- 1Department of Neonatology, KK Women's and Children's Hospital, Singapore, Singapore
- 2KK Research Centre, KK Women's and Children's Hospital, Singapore, Singapore
- 3Centre for Quantitative Medicine, Duke-NUS Medical School, Singapore, Singapore
- 4Duke-NUS Medical School, Singapore, Singapore
- 5Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore
- 6Lee Kong Chian School of Medicine, Singapore, Singapore
Objective: To determine the risk factors for mortality associated with late onset sepsis (LOS) among preterm very-low-birthweight (VLBW) infants.
Study Design: We performed a retrospective cohort study of infants born <32 weeks gestation and <1,500 gm admitted to a Singaporean tertiary-level neonatal intensive care unit. We determined the clinical, microbial, and laboratory risk factors associated with mortality due to culture-positive LOS in this cohort.
Results: A total of 1,740 infants were admitted, of which 169 (9.7%) developed LOS and 27 (16%) died. Compared to survivors, those who died had lower birth gestational age (median 24 vs. 25 weeks, p = 0.02) and earlier LOS occurrence (median 10 vs. 17 days, p = 0.007). There was no difference in the incidence of meningitis (11.1 vs. 16.9%, p = 0.3), NEC (18.5 vs. 14.8%, p = 0.6), or intestinal surgery (18.5 vs. 23.3%, p = 0.6) among infants who died compared to survivors. Gram-negative bacteria accounted for 21/27 (77.8%) LOS-associated deaths and almost all (13/14, 93%) fulminant episodes. The presence of multiorgan failure, as evidenced by the need for mechanical ventilation (100 vs. 79.0%, p = 0.008), elevated lactate (12.4 vs. 2.1 mmol/L, p < 0.001), and inotropic support (92.6 vs. 37.5%, p < 0.001), was significantly associated with mortality. Infants who died had significantly lower white blood cell (WBC) counts (median 4.2 × 109/L vs. 9.9 × 109/L, p = 0.001), lower platelet count (median 40 × 109/L vs. 62 × 109/L, p = 0.01), and higher immature to total neutrophil (I: T) ratio (0.2 vs. 0.1, p = 0.002). Inotrope requirement [AOR 22.4 (95%CI 2.9, 103.7)], WBC <4 × 109/L [AOR 4.7 (1.7, 13.2)], and I: T ratio >0.3 [AOR 3.6 (1.3, 9.7)] were independently associated with LOS mortality.
Conclusions: In a setting with predominantly Gram-negative bacterial infections, the need for inotropic support, leukopenia, and elevated I: T ratio were significantly associated with LOS mortality among preterm VLBW infants.
Introduction
Even as neonatal intensive care continues to improve, sepsis remains a significant cause of mortality and morbidity among preterm infants (1, 2). Based on published reports over the past decade, ~12–28% of VLBW infants developed LOS (3–5) and up to 18% of those infected died (1). Recent data on LOS incidences have revealed inconsistent incidence trends in different settings—with some reporting a decline (6, 7), where others have shown a stable to increased incidence (8–12).
Gram-positive bacteria, especially coagulase-negative staphylococci, are the prevalent group of pathogens reported as the cause of LOS in many neonatal intensive care units (NICUs) (1, 3, 13–16). Even so, there have been multiple reports of units with predominant, and increasing incidence of Gram-negative LOS infections among VLBW infants from different geographical settings (4, 10, 17–21). This difference in microbial distribution is particularly important as Gram-negative infections among these high-risk infants have been associated with increased risk of fulminant sepsis and morbidities including bronchopulmonary dysplasia and neurological impairment, compared to other microorganism subtypes (1, 9, 20, 22–24). Increasing Gram-negative neonatal bacterial infections are also particularly concerning in low- and middle-income countries due to growing concerns of increasing levels of multi-drug bacterial resistance in these settings (25).
An understanding of the risk factors associated with mortality among VLBW infants with LOS is important in guiding the formulation of strategies for the treatment and prognostication of outcomes due to LOS. As such, this current study aims to determine the clinical, microbial, and laboratory risk factors associated with mortality due to LOS among VLBW infants.
Methods
Study Design, Setting, and Participants
We performed a retrospective cohort study of VLBW (<1,500 g) infants born <32 completed weeks gestational age and admitted to the Neonatal Intensive Care Unit (NICU) at KK Women's and Children's Hospital (KKH), Singapore, over an 11-year period (Jan 1, 2006–Dec 31, 2016). Infants with major congenital anomalies, stillbirths, and labor-room deaths were excluded. KKH is an 830-bed referral hospital and is the largest tertiary-level perinatal center in Singapore, with 40 NICU and 50 special care nursery beds. KKH provides care for ~11,500 pregnant women and 1,200 preterm infants annually.
Data Sources
The KKH VLBW clinical database records maternal, perinatal, and neonatal information using a standardized data collection form for all live-born infants <1,500 g in the hospital. The data are cross-checked for completeness by an audit officer before submission into an electronic database. We identified all VLBW infants with positive blood and cerebrospinal (CSF) culture results from our hospital microbiology database and performed a data linkage with our VLBW database using unique national identification numbers (allocated to every baby born in Singapore).
Variables and Definitions
LOS was defined as clinical episodes with ≥1 positive blood and/or CSF culture obtained >72 h of life, in the presence of signs or symptoms suggestive of infection. The inclusion of positive CSF cultures in the definition is based on reports where 38% of infants with pathogens isolated from CSF had negative blood cultures (13, 26). Positive cultures with coagulase-negative staphylococci, Micrococcus, Bacillus, Corynebacterium, and Propionibacterium species were considered contaminants unless ≥2 cultures were positive for the organism and/or the infant showed signs of sepsis and received intravenous antibiotics for ≥5 days. The decision to obtain blood cultures and to commence antibiotics was at the discretion of the attending physician. A minimum of 1 ml of peripheral blood was routinely obtained using an aseptic technique from the infant for blood cultures. A separate LOS episode was considered if the infant developed signs of sepsis with a positive blood culture after completion of >10-days of appropriate antibiotics. Meningitis was diagnosed if there were CNS-related symptoms and either a positive CSF culture or increased CSF white cell count (>20 × 106/μL) with a positive blood culture. Mortality was attributed to LOS if it was designated as the primary cause of death by the attending physician. Fulminant sepsis was defined as death due to sepsis that occurred <72 h from antibiotics commencement (27). The empiric antibiotic regimen for LOS in our department typically includes cloxacillin and gentamicin, with escalation to cephalosporin or carbapenem if clinically indicated.
Gestational age is defined as the best obstetric estimate of completed weeks based on obstetric history, clinical examination, and antenatal ultrasound. An infant is small for gestational age if birth weight is <10th percentile according to the Fenton growth charts (28). Histologic chorioamnionitis is defined as the presence of inflammatory cells in the chorioamniotic membrane, umbilical cord, and/or the placental disc (29). Necrotizing enterocolitis (NEC) was confirmed by diagnosis at surgery/postmortem or by radiologic diagnosis with a consistent clinical history (30). Severe intraventricular hemorrhage was defined as Stage 3 and above (31).
Data on clinical course during the LOS episode was collected over a 72-h period (1 day prior to sepsis onset up to 1 day after). In this study, tachycardia was defined as heart-rate >180/min without any external stimulus or influence from drug therapy, and considered persistent if there were >2 episodes recorded within a 4-h period (32, 33). Apnea was defined as cessation of breathing for >20 s, or >10 s in the presence of bradycardia and/or desaturation (34). Temperature instability was defined as core body temperature <36°C or >38.5°C (35). Abnormal glucose levels were noted if <2.2 mmol/L or >7.8 mmol/L (35). Inotropic drug support during LOS included the usage of common inotropic agents (dopamine, dobutamine, adrenaline, and vasopressin) and/or the provision of corticosteroids for presumed adrenal insufficiency or catecholamine-resistant shock (36). Laboratory parameters, including the lowest and highest white blood cell count (WBC), platelet count, absolute neutrophil count (ANC), and the highest immature to total neutrophil (I: T) ratio during each LOS episode, were recorded. Multi-drug resistance was defined as antimicrobial resistance to at least three antimicrobial classes.
Statistical Analysis
Differences between categories were tested using χ2 or Fisher's exact test (where appropriate) and Mann-Whitney U-test for categorical and continuous variables, respectively. Subgroup analysis was performed for fulminant sepsis and microorganism subtype. Univariate and multivariable logistic regression analysis was performed to determine associated factors for death due to sepsis. Variables with p-value < 0.05 were selected for the multivariable model. Stepwise variable selection methods were used to finalize the multivariable model. Continuous variables were converted to categorical variable using Youden's J–index. Quantitative associations from logistic regression were reported as odds ratio (OR) or adjusted odds ratios (AOR) with 95% confidence intervals (95% CI). Analysis of incidence trends was performed using χ2 test for non-linear trend. All tests were two-sided and p–value < 0.05 was considered statistically significant. Statistical analyses were performed using IBM SPSS Statistics version 23.0 (IBM Corporation, New York).
Results
Perinatal Characteristics
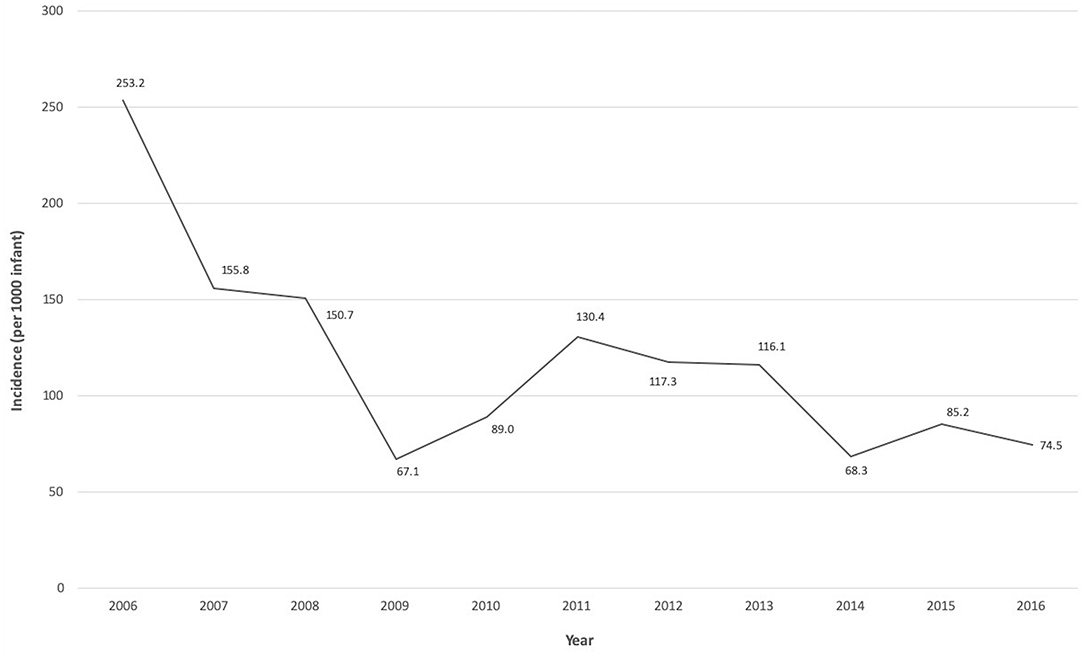
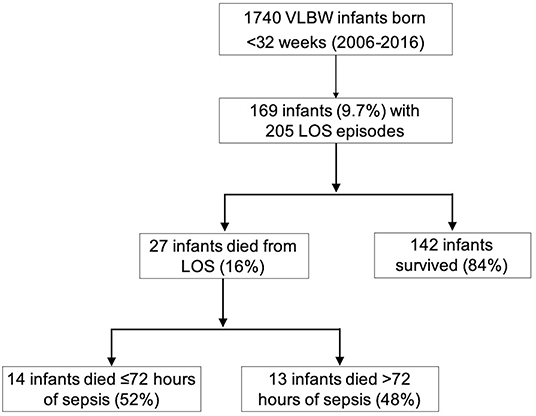
Over the 11-year period, 1,740 VLBW infants <32 weeks gestation were admitted to the NICU. A total of 169 infants (9.7%) developed LOS, with an overall rate of 118.9 infections [standard deviation (SD) 54.5] per 1,000 infants. The LOS incidence significantly decreased over the study period from 253.2 per 1,000 infants in 2006 to 74.5 per 1,000 infants in 2016 (p = 0.009 for analysis of trends) (Figure 1). About half of the infants with LOS (54.4%) were born via vaginal delivery, 79.3% were <28 weeks gestation, 85.2% had birthweight ≤ 1,000 g, 59.8% were male and 94.7% had insertion of a central venous catheter. Up to 88.2% of mothers from these infants received antenatal steroids, 51.5% had histologic placental chorioamnionitis, and 46.2% received intrapartum antibiotics. A total of 32 infants (19%) had ≥2 LOS episodes and 4 infants (2.4%) had 3 episodes. Among those with multiple LOS, all were <28 weeks gestation with median birthweight of 726 g [Interquartile range (IQR) 646–810]. All infants in this study (n = 169) received an average of 4 separate (SD 2) exposures to antibiotics for concerns of infections prior to initial discharge or death.
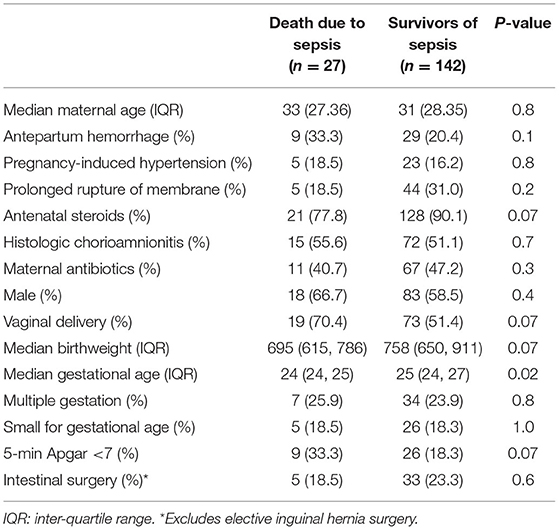
A total of 27 (16%) infants died due to complications of LOS (Figure 2). The proportion of LOS deaths over the total number of LOS cases over the first 5 years was not different compared to the latter 5 years [14/95 (14.7%) vs. 11/64 (17.3%), p = 0.8). Compared to LOS survivors, those who died were born at lower gestational age (median 24 vs. 25 weeks, p = 0.02) (Table 1), and had earlier LOS occurrence (median age 10 vs. 17 days, p = 0.007). There was no difference in the incidence of meningitis (11.1 vs. 16.9%, p = 0.3), NEC (18.5 vs. 14.8%, p = 0.6), or intestinal surgery (18.5 vs. 23.3%, p = 0.6) among infants who died compared to survivors (Table 1; Supplementary Table 1). Slightly more than half of the LOS deaths (14/27, 52%) were fulminant. Comparing infants with fulminant and non-fulminant sepsis, there were generally no significant differences in pregnancy complications, antenatal interventions, and neonatal characteristics (Supplementary Table 2), and no difference in bacteremia onset (median 8.5 vs. 9 days of life, respectively, p = 0.5). A smaller proportion of infants with fulminant sepsis were born via vaginal delivery compared to those without (50 vs. 92.3%, p = 0.02). Among infants who died from non-fulminant sepsis, the median duration from sepsis onset to death for this group was 5 days (range 4–18).
Microorganisms
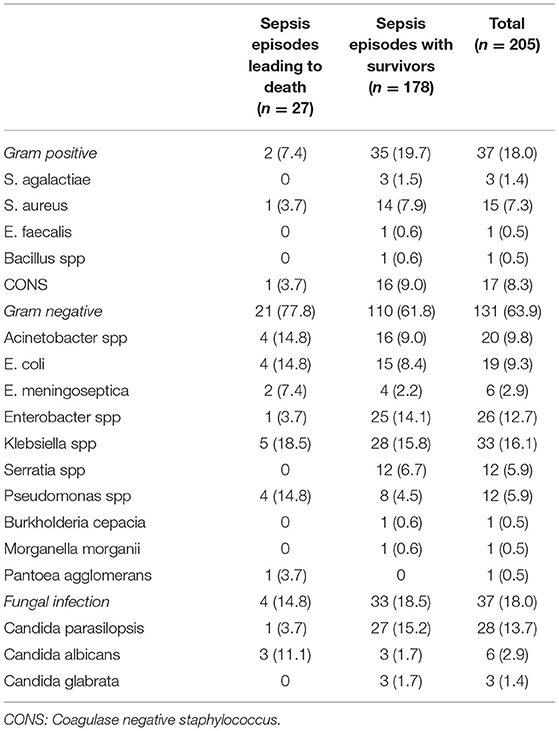
Gram-negative bacteria were the most common microorganisms isolated with each LOS episode (131/205, 64%), with similar proportions of Gram-positive and fungal organisms (37/205, 18%) (Table 2). Gram-negative infections were also more common among sepsis episodes leading to death, accounting for 77.8% (21/27) of such episodes compared to 61.8% (110/178) of episodes among survivors (p = 0.1). Klebsiella spp was the most common causative bacteria in both groups. Gram-positive infections were more common among LOS episodes with survivors compared to those resulting in death (19.7 vs. 7.4%, p = 0.2), with Coagulase negative staphylococcus being the predominant organism. Candida spp accounted for all the fungal infections in our population with Candida parasilopsis being the most common. Among fulminant LOS episodes, almost all were Gram-negative bacteria (13/14, 93%) with Acinetobacter, Klebsiella, Pseudomonas spp (2 infections (21.4%) each) being the most common.
There was no difference in the incidence of multi-drug resistance in infecting microorganism between infants who died compared to survivors of LOS (26 vs. 17%, p = 0.2). For the six most common groups of Gram-negative bacteria isolated (Acinetobacter spp, E. coli, Enterobacter spp, Klebsiella spp, Serratia spp, Pseudomonas spp), there was no significant difference in the incidence of gentamicin resistance (47.6 vs. 38.9%, p = 0.5) and cefotaxime/ceftriaxone resistance (61.8 vs. 44.4%, p = 0.2) between infants who died compared to survivors, respectively. Only 1 out of 37 (2.7%) fungal LOS episode had prior exposure to cephalosporins therapy, compared to 45 out of 168 (26.8%) bacterial LOS episodes.
Clinical Course During LOS Episodes
Compared to those with survivors, episodes resulting in death were more likely to be complicated by seizures (59.3 vs. 6.3%, p ≤ 0.001), require mechanical ventilation (100 vs. 79%, p = 0.008), require inotropic support (92.6 vs. 37.5%, p ≤ 0.001), and have higher median lactate values (12.4 vs. 2.1 mmol/L, p < 0.001) (Table 3). The median number of inotropic agents required was also significantly higher in episodes leading to death compared to those with survivors (2 vs. 0, p ≤ 0.001).
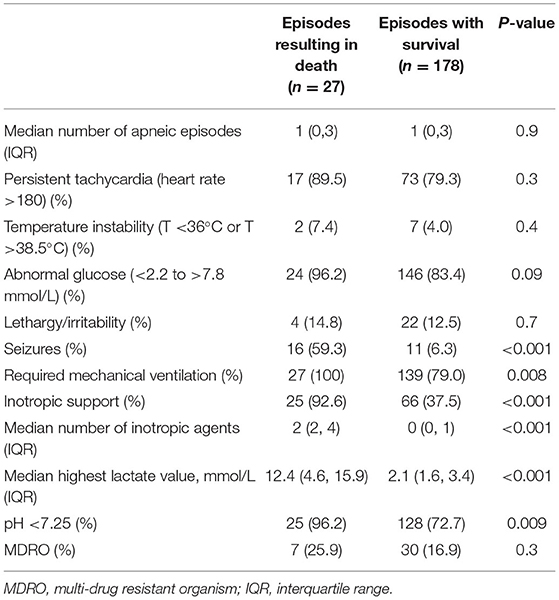
Table 3. Clinical course during late onset sepsis episodes stratified by those with survival and those resulting in death (n = 205).
Laboratory Findings
Compared to survivors, infants who died had significantly lower WBC [median 4.2 × 109/L (IQR 2.4–7.7) vs. 9.9 × 109/L (IQR 4.9–13.4), p = 0.001), lower platelet count [median 40 × 109/L (IQR 19–57) vs. 62 × 109/L (32–121), p = 0.01], lower ANC [median 1.2 cells/mm3 (IQR 0.6–3.6) vs. 4.2 cells/mm3 (IQR 1.7–7.3), p = 0.001], and higher I:T ratio [median 0.2 (IQR 0.2–0.4) vs. 0.1 (IQR 0.1–0.2), p = 0.002] (Figure 3). Infants with Gram-negative and invasive fungal infections were also found to have lower white cell count, lower platelet count, lower absolute neutrophil count, and higher I:T ratio compared to those with Gram-positive bacteremia (Supplementary Figure 1).

Figure 3. Comparisons of laboratory parameters between VLBW infants who died and infants who survived late onset sepsis. Comparisons of the following parameters during LOS episode: (A) lowest WBC, (B) lowest platelets, (C) lowest absolute neutrophil count, (D) highest I: T ratio. The open circles in the graphs indicate outliers.
Predictors of Death due to LOS
Using multivariable logistic regression analysis, we determined that inotrope requirement [AOR 22.4 (2.9, 103.7)], WBC <4 × 109/L [AOR 4.7 (1.7, 13.2)] and I: T ratio ≥0.3 [AOR 3.6 (1.3, 9.7)] were significantly associated with mortality from LOS in VLBW infants (Table 4).
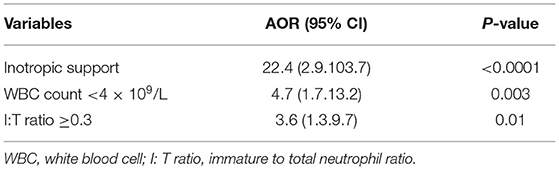
Table 4. Multivariable logistic regression model of clinical, microbiologic, and laboratory parameters associated with death due to LOS.
Discussion
Concurrent with trends of improved survival of VLBW infants over the past few decades (37) there have been varying reports on LOS incidence trends in this group of infants (6–12). Our decreasing LOS trends and the overall incidence of 118.9 per 1,000 infants over 11 years is comparable to other recent reports, with reported LOS incidence ranging from 72 to 339 episodes per 1,000 admissions (3–5, 20, 22). Similarly, the LOS mortality rate of 16% seen in our study is within the range seen in other reports, from 9 to 21% (1–4, 18, 38). Importantly, the proportion of LOS due to Gram-negative bacteria in our study (64%) is significantly higher than that in other reports (16–48%) (20, 24).
Available reports on LOS from individual neonatal units and neonatal networks from the US, Europe, Australia, and New Zealand (1–4, 6, 14, 16, 39) have reported a predominance of Gram-positive bacteria as a causal LOS microorganism among VLBW infants, with rates ranging from 55 to 84%. These data are in sharp contrast to our study, where Gram-negative bacteria is seen in up to 64% of LOS episodes and is associated with 78% of LOS mortality. Importantly, almost all (13/14, 93%) who had fulminant LOS were infected by a Gram-negative organism. The predominance of Gram-negative organisms in LOS have been reported in other units, many from NICUs in low- and middle-income countries (5, 18, 25, 40). Shah et al. hypothesized that the increasing usage of antibiotics in the antepartum and neonatal period may have contributed to the increasing trend of Gram-negative infections seen in these units (10). The antibiotic exposure during early life could lead to altered neonatal mucosal colonization and a predominance of Gram-negative bacteria, resulting in an increased risk of Gram-negative infections (41, 42). Also, prolonged and inappropriate antibiotic usage could lead to emergence of multidrug resistance Gram-negative bacteria which have been associated with increased risk of infectious complications and death (43). In our study, there was a relatively high exposure to antibiotics among our preterm infants, where almost half of our infants (46.1%) had exposure to intrapartum antibiotics and each infant received an average of four courses of antibiotics during their admission. However, we did not find significant difference in the rates of multi-drug resistance between infants who died compared to survivors of sepsis.
Previous studies have associated Gram-negative LOS infections with increased risk of fulminant sepsis (1, 24). In a retrospective 10-year single-center study from the US, Karlowicz et al. reported that Gram-negative organisms accounted for 69% of fulminant sepsis cases with Pseudomonas spp accounting for up to 56% of fulminant LOS episodes (24). These findings are consistent with our study which found that Pseudomonas, Klebsiella, and Acinetobacter spp accounted for up to 64% of all fulminant LOS episodes. The independent effect of infecting Gram-negative organisms on early LOS mortality was previously reported, with Pseudomonas, Klebsiella, and Serratia being associated with the highest risk of early mortality (44). The differential risks for fulminant mortality may be related to the potential of selected organism types to form biofilms, exhibit multi-drug resistance, and demonstrate hypervirulence (45).
Studies have reported an invasive fungal infection incidence of 2.4–18%, and a mortality rate of 26–33% (1, 3–5, 14, 23, 38). The incidence of fungal sepsis in this study was comparable but we reported a lower mortality rate. The incidence of fungal infection in our cohort is likely modulated by the introduction of fluconazole prophylaxis in our center in 2009. Most episodes of fungal sepsis occurred prior to this change, with only 3 episodes occurring after the start of fungal prophylaxis of all infants <800 g and/or those with invasive intravenous central catheter. This observation is consistent with current evidence indicating the efficacy of antifungal prophylaxis in reducing invasive candidiasis in preterm infants.
Infants who died from LOS were of significantly lower gestational age and had earlier occurrence of sepsis. It is well-established that infants of lower gestational age are more likely to require mechanical ventilation (46), longer duration of central venous catheter (15), increased antimicrobial exposure (47), and longer duration of hospitalization, which renders them more susceptible to nosocomial infections. Even so, gestational age or timing of LOS were not independently associated with death due to sepsis in our analysis. We determined that the need for inotropes, significant neutropenia, and an elevated I: T ratio were all independently associated with death due to LOS. Progression toward multi-organ injury and dysfunction have been recently evaluated to predict LOS mortality among preterm, VLBW infants (27, 36, 48). We similarly found that LOS episodes resulting in death were associated with increased need for mechanical ventilation and inotropic support. Additionally, we noted a higher likelihood of neurological dysfunction with seizures and early evidence of metabolic dysfunction with elevated lactate and significant acidosis among those who died. In our analysis, the need for inotropic support was the most important factor associated with the risk of death among preterm VLBW infants with LOS, consistent with findings in other studies (36, 48).
Compared to survivors of LOS in our study, infants who died had significantly lower white cell counts, lower absolute neutrophil counts, lower platelet counts, and higher I:T ratio. These changes are attributed to increased neutrophil demargination during infection combined with increased cellular destruction in the peripheral circulation and bone marrow depression, resulting in reduced numbers of mature neutrophils (38). The preterm infant's reduced ability to accelerate neutrophil and thrombocyte production during infection likely compounds the derangement in the counts (23, 49–51). The low G-CSF levels seen in neonatal sepsis also likely contributes to these findings (51). Consistent with our results, previous studies have also reported that infants had higher incidence of neutropenia (52), higher I:T ratio (53), and lower platelet count (49), especially in Gram-negative infections. These derangements in laboratory parameters are potentially important in the clinical setting as an indicator of increased risk of disease severity and mortality in LOS.
Our study was limited by the small sample size and the single center design. Even so, our cohort involving >200 LOS episodes analyzed over an 11-year period provided a reasonable number from which detailed perinatal, clinical data from the LOS episodes could be obtained and important inferences can be made. However, the lower numbers of LOS bacteremia precluded us from undertaking further analysis to determine differential risk of infection and mortality between different types and subgroups of bacteria. Other limitations include the lack of data on breastmilk intake rates, which is known to reduce the risk of sepsis and allow for the development of the appropriate intestinal flora and microbial predominance among preterm infants.
Conclusion
In our setting with a predominance of Gram-negative bacterial LOS among preterm VLBW infants, having any requirement for inotropic support, low WBC <4 × 109/L and an I: T ratio ≥0.3 are significantly associated with increased risk of death. Our data also suggests that empiric antimicrobial coverage for LOS in similar settings needs to treat Gram-negative organisms broadly and effectively.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by SingHealth Centralized Institutional Review Board (CIRB No. 2017/2761). Written informed consent from the participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author Contributions
GG and KY conceptualized and designed the study, coordinated and acquired the data, carried out the initial analyses, drafted the initial manuscript, and critically revised the manuscript. CL and RD coordinated and contributed to the acquisition of data, and critically revised the manuscript. RS was responsible for the statistical analysis, data interpretation, and critical revision of the manuscript. VR contributed to the analysis, interpretation of data, and critical revision of the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank the patients, physicians, and nurses for contributing to the very low-birth-weight database at the Department of Neonatology, KK Women's and Children's Hospital. Also, we would like to acknowledge Prof. Ju-Lee Oei for her constructive feedback on earlier versions of the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2021.801955/full#supplementary-material
Supplementary Table 1. Selected major neonatal morbidities of VLBW infants with late onset sepsis.
Supplementary Table 2. Comparison of perinatal characteristics of VLBW infants with fulminant and non-fulminant late onset sepsis (n = 27).
Supplementary Figure 1. Comparisons of laboratory parameters associated with different infecting microorganism subtypes. Comparisons of the following parameters during LOS episode: (A) lowest WBC, (B) lowest platelets, (C) lowest absolute neutrophil count, (D) highest I: T ratio.
References
1. Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD neonatal research network. Pediatrics. (2002) 110:285–91. doi: 10.1542/peds.110.2.285
2. Gordon A, Isaacs D. Late onset neonatal gram-negative bacillary infection in Australia and New Zealand: 1992-2002. Pediatr Infect Dis J. (2006) 25:25–9. doi: 10.1097/01.inf.0000195628.35980.2e
3. Hornik CP, Fort P, Clark RH, Watt K, Benjamin DK Jr, Smith PB, et al. Early and late onset sepsis in very-low-birth-weight infants from a large group of neonatal intensive care units. Early Hum Dev. (2012) 88(Suppl. 2):S69–74. doi: 10.1016/S0378-3782(12)70019-1
4. Ran NC, van den Hoogen A, Hemels MAC. Gram-negative late-onset sepsis in extremely low birth weight infants is emerging in the netherlands despite quality improvement programs and antibiotic stewardship! Pediatr Infect Dis J. (2019) 38:952–7. doi: 10.1097/INF.0000000000002408
5. Boo NY, Cheah IG. Factors associated with inter-institutional variations in sepsis rates of very-low-birth-weight infants in 34 Malaysian neonatal intensive care units. Singapore Med J. (2016) 57:144–52. doi: 10.11622/smedj.2016056
6. Greenberg RG, Kandefer S, Do BT, Smith PB, Stoll BJ, Bell EF, et al. Late-onset sepsis in extremely premature infants: 2000-2011. Pediatr Infect Dis J. (2017) 36:774–9. doi: 10.1097/INF.0000000000001570
7. Kaplan HC, Lannon C, Walsh MC, Donovan EF, Ohio Perinatal Quality C. Ohio statewide quality-improvement collaborative to reduce late-onset sepsis in preterm infants. Pediatrics. (2011) 127:427–35. doi: 10.1542/peds.2010-2141
8. Dong Y, Speer CP. Late-onset neonatal sepsis: recent developments. Arch Dis Child Fetal Neonatal Ed. (2015) 100:F257–63. doi: 10.1136/archdischild-2014-306213
9. Tsai MH, Hsu JF, Chu SM, Lien R, Huang HR, Chiang MC, et al. Incidence, clinical characteristics and risk factors for adverse outcome in neonates with late-onset sepsis. Pediatr Infect Dis J. (2014) 33:e7–13. doi: 10.1097/INF.0b013e3182a72ee0
10. Shah SS, Ehrenkranz RA, Gallagher PG. Increasing incidence of gram-negative rod bacteremia in a newborn intensive care unit. Pediatr Infect Dis J. (1999) 18:591–5. doi: 10.1097/00006454-199907000-00005
11. Bizzarro MJ, Raskind C, Baltimore RS, Gallagher PG. Seventy-five years of neonatal sepsis at yale: 1928-2003. Pediatrics. (2005) 116:595–602. doi: 10.1542/peds.2005-0552
12. van den Hoogen A, Gerards LJ, Verboon-Maciolek MA, Fleer A, Krediet TG. Long-term trends in the epidemiology of neonatal sepsis and antibiotic susceptibility of causative agents. Neonatology. (2010) 97:22–8. doi: 10.1159/000226604
13. Berardi A, Sforza F, Baroni L, Spada C, Ambretti S, Biasucci G, et al. Epidemiology and complications of late-onset sepsis: an Italian area-based study. PLoS ONE. (2019) 14:e0225407. doi: 10.1371/journal.pone.0225407
14. Makhoul IR, Sujov P, Smolkin T, Lusky A, Reichman B. Epidemiological, clinical, and microbiological characteristics of late-onset sepsis among very low birth weight infants in Israel: a national survey. Pediatrics. (2002) 109:34–9. doi: 10.1542/peds.109.1.34
15. Sanderson E, Yeo KT, Wang AY, Callander I, Bajuk B, Bolisetty S, et al. Dwell time and risk of central-line-associated bloodstream infection in neonates. J Hosp Infect. (2017) 97:267–74. doi: 10.1016/j.jhin.2017.06.023
16. Vergnano S, Menson E, Kennea N, Embleton N, Russell AB, Watts T, et al. Neonatal infections in England: the NeonIN surveillance network. Arch Dis Child Fetal Neonatal Ed. (2011) 96:F9–14. doi: 10.1136/adc.2009.178798
17. Hammoud MS, Al-Taiar A, Al-Abdi SY, Bozaid H, Khan A, AlMuhairi LM, et al. Late-onset neonatal sepsis in Arab states in the gulf region: two-year prospective study. Int J Infect Dis. (2017) 55:125–30. doi: 10.1016/j.ijid.2017.01.006
18. Tiskumara R, Fakharee SH, Liu CQ, Nuntnarumit P, Lui KM, Hammoud M, et al. Neonatal infections in Asia. Arch Dis Child Fetal Neonatal Ed. (2009) 94:F144–8. doi: 10.1136/adc.2008.139865
19. Bai X, Wei Q, Duan T, Yi Y, Peng H, Hu L. Predominance of gram-negative infections a cause of neonatal sepsis among low birth weight preterm infants. J Lab Med. (2021) 45:7–12. doi: 10.1515/labmed-2020-0022
20. Dong Y, Glaser K, Speer CP. Late-onset sepsis caused by gram-negative bacteria in very low birth weight infants: a systematic review. Expert Rev Anti Infect Ther. (2019) 17:177–88. doi: 10.1080/14787210.2019.1568871
21. Al-Taiar A, Hammoud MS, Cuiqing L, Lee JK, Lui KM, Nakwan N, et al. Neonatal infections in China, Malaysia, Hong Kong and Thailand. Arch Dis Child Fetal Neonatal Ed. (2013) 98:F249–55. doi: 10.1136/archdischild-2012-301767
22. Shah J, Jefferies AL, Yoon EW, Lee SK, Shah PS, Canadian Neonatal N. Risk factors and outcomes of late-onset bacterial sepsis in preterm neonates born at <32 weeks' gestation. Am J Perinatol. (2015) 32:675–82. doi: 10.1055/s-0034-1393936
23. Kaufman D, Fairchild KD. Clinical microbiology of bacterial and fungal sepsis in very-low-birth-weight infants. Clin Microbiol Rev. (2004) 17:638–80. doi: 10.1128/CMR.17.3.638-680.2004
24. Karlowicz MG, Buescher ES, Surka AE. Fulminant late-onset sepsis in a neonatal intensive care unit, 1988-1997, and the impact of avoiding empiric vancomycin therapy. Pediatrics. (2000) 106:1387–90. doi: 10.1542/peds.106.6.1387
25. Wen SCH, Ezure Y, Rolley L, Spurling G, Lau CL, Riaz S, et al. Gram-negative neonatal sepsis in low- and lower-middle-income countries and WHO empirical antibiotic recommendations: a systematic review and meta-analysis. PLoS Med. (2021) 18:e1003787. doi: 10.1371/journal.pmed.1003787
26. Shane AL, Stoll BJ. Recent developments and current issues in the epidemiology, diagnosis, and management of bacterial and fungal neonatal sepsis. Am J Perinatol. (2013) 30:131–41. doi: 10.1055/s-0032-1333413
27. Wynn JL, Kelly MS, Benjamin DK, Clark RH, Greenberg R, Benjamin DK, et al. Timing of multiorgan dysfunction among hospitalized infants with fatal fulminant sepsis. Am J Perinatol. (2017) 34:633–9. doi: 10.1055/s-0036-1597130
28. Fenton TR, Kim JH. A systematic review and meta-analysis to revise the fenton growth chart for preterm infants. BMC Pediatr. (2013) 13:59. doi: 10.1186/1471-2431-13-59
29. Khong TY, Mooney EE, Ariel I, Balmus NC, Boyd TK, Brundler MA, et al. Sampling and definitions of placental lesions: amsterdam placental workshop group consensus statement. Arch Pathol Lab Med. (2016) 140:698–713. doi: 10.5858/arpa.2015-0225-CC
30. Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am. (1986) 33:179–201. doi: 10.1016/S0031-3955(16)34975-6
31. Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. (1978) 92:529–34. doi: 10.1016/S0022-3476(78)80282-0
32. Hofer N, Zacharias E, Muller W, Resch B. Performance of the definitions of the systemic inflammatory response syndrome and sepsis in neonates. J Perinat Med. (2012) 40:587–90. doi: 10.1515/jpm-2011-0308
33. Wynn JL, Wong HR. Pathophysiology and treatment of septic shock in neonates. Clin Perinatol. (2010) 37:439–79. doi: 10.1016/j.clp.2010.04.002
34. Eichenwald EC. American Academy of Pediatrics Committee on Fetal and Newborn. Apnea of Prematurity. Pediatrics. (2016) 137:e20153757. doi: 10.1542/peds.2015-3757
35. Husada D, Chanthavanich P, Chotigeat U, Sunttarattiwong P, Sirivichayakul C, Pengsaa K, et al. Predictive model for bacterial late-onset neonatal sepsis in a tertiary care hospital in Thailand. BMC Infect Dis. (2020) 20:151. doi: 10.1186/s12879-020-4875-5
36. Wynn JL, Polin RA. A neonatal sequential organ failure assessment score predicts mortality to late-onset sepsis in preterm very low birth weight infants. Pediatr Res. (2020) 88:85–90. doi: 10.1038/s41390-019-0517-2
37. Hug L, Alexander M, You D, Alkema L, Estimation UNI-aGfCM. National, regional, and global levels and trends in neonatal mortality between 1990 and 2017, with scenario-based projections to 2030: a systematic analysis. Lancet Glob Health. (2019) 7:e710–20. doi: 10.1016/S2214-109X(19)30163-9
38. Levit O, Bhandari V, Li FY, Shabanova V, Gallagher PG, Bizzarro MJ. Clinical and laboratory factors that predict death in very low birth weight infants presenting with late-onset sepsis. Pediatr Infect Dis J. (2014) 33:143–6. doi: 10.1097/INF.0000000000000024
39. Piening BC, Geffers C, Gastmeier P, Schwab F. Pathogen-specific mortality in very low birth weight infants with primary bloodstream infection. PLoS ONE. (2017) 12:e0180134. doi: 10.1371/journal.pone.0180134
40. Chaurasia S, Sivanandan S, Agarwal R, Ellis S, Sharland M, Sankar MJ. Neonatal sepsis in South Asia: huge burden and spiralling antimicrobial resistance. BMJ. (2019) 364:k5314. doi: 10.1136/bmj.k5314
41. Smith A, Saiman L, Zhou J, Della-Latta P, Jia H, Graham PL 3rd. Concordance of gastrointestinal tract colonization and subsequent bloodstream infections with gram-negative bacilli in very low birth weight infants in the neonatal intensive care unit. Pediatr Infect Dis J. (2010) 29:831–5. doi: 10.1097/INF.0b013e3181e7884f
42. Patel SJ, Green N, Clock SA, Paul DA, Perlman JM, Zaoutis T, et al. Gram-negative bacilli in infants hospitalized in the neonatal intensive care unit. J Pediatric Infect Dis Soc. (2017) 6:227–30. doi: 10.1093/jpids/piw032
43. Tsai MH, Chu SM, Hsu JF, Lien R, Huang HR, Chiang MC, et al. Risk factors and outcomes for multidrug-resistant gram-negative bacteremia in the NICU. Pediatrics. (2014) 133:e322–9. doi: 10.1542/peds.2013-1248
44. Makhoul IR, Sujov P, Smolkin T, Lusky A, Reichman B, Israel Neonatal N. Pathogen-specific early mortality in very low birth weight infants with late-onset sepsis: a national survey. Clin Infect Dis. (2005) 40:218–24. doi: 10.1086/426444
45. Khaertynov KS, Anokhin VA, Rizvanov AA, Davidyuk YN, Semyenova DR, Lubin SA, et al. Virulence factors and antibiotic resistance of klebsiella pneumoniae strains isolated from neonates with sepsis. Front Med. (2018) 5:225. doi: 10.3389/fmed.2018.00225
46. Rich W, Finer NN, Vaucher YE. Ten-year trends in neonatal assisted ventilation of very low-birthweight infants. J Perinatol. (2003) 23:660–3. doi: 10.1038/sj.jp.7210995
47. Schulman J, Dimand RJ, Lee HC, Duenas GV, Bennett MV, Gould JB. Neonatal intensive care unit antibiotic use. Pediatrics. (2015) 135:826–33. doi: 10.1542/peds.2014-3409
48. Fleiss N, Coggins SA, Lewis AN, Zeigler A, Cooksey KE, Walker LA, et al. Evaluation of the neonatal sequential organ failure assessment and mortality risk in preterm infants with late-onset infection. JAMA Netw Open. (2021) 4:e2036518. doi: 10.1001/jamanetworkopen.2020.36518
49. Guida JD, Kunig AM, Leef KH, McKenzie SE, Paul DA. Platelet count and sepsis in very low birth weight neonates: is there an organism-specific response? Pediatrics. (2003) 111:1411–5. doi: 10.1542/peds.111.6.1411
50. Melvan JN, Bagby GJ, Welsh DA, Nelson S, Zhang P. Neonatal sepsis and neutrophil insufficiencies. Int Rev Immunol. (2010) 29:315–48. doi: 10.3109/08830181003792803
51. Carr R, Modi N, Dore C. G-CSF and GM-CSF for treating or preventing neonatal infections. Cochrane Database Syst Rev. (2003) 3:CD003066. doi: 10.1002/14651858.CD003066
52. Sarkar S, Bhagat I, Hieber S, Donn SM. Can neutrophil responses in very low birth weight infants predict the organisms responsible for late-onset bacterial or fungal sepsis? J Perinatol. (2006) 26:501–5. doi: 10.1038/sj.jp.7211554
Keywords: neonatal sepsis, premature infant, mortality, risk factors, multi-organ dysfunction
Citation: Goh GL, Lim CSE, Sultana R, De La Puerta R, Rajadurai VS and Yeo KT (2022) Risk Factors for Mortality From Late-Onset Sepsis Among Preterm Very-Low-Birthweight Infants: A Single-Center Cohort Study From Singapore. Front. Pediatr. 9:801955. doi: 10.3389/fped.2021.801955
Received: 26 October 2021; Accepted: 22 December 2021;
Published: 31 January 2022.
Edited by:
Bernhard Resch, Medical University of Graz, AustriaReviewed by:
Mary Catherine Harris, University of Pennsylvania, United StatesAlison Bedford Russell, Liverpool Women's Hospital, United Kingdom
Copyright © 2022 Goh, Lim, Sultana, De La Puerta, Rajadurai and Yeo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kee Thai Yeo, eWVvLmtlZS50aGFpQHNpbmdoZWFsdGguY29tLnNn
 Guan Lin Goh
Guan Lin Goh Charis Shu En Lim2
Charis Shu En Lim2 Rowena De La Puerta
Rowena De La Puerta Victor Samuel Rajadurai
Victor Samuel Rajadurai Kee Thai Yeo
Kee Thai Yeo