnior A, Dias EP. Association between socioeconomic status and HIV-associated oral lesions in Rio de Janeiro from 1997 to (2004). Braz Oral Res. (2009) 23:149–54. doi: 10.1590/S1806-83242009000200010
62. Naidu GS, Thakur R, Singh AK, Rajbhandary S, Mishra RK, Sagtani A. Oral lesions and immune status of HIV infected adults from eastern Nepal. J Clin Exp Dent. (2013) 5:e1–7. doi: 10.4317/jced.50888
63. Patel PK, Erlandsen JE, Kirkpatrick WR, Berg DK, Westbrook SD, Louden C, et al. The changing epidemiology of oropharyngeal candidiasis in patients with HIV/AIDS in the era of antiretroviral therapy. AIDS Res Treat. (2012) 2012:262471. doi: 10.1155/2012/262471
64. Tirwomwe JF, Rwenyonyi CM, Muwazi LM, Besigye B, Mboli F. Oral manifestations of HIV/AIDS in clients attending TASO clinics in Uganda. Clin Oral Invest. (2007) 11:289–92. doi: 10.1007/s00784-007-0118-z
65. Nugraha AP, Ernawati DS, Parmadiati AE, Soebadi B, Triyono EA, Prasetyo RA, et al. Prevalence of candida species in oral candidiasis and correlation with CD4+ count in HIV/AIDS patients at Surabaya, Indonesia. J Int Dent Medical Res. (2018) 11:81–5.
66. Shetty K, Leigh J. The changing face of oral lesions in HIV/AIDS patients undergoing highly active antiretroviral treatment. AIDS Patient Care STDS. (2000) 14:627–35. doi: 10.1089/10872910050206540
67. Eyeson JD, Warnakulasuriya KA, Johnson NW. Prevalence and incidence of oral lesions - the changing scene. Oral Dis. (2000) 6:267–73. doi: 10.1111/j.1601-0825.2000.tb00137.x
68. Marcus M, Maida CA, Freed JR, Younai F, Coulter ID, Der-Martirosian C, et al. Oral white patches in a national sample of medical HIV patients in the era of HAART. Community Dent Oral Epidemiol. (2005) 33:99–106. doi: 10.1111/j.1600-0528.2004.00171.x
69. Ramos-Gomez FJ, Flaitz C, Catapano P, Murray P, Milnes AR, Dorenbaum A. Classification, diagnostic criteria, and treatment recommendations for orofacial manifestations in HIV-infected pediatric patients. collaborative workgroup on oral manifestations of pediatric HIV infection. J Clin Pediatr Dent. (1999) 23:85–96.
70. Sasani E, Rafat Z, Ashrafi K, Salimi Y, Zandi M, Soltani S, et al. Vulvovaginal candidiasis in Iran: a systematic review and meta-analysis on the epidemiology, clinical manifestations, demographic characteristics, risk factors, etiologic agents and laboratory diagnosis. Microb pathog. (2021) 154:104802. doi: 10.1016/j.micpath.2021.104802
Keywords: HIV, aids, pediatrics, children, infants, oral candidiasis, prevalence, meta-analysis
Citation: Rafat Z, Sasani E, Salimi Y, Hajimohammadi S, Shenagari M and Roostaei D (2021) The Prevalence, Etiological Agents, Clinical Features, Treatment, and Diagnosis of HIV-Associated Oral Candidiasis in Pediatrics Across the World: A Systematic Review and Meta-Analysis. Front. Pediatr. 9:805527. doi: 10.3389/fped.2021.805527
Received: 30 October 2021; Accepted: 07 December 2021;
Published: 24 December 2021.
Edited by:
Tauqeer Hussain Mallhi, Al Jouf University, Saudi ArabiaReviewed by:
Hamid Badali, Mazandaran University of Medical Sciences, IranShi-Bing Liang, Beijing University of Chinese Medicine, China
Copyright © 2021 Rafat, Sasani, Salimi, Hajimohammadi, Shenagari and Roostaei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammad Shenagari, c2hlbmFnYXJpQGd1bXMuYWMuaXI=; c2hlbmFnYXJpQGdtYWlsLmNvbQ==
 Zahra Rafat
Zahra Rafat Elahe Sasani
Elahe Sasani Yahya Salimi
Yahya Salimi Samaneh Hajimohammadi
Samaneh Hajimohammadi Mohammad Shenagari
Mohammad Shenagari Davoud Roostaei
Davoud Roostaei