- 1Department of Neonatology, Fujian Maternity and Child Health Hospital, Affiliated Hospital of Fujian Medical University, Fuzhou, China
- 2Fujian Key Laboratory of Women and Children's Critical Diseases Research, Fujian Maternity and Child Health Hospital, Fuzhou, China
- 3Fujian Branch of Shanghai Children's Medical Center, Fuzhou, China
- 4Fujian Children's Hospital, Fuzhou, China
Objectives: The aim of this study was to investigate the safety and feasibility of nHFOV as initial respiratory support in preterm infants with RDS.
Methods: This study retrospectively analyzed the clinical data of 244 premature infants with RDS who were treated in our hospital from January 2016 to January 2019 and divided into the nHFOV group (n = 115) and the BiPAP group (n = 129) based on the initial respiratory support method.
Results: Respiratory outcomes showed that the rate of NIV failure during the first 72 hours of life in the nHFOV group was significantly lower than that in the BiPAP group. The time of NIV in the nHFOV group was significantly shorter than that in the BiPAP group. The time of supplemental oxygen in the nHFOV group was significantly shorter than that in the BiPAP group. The incidence of air leakage syndrome in the nHFOV group was significantly lower than that in the BiPAP group, and the length of hospital stay of the nHFOV group was also significantly shorter than that in the BiPAP group. Although the rate of infants diagnosed with BPD was similar between the two groups, the rate of severe BPD in the nHFOV group was significantly lower than that in the BiPAP group.
Conclusion: This study showed that nHFOV as initial respiratory support for preterm infants with RDS was feasible and safe compared to BiPAP. Furthermore, nHFOV can reduce the need for IMV and reduce the incidence of severe BPD and air leak syndrome.
Introduction
Respiratory distress syndrome (RDS) is one of the most common complications in preterm infants and the most common reason for premature death. A large proportion of preterm infants with RDS require invasive mechanical ventilation (IMV) at an early stage of life. Although ventilation is usually life-saving, it can also cause many complications, such as air leak syndrome, lung injury, and neurodevelopmental impairment (1–3). Neonatologists are increasingly using non-invasive ventilation (NIV) in the neonatal intensive care unit (NICU) to reduce these adverse effects of IMV; among them, biphasic positive airway pressure (BiPAP) is the classic non-invasive ventilation mode. Reports have shown that NIV is feasible in clinical practice and is associated with reducing the need for intubation and decreasing ventilator-related lung injury and other complications (2, 4). Non-invasive high-frequency oscillatory ventilation (nHFOV) is a promising new mode of NIV that can reduce the risk and complications of IMV (5, 6). However, there are few reports on the application of nHFOV for the treatment of premature infants with RDS. We hypothesized that nHFOV was safe and effective as an initial respiratory support for preterm infants with RDS and had more advantages than BiPAP. We conducted a retrospective controlled study to evaluate the efficacy, safety and advantages of nHFOV for the treatment of premature infants with RDS.
Methods
The present study was approved by the ethics committee of our hospital and adhered to the tenets of the Declaration of Helsinki. Additionally, all parents of the patients signed the consent form before participating in the study.
Patients
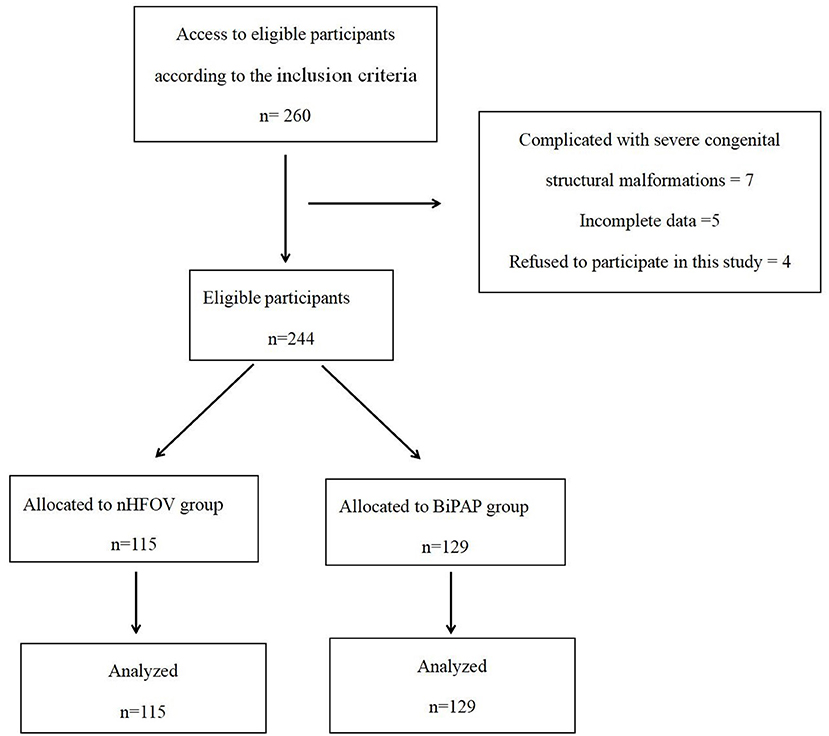
Our hospital started using nHFOV in January 2018, and then RDS initial respiratory support was gradually transitioned from the previous BiPAP to nHFOV. Therefore, BiPAP respiratory support was also used in some patients from 2018 and 2019. From January 2016 to January 2019, there was no change in other treatment except respiratory support. This study retrospectively analyzed the clinical data of 244 premature infants with RDS who were treated in our hospital from January 2016 to January 2019 and were divided into two groups based on the initial respiratory support methods. The nHFOV group had 115 premature infants who received nHFOV as initial respiratory support, and the BiPAP group had 129 premature infants who received BiPAP as initial respiratory support (Figure 1). All the patients were definitely diagnosed with RDS based on the diagnostic criteria. The diagnostic criteria of RDS are as follows (6): (1) High-risk factors: maternal diabetes during pregnancy, intrauterine infection, premature delivery, premature rupture of membranes for more than 24 h, intrauterine distress, asphyxia during delivery, etc. (2) Clinical symptoms: progressive aggravation of tachypnea within 6 h after birth (>60 times/min); cyanosis, three depressions in inhalation and obvious expiratory moans, irregular breathing, and apnoea; and decreased respiratory sounds in both lungs were detected on auscultation. (3) Typical chest X-ray features.
The inclusion criteria were as follows: (1) premature infants with gestational age of 25–34 weeks; (2) diagnosed with RDS within 24 h of birth and received nHFVO or BiPAP as initial respiratory support. The exclusion criteria were as follows: (1) complications involving severe congenital structural malformations such as congenital heart disease, congenital diaphragmatic hernia, respiratory tract malformation, and severe digestive tract malformation; (2) incomplete data; and (3) the parents of the infants refused to participate in this study.
Management in the Delivery Room
Drying, maintaining warmth and treating the umbilical cord immediately after birth were performed in the delivery room. Then, based on the breathing condition of the premature baby, we started respiratory support in the delivery room with nasal continuous positive airway pressure support or intermittent positive pressure ventilation after intubation. The initial management in the delivery room for all the babies in the two groups was the same.
Respiratory Management
Daily care was performed as needed for preterm infants in the NICU, along with continuous monitoring of pulse oxygen saturation, respiratory rate, and heart rate. nHFOV or BiPAP was started within 24 h of life with clinical signs of respiratory distress. Short binasal prongs (Infant Flow, CareFusion, California, USA) were used as the interface for the two NIV devices. The orogastric tube was kept open to decompress the stomach and to facilitate feeding. All of the preterm infants were given prophylactic caffeine on the first day of life. Caffeine was administered at a loading dose of 20 mg/kg caffeine citrate, and then a daily maintenance dose of 5–10 mg/kg coffee citrate was given. Our unit did not implement preventive use of surfactants. Surfactant requirements were assessed for all preterm infants. Porcine surfactant (Curosurf, Chiesi Farmaceutici, Parma, Italy) was administered as a rescue therapy via the intubation, surfactant therapy, extubation (INSURE) method if the infant required ≥ 0.40 fraction of inspired oxygen (FiO2) to maintain the target oxygen saturation level of 90–95%. The first dose of surfactant therapy was 200 mg/kg. Additional surfactant doses of 100 mg/kg were administered if the infants still required ≥ 0.40 FiO2 to maintain the target oxygen saturation.
In the nHFOV group, a neonatal non-invasive high-frequency ventilator from Medin CNO (Medical Innovations GmbH, Puchheim, Germany) with an output oxygen concentration range of 21 to 100% was used. The initial parameters were as follows: average airway pressure (MAP) was 8 cmH2O, frequency was 9 Hz, amplitude was adjusted to achieve sufficient chest oscillation at rest, and FiO2 was 0.4. Based on blood gas analysis and transcutaneous oxygen saturation (SpO2) adjustment parameters, FiO2 was adjusted by 0.05, MAP was adjusted by 1 cmH2O, and frequency was adjusted by 1 Hz each time.
For infants in the BiPAP group, a baseline PEEP of 5 cmH2O was established, with a high PEEP of 9 cmH2O, a respiratory rate of 30/min, and an initial inspiratory time of 0.5 s (CareFusion, California, USA). The baseline PEEP was adjusted to 4–7 cmH2O, and the high PEEP was adjusted to 7–10 cmH2O.
If the infant remained clinically stable at minimum respiratory parameters with nHFOV (MAP: 6 cmH2O; FiO2: 0.30) and BiPAP (cycle rate: 15 times/min, lower CPAP: 3 cmH2O; higher CPAP: 5 cmH2O; FiO2: 0.30), good respiratory effort and maintenance of an oxygen saturation level of 90–95%, conditions were switched to a heated humidified high-flow nasal cannula or nasal continuous positive airway pressure (NCPAP). If the infant under NCPAP had a CPAP level of 3 cmH2O and FiO2 <0.25 and tolerated the treatment well for at least 24 h with no evidence of apnoea, the infant was weaned to supplemental oxygen or room air.
Failure of NIV was defined as at least one of the following (7): severe respiratory acidosis (pH ≤ 7.20 and PaCO2 ≥ 60 mmHg); hypoxemia (PaO2 ≤ 50 mmHg and FiO2 ≥ 0.6); recurrent apnoea associated with bradycardia ≥ 3 times/hour; or a single episode of apnoea that required bag-and-mask ventilation, pneumothorax or intestinal perforation, severe respiratory distress, or pulmonary hemorrhage.
The definition of bronchopulmonary dysplasia (BPD) was any oxygen dependence (FiO2 > 21%) of the newborn lasting more than 28 days. Based on the judgement of oxygen dependence and different types of respiratory support measures, BPD can be categorized into three degrees: mild, moderate and severe. Preterm infants with a gestational age less than 32 weeks were assessed at 36 weeks postmenstrual or discharge, and preterm infants with a gestational age more than 32 weeks were assessed at 56 days after birth or discharge. BPD was considered (1) mild for patients without oxygen inhalation; (2) moderate for those requiring oxygen inhalation and FiO2 was <30%; and (3) severe for those requiring oxygen inhalation, FiO2 was ≥ 30% or positive pressure ventilation was needed (8).
Statistical Analysis
SPSS 25.0 was used for statistical analysis. Continuous data are presented as the mean ± standard deviation and range. With all continuous data, the normality of the distribution was tested, and they followed a normal distribution. Clinical parameters between the two groups were compared with independent samples t tests. χ2 tests were used to categorize the variables. A p value of < 0.05 was defined as significant.
Results
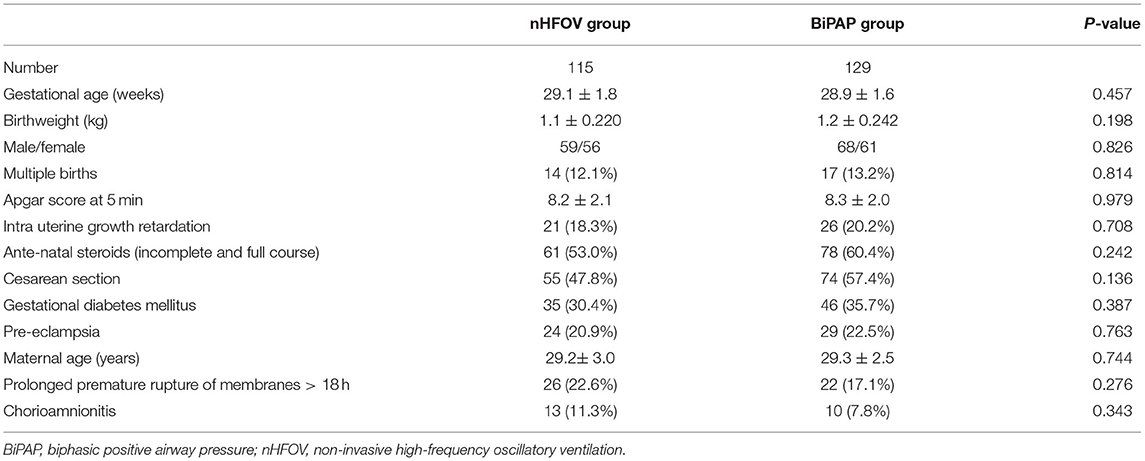
The general data, birth situation and perinatal data of the participants in the two groups are shown in Table 1; there were no statistically significant differences between the two groups, which indicated that the baseline characteristics between the two groups were similar (Table 1).
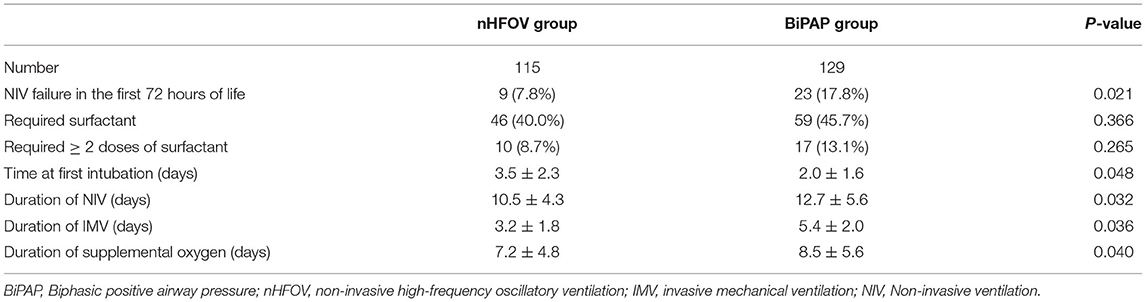
The respiratory outcomes showed that the rate of NIV failure in the first 72 h of life was significantly lower in the nHFOV group than in the BiPAP group (9 vs. 23, P = 0.021). The time of NIV in the nHFOV group was shorter than that in the BiPAP group (10.5 ± 4.3 vs. 12.7 ± 5.6, P = 0.032). Among infants with NIV failure, the time of first intubation in the BiPAP group was significantly earlier than that in the nHFOV group (2.0 ± 1.6 vs. 3.5 ± 2.3, P = 0.048), and the time of invasive ventilation in the nHFOV group was significantly shorter than that in the BiPAP group (3.2 ± 1.8 vs. 5.4 ± 2.0, P = 0.036). The time of supplemental oxygen in the nHFOV group was shorter than that in the BiPAP group (7.2 ± 4.8 vs. 8.5 ± 5.6, P = 0.040). The rate of the required surfactant was similar between the two groups (Table 2).
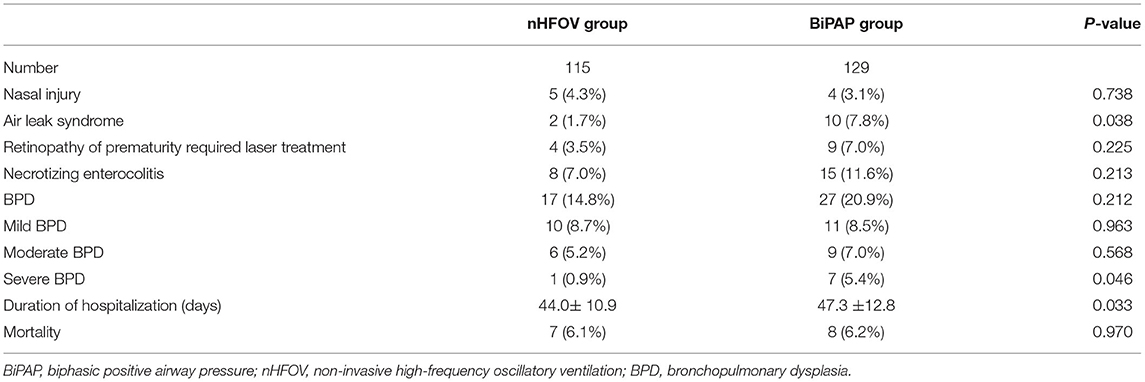
A comparison of the complications between the two groups showed that the incidence of air leakage syndrome in the nHFOV group was significantly lower than that in the BiPAP group (2 vs. 10, P = 0.038), and the length of hospital stay was also significantly shorter in the nHFOV group than in the BiPAP group (44.0 ± 10.9 vs. 47.3 ± 12.8, P = 0.033). Although the rate of infants diagnosed with BPD was similar between the two groups, the rate of severe BPD in the nHFOV group was significantly lower than that in the BiPAP group (1 vs. 7, P = 0.046). The incidence of nasal injury, retinopathy of prematurity requiring laser treatment, necrotizing enterocolitis, and mortality were similar between the two groups (Table 3).
Discussion
Various forms of NIV are increasingly being used by neonatologists because of the potential adverse consequences of IMV, particularly ventilator-induced lung injury, and this restricted use of IMV in preterm infants might decrease lung inflammation and reduce the incidence of BPD and death (9). The most commonly used NIV method is BiPAP, and BiPAP is an effective therapy in the early management of RDS in preterm infants (10). nHFOV, as a novel mode of non-invasive ventilation, can improve the removal of carbon dioxide with the advantages of high-frequency ventilation and NIV (11).
Studies have shown that nHFOV can reduce the need for IMV compared to other NIV techniques (12–14). In this study, we found that compared to BiPAP, nHFOV as initial respiratory support reduced the need for IMV within the first 72 h of life. We also found that nHFOV decreased the rate of intubation, shortened the duration of IMV and postponed the first intubation. Compared with other forms of NIV, nHFOV retains a non-invasive interface and maintains continuous airway pressure, and it can also increase functional residual volume, maintain the opening of the upper airway, and prevent alveolar collapse, which can improve ventilation and oxygenation (15). Mukerji et al. used a lung model to compare nHFOV with other forms of NIV, and the results showed that nHFOV could remove carbon dioxide more effectively, promote alveolar revascularization and reduce the rate of tracheal intubation compared with other forms of NIV (16). We suggested that the higher NIV failure rate in the BiPAP group than in the nHFOV group might also be due to BiPAP offering synchronized nasal intermittent positive pressure ventilation using an abdominal capsule, which may be difficult to synchronize with infant breathing and does not result in larger tidal volumes (17). However, with nHFOV, there is no need for synchronization. nHFOV may improve ventilation by enhancing alveolar recruitment by applying higher MAP, and the functional residual capacity was increased (18). On the other hand, despite restricting the use of MAP on nHFOV to 10 cmH2O, this was still higher than BiPAP for the corresponding FiO2 levels. Binmanee et al. (19) proved that the use of high NIV (MAP ≥ 10 cmH2O) resulted in the avoidance of intubation in the majority of cases, without adverse effects. Yaser et al. (20) also found a higher MAP in the successful group than in the failure group as a prophylactic or rescue mode of NIV following extubation. Therefore, these differences may be due to the airway pressure itself rather than the pressure waveform generated from the two NIV modes.
The nHFOV group had a lower rate of air leak syndrome than the BiPAP group, and we suspected that this may be due to the small tidal volumes used, which results in less pressure-and-volume trauma from nHFOV (21). High-frequency oscillation ventilation is a type of oscillation with a high frequency of air flow. Through a diffusion mechanism, a small amount of gas was sent into or out of the airway ventilation method, and there was no synchronization and no man-machine confrontation (22). However, BiPAP offers synchronized nasal intermittent positive pressure ventilation using an abdominal capsule, which may be difficult to synchronize with infant breathing. This situation makes it very easy to create man-machine confrontation; even if the MAP was lower, air leak syndrome was more likely to occur.
BPD is a common and serious complication in premature infants (23). Studies have shown that IMV and high oxygen exposure are high-risk factors for BPD (24, 25). Although the rate of infants diagnosed with BPD was similar between the two groups, the rate of severe BPD in the nHFOV group was significantly lower than that in the BiPAP group. This difference may have resulted from the facilitation of gas exchange in neonates treated with nHFOV, and nHFOV was able to sustain oxygenation and ventilation while leading to improved alveolar or lung development (6, 26–28). These effects on lung development raise the possibility that nHFOV may reduce neonatal chronic lung disease. nHFOV reduces the duration of supplemental oxygen and the use of IMV, which can also help reduce the incidence of severe BPD.
nHFOV reduced the duration of supplemental oxygen and hospitalization, which may have benefitted from the reduction in airway inflammation, lung injury, and incidence of severe BPD by nHFOV. Of concern was the decrease in NEC and retinopathy of prematurity requiring laser treatment in thed nHFOV group, which may have been due to nHFOV reducing the duration of supplemental oxygen and IMV and the fluctuations in blood oxygen saturation, although there were no statistically significant differences.
Our study had some limitations, which may affect the validity of our findings. First, it was a retrospective study, and it was not a prospective randomized controlled study, and therefore, the study's objectivity was somewhat limited. Second, it was a single-center study with a small sample size. Third, the follow-up time was not long enough. Fourth, the study infants were not stratified by birth weight or gestational age, and there was a lack of infants at less than 25-weeks of gestation.
Conclusion
This study showed that nHFOV as initial respiratory support in preterm infants with RDS was feasible and safe compared to BiPAP. Furthermore, nHFOV can reduce the need for IMV and reduce the incidence of severe BPD and air leak syndrome.
Data Availability Statement
The data analyzed in this study is subject to the following licenses/restrictions: The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions. Requests to access these datasets should be directed to Shu-Hua Lai, bGFpc2h1aHVhMjAxNEAxNjMuY29tLg==
Author Contributions
S-HL, Y-FL, and Y-RZ designed the study, collected the clinical data, performed the statistical analysis, participated in the operation, and drafted the manuscript. Y-LX , Z-QC, RC, W-HC, and L-CW participated in the operation and revised the article. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We appreciated all doctors in our center for fruitful advice and discussions.
Abbreviations
RDS, Respiratory distress syndrome; IMV, Invasive mechanical ventilation; NIV, Non-invasive ventilation; NICU, Neonatal intensive care unit; BiPAP, Biphasic positive airway pressure; nHFOV, Non-invasive high-frequency oscillatory ventilation; FiO2, Inspired oxygen; MAP, Average airway pressure; NCPAP, Nasal continuous positive airway pressure; BPD, Bronchopulmonary dysplasia.
References
1. Miller JD, Carlo WA. Pulmonary complications of mechanical ventilation in neonates. Clin Perinatol. (2008) 35:273–81. doi: 10.1016/j.clp.2007.11.004
2. Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, Te Pas A, et al. European consensus guidelines on the management of respiratory distress syndrome - 2019 update. Neonatology. (2019) 115:432–50. doi: 10.1159/000499361
3. Walsh MC, Morris BH, Wrage LA, Vohr BR, Poole WK, Tyson JE, et al. Extremely low birth weight neonates with protracted ventilation: mortality and 18-month neurodevelopmental outcomes. J Pediatr. (2005) 146:798–804. doi: 10.1016/j.jpeds.2005.01.047
4. Vendettuoli V, Bellu R, Zanini R, Mosca F, Gagliardi L. Italian Neonatal Network: Changes in ventilator strategies and outcomes in preterm infants. Arch Dis Child Fetal Neonatal Ed. (2014) 99:F321–4. doi: 10.1136/archdischild-2013-305165
5. Zhu XW, Zhao JN, Tang SF, Yan J, Shi Y. Noninvasive high-frequency oscillatory ventilation versus nasal continuous positive airway pressure in preterm infants with moderate-severe respiratory distress syndrome: a preliminary report. Pediatr Pulmonol. (2017) 52:1038–42. doi: 10.1002/ppul.23755
6. Null DM, Alvord J, Leavitt W, Wint A, Dahl MJ, Presson AP, et al. High-frequency nasal ventilation for 21 d maintains gas exchange with lower respiratory pressures and promotes alveolarization in preterm lambs. Pediatr Res. (2014) 75:507–16. doi: 10.1038/pr.2013.254
7. Shi Y, De Luca D. Continuous positive airway pressure (CPAP) vs noninvasive positive pressure ventilation (NIPPV) vs noninvasive high frequency oscillation ventilation (NHFOV) as post-extubation support in preterm neonates: protocol for an assessor-blinded, multicenter, randomized controlled trial. BMC Pediatr. (2019) 19:256. doi: 10.1186/s12887-019-1625-1
8. Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. (2001) 163:1723–9. doi: 10.1164/ajrccm.163.7.2011060
9. Vliegenthart RJS, Onland W, van Wassenaer-Leemhuis AG et al. Restricted ventilation associated with reduced neurodevelopmental impairment in preterm infants. Neonatology. (2017) 112:172–9. doi: 10.1159/000471841
10. Buyuktiryaki M, Okur N, Sari FN, Bekmez BO, Bezirganoglu H, Cakir U, et al. Comparison of three different noninvasive ventilation strategies as initial respiratory support in very low birth weight infants with respiratory distress syndrome: a retrospective study. Arch Pediatr. (2020) 27:322–7. doi: 10.1016/j.arcped.2020.06.002
11. Bottino R, Pontiggia F, Ricci C, Gambacorta A, Paladini A, Chijenas V, et al. Nasal high-frequency oscillatory ventilation and CO2 removal: a randomized controlled crossover trial. Pediatr Pulmonol. (2018) 53:1245–51. doi: 10.1002/ppul.24120
12. Mukerji A, Singh B, Helou SE, Fusch C, Dunn M, Belik J, et al. Use of noninvasive high-frequency ventilation in the neonatal intensive care unit: a retrospective review. Am J Perinatol. (2015) 30:171–6. doi: 10.1055/s-0034-1381317
13. Ali YAH, Seshia MM, Ali E, Alvaro R. Noninvasive High- Frequency Oscillatory Ventilation: A Retrospective Chart Review. Am J Perinatol. (2020). doi: 10.1055/s-0040-1718738 [Epub ahead of print].
14. Cao H, Li H, Zhu X, Wang L, Yi M, Li C, Chen L, Shi Y. Three non-invasive ventilation strategies for preterm infants with respiratory distress syndrome: a propensity score analysis. Arch Med Sci. (2020) 16:1319–26. doi: 10.5114/aoms.2020.93541
15. Haidar Shehadeh AM. Non-invasive high flow oscillatory ventilation in comparison with nasal continuous positive pressure ventilation for respiratory distress syndrome, a literature review. J Matern Fetal Neonatal Med. (2021) 34:2900–9. doi: 10.1080/14767058.2019.1671332
16. Mukerji A, Finelli M, Belik J. Nasal high-frequency oscillation for lung carbon dioxide clearance in the newborn. Neonatology. (2013) 103:161–5. doi: 10.1159/000345613
17. Owen LS, Morley CJ, Davis PG. Effects of synchronization during SiPAP-generated nasal intermittent positive pressure ventilation (NIPPV) in preterm infants. Arch Dis Child Fetal Neonatal Ed. (2015) 100:F24–30. doi: 10.1136/archdischild-2013-305830
18. De Luca D, Dell'Orto V. Non-invasive high-frequency oscillatory ventilation in neonates: review of physiology, biology, and clinical data. Arch Dis Child Fetal Neonatal Ed. (2016) 101:F565–70. doi: 10.1136/archdischild-2016-310664
19. Binmanee A, El Helou S, Shivananda S, Fusch C, Mukerji A. Use of high noninvasive respiratory support pressures in preterm neonates: a single-center experience. J Matern Fetal Neonatal Med. (2017) 30:2838–43. doi: 10.1080/14767058.2016.1265931
20. Mukerji A, Sarmiento K, Lee B, Hassall K, Shah V. Non-invasive high-frequency ventilation versus bi-phasic continuous positive airway pressure (BP-CPAP) following CPAP failure in infants <1250 g: a pilot randomized controlled trial. J Perinatol. (2017) 37:49–53. doi: 10.1038/jp.2016.172
21. Gaertner VD, Waldmann AD, Davis PG, Bassler D, Springer L, Thomson J, et al. Transmission of oscillatory volumes into the preterm lung during noninvasive high-frequency ventilation. Am J Respir Crit Care Med. (2021) 203:998–1005. doi: 10.1164/rccm.202007-2701OC
22. Courtney SE, Asselin JM. High-frequency jet and oscillatory ventilation for neonates: which strategy and when? Respir Care Clin N Am. (2006) 12:453–67. doi: 10.1016/j.rcc.2006.06.005
23. Horbar JD, Carpenter JH, Badger GJ, Kenny MJ, Soll RF, Morrow KA, et al. Mortality and neonatal morbidity among infants 501 to 1500 grams from 2000 to 2009. Pediatrics. (2012) 129:1019–26. doi: 10.1542/peds.2011-3028
24. Keszler M, Sant'Anna G. Mechanical ventilation and bronchopulmonary dysplasia. Clin Perinatol. (2015) 42:781–96. doi: 10.1016/j.clp.2015.08.006
25. Kapadia VS, Chalak LF, Sparks JE, Allen JR, Savani RC, Wyckoff MH. Resuscitation of preterm neonates with limited versus high oxygen strategy. Pediatrics. (2013) 132:e1488–96. doi: 10.1542/peds.2013-0978
26. Reyburn B, Li M, Metcalfe DB, Kroll NJ, Alvord J, Wint A, et al. Nasal ventilation alters mesenchymal cell turnover and improves alveolarization in preterm lambs. Am J Respir Crit Care Med. (2008) 178:407–18 doi: 10.1164/rccm.200802-359OC
27. Sahni M, Bhandari V. Recent advances in understanding and management of bronchopulmonary dysplasia. F1000Res. (2020) 9:F1000. doi: 10.12688/f1000research.25338.1
Keywords: non-invasive high-frequency oscillatory ventilation, biphasic positive airway pressure, preterm infants, respiratory distress syndrome, non-invasive ventilation
Citation: Lai SH, Xie YL, Chen ZQ, Chen R, Cai WH, Wu LC, Lin YF and Zheng YR (2022) Non-invasive High-Frequency Oscillatory Ventilation as Initial Respiratory Support for Preterm Infants With Respiratory Distress Syndrome. Front. Pediatr. 9:792160. doi: 10.3389/fped.2021.792160
Received: 09 October 2021; Accepted: 17 December 2021;
Published: 11 January 2022.
Edited by:
Deb Strickland, University of Western Australia, AustraliaReviewed by:
Daniel Klotz, University of Freiburg, GermanyGianluca Lista, Ospedale dei Bambini Vittore Buzzi, Italy
Copyright © 2022 Lai, Xie, Chen, Chen, Cai, Wu, Lin and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yun-Feng Lin, bGlueWYyMDAzQHFxLmNvbQ==; Yi-Rong Zheng, emhlbmd5aXJvbmcyMDIwQDE2My5jb20=
 Shu-Hua Lai1,2
Shu-Hua Lai1,2 Zhi-Qing Chen
Zhi-Qing Chen Yun-Feng Lin
Yun-Feng Lin Yi-Rong Zheng
Yi-Rong Zheng