- Department of Pediatric Surgery, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy
Aim: The use of robotics-assisted surgery in oncology has been proved effective and safe in adults. Despite these results, the use of robotics has been rarely reported for pediatric oncology. Our review aims to evaluate the safety and feasibility of robotics-assisted surgery in this field, analyzing our experience and performing a systematic review of the most recent studies.
Methods: We reviewed all patients affected by an oncological disease who underwent a robotics-assisted procedure at our institute. We performed a systematic review of the literature from 2012 to 2021 on the subjects.
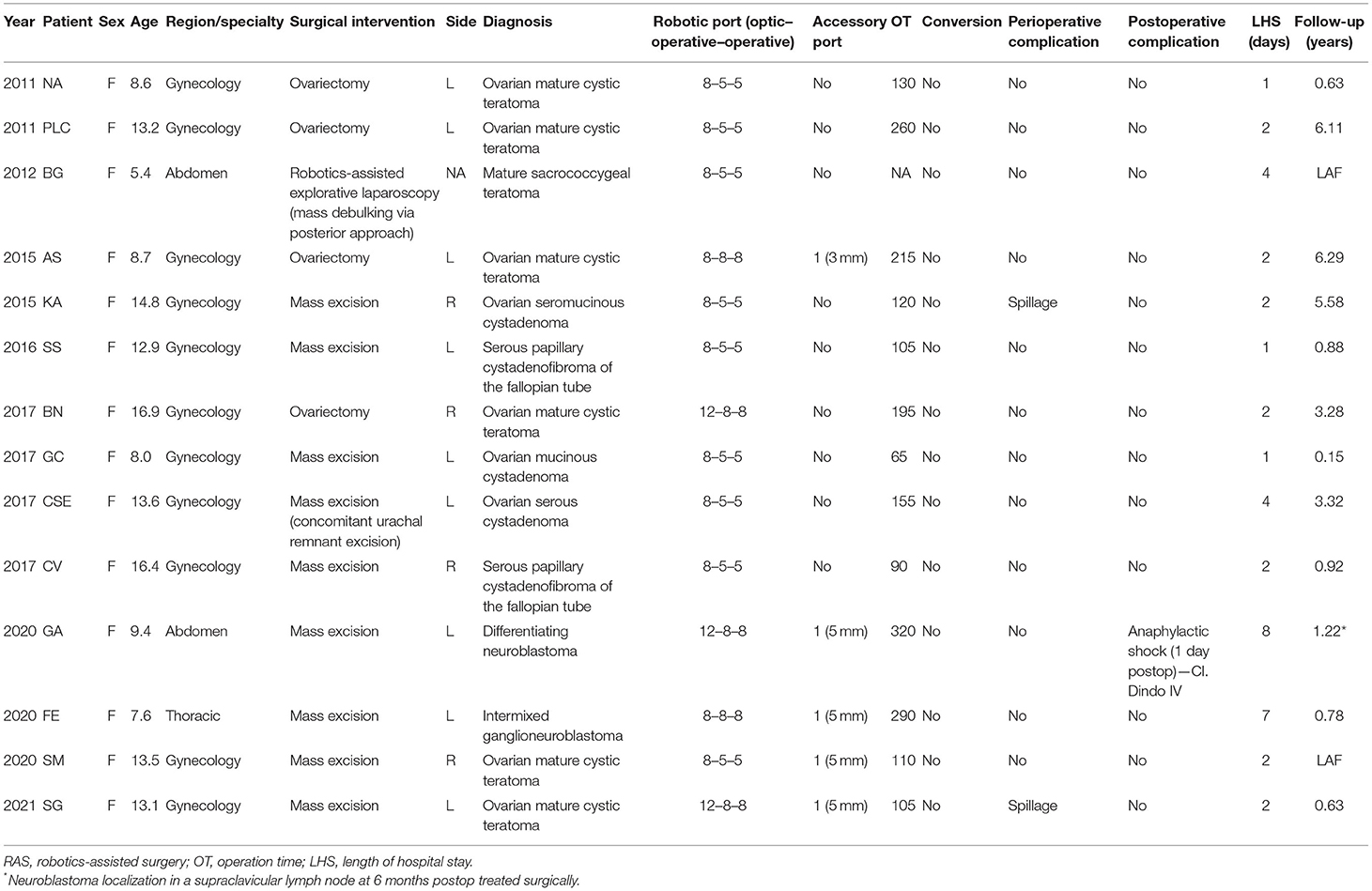
Findings: A total of 14 patients underwent robotics-assisted tumor resection. Eleven procedures (median age 13.2-years old) were carried out in children with adnexal lesions (seven tumor excision and four ovariectomies). Histological diagnosis was mature teratoma (six), serous papillary cystadenofibromas of the fallopian tube (two), ovarian serous cystadenoma (one), ovarian mucinous cystadenoma (one), and ovarian seromucinous cystadenoma. The median length of stay was 2 days. No recurrences or complications at a median follow-up of 2.1-years were observed. A 5-year-old girl underwent a complete posterior resection of a type 3 sacrococcygeal tumor with a robotics-assisted approach for the dissection of a possible intraabdominal residual component of the lesion. No intra- and postoperative complications were recorded. Complete excision of a recurrent differentiating neuroblastoma of the left para-renal region was performed on a 9-year-old girl. An idiopathic anaphylactic shock occurred 1 day after the procedure. At 9 months' follow-up, no local recurrences of the lesion were observed. Overall, we reported no conversion to open surgery. Lastly, a robotic excision of a growing left superior mediastinal intermixed ganglioneuroblastoma was performed on an 8-year-old girl with no postoperative complications. Follow-up was uneventful (7 months). In the literature, the rate of complications ranges from 0 to 28%, mainly related to difficult dissection and impaired anatomy. Conversion is reported in 5% of all oncological procedures, due to more invading tumors and altered anatomical features. No robotics-related complications were reported.
Conclusion: Robotics-assisted surgery in pediatric oncology has proven to be feasible. Nevertheless, its use should be limited to selected cases and performed by highly trained oncological surgeons. Preparation and patient positioning, alongside a correct port placement, are crucial to carrying out these procedures. Further innovations in robotics may allow a wider application of this technology in pediatric oncology.
Introduction
Robotics-assisted surgery (RAS) represents one of the most important advancements in minimally invasive surgery (MIS) in recent years and has progressively gained a predominant role in many fields of adult surgery. The da Vinci surgical system (Intuitive Surgical, Inc., Sunnyvale, CA, USA) is actually present in 67 countries, and more than 5,500 robots are used worldwide (1).
Well-known advantages are a stable magnified 3D view, tremor filtering, and motion scaling, which allow precise intracorporeal exposure and suturing (2).
RAS in pediatric surgery has initially struggled due to some limitations, notably port and arm dimensions, as well as high costs (3). Nevertheless, the growing number of case reports and series published every year reveals how RAS is increasingly applied for children (4).
Despite this spread, its use for pediatric oncology is still limited, and few studies have been conducted on the subject. The reasons are represented by the characteristics of pediatric tumors, as each type may be considered a rare disease. Moreover, most pediatric malignancies are embryonal tumors with rapid growth, which require frequently other therapies as neoadjuvant chemotherapy. All these distinctive features limit the creation of guidelines for the robotic approach. Nonetheless, accepted recommendations require an evaluation by a multidisciplinary tumor board and respecting oncological protocols for open surgery for each specific pathology (5).
We performed a retrospective study to critically review our experience in RAS.
In order to compare our results with those from the literature, we performed a systematic review, focusing on technical skills that could help pediatric surgeons to avoid intra- and postoperative complications.
Materials and Methods
We performed a retrospective review of all pediatric oncological patients who underwent RAS at our institution from 2010 to 2021. From 2017 to 2020, the use of the robotic platform has been suspended due to technical reasons.
Patients over 18-years old were excluded, as well as all malignancies not treated with RAS.
We analyzed demographic data, including age at surgery, sex, pathology, possible comorbidities, operation time (OT), length of hospital stay (LHS), perioperative complications, and postoperative outcomes. Postoperative complications were classified according to the Clavien–Dindo classification and graded from I to V.
All procedures were carried out using the da Vinci Si Surgical System (Intuitive Surgical, Sunnyvale, CA, USA). All surgeries were performed with three robotic arms, placed accordingly depending on the lesion site and size. Some procedures required an accessory port (3 or 5 mm).
To compare our results with those of the literature, a systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria.
We selected articles reporting RAS in oncological pediatric patients between 2012 and September 2021 in MEDLINE and EMBASE using the following keywords: “(pediatric) or (children) and (robot) or (robotic) and (oncology) or (tumor).”
Inclusion criteria were as follows:
- Articles published between January 2012 and September 2021
- Articles written in English
- Median/mean age <18-years
- Case series with more than 3 patients
- Articles where data concerning demographics, surgical indications, complication, and conversion rates were clearly deductible.
All data were elaborated using the statistical software “R,” version 3.4.1. Descriptive statistics were used to present findings, and quantitative variables were expressed as median (range) to express our data. Data elaborated from the literature review were expressed as median (range) or mean ± SDs depending on the reference found in the original articles.
Results
A total of 14 pediatric patients underwent RAS for oncological pathologies from 2010 to 2021 at our institute. All data are displayed in Table 1. No patients required a conversion to open surgery.
Among our cohort, 11 gynecological procedures were performed (7 mass excisions and 4 ovariectomies) for the following tumors: 6 ovarian mature cystic teratomas (Figure 1), 2 serous papillary cystadenofibroma of the fallopian tube, 1 ovarian mucinous cystadenoma, 1 ovarian serous cystadenoma, and 1 ovarian seromucinous cystadenoma. The median age at surgery was 13.2 [8.0–16.9], with median operative time including docking time 120 [65–260]. Most of the procedures were carried out using an 8-mm optic port and two 5-mm operative ports. In only three patients, an additional accessory port (either 5 or 3 mm) was positioned. In two cases (14.2%), intraoperative spillage was reported. No other perioperative complications nor conversion were reported. Follow-up was uneventful for all patients (median follow-up 2.1-years [0.2–6.3]). The median length of hospitalization was 2 days (1–4).
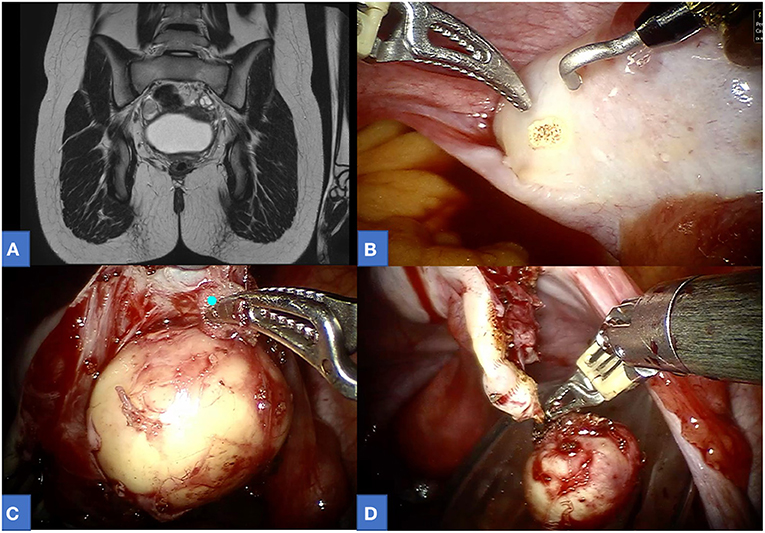
Figure 1. A 13-year-old affected by right ovarian mature teratoma. (A) Preoperative MRI. (B–D) Intraoperative view.
We performed one thoracic procedure on a 7-year-old girl for a growing intermixed ganglioneuroblastoma located on the supero-posterior mediastinum encasing the subclavian vessels. An 8-mm optic port was positioned in the sixth intercostal space on the midaxillary line. Two 8-mm operative ports were positioned 8 cm away from the optic port, in the fifth intercostal space on the anterior axillary line and in the seventh intercostal space on the paravertebral line. Finally, a 5-mm auxiliary port was placed in the fourth intercostal space on the anterior axillary line. No postoperative complications were reported.
We report one robotics-assisted explorative laparoscopy on a 5-year-old girl who previously underwent posterior excision of a type 3 mature sacrococcygeal teratoma, as the preoperative imaging showed suspicion of tumor extension in the pelvis. The robotics-assisted exploration result was negative.
We completed an excision of a left perirenal recurrence of neuroblastoma in a 9-year-old girl. A 12-mm optic port was placed trans-umbilically, whereas two 8-mm operative ports were placed in the left hypochondrium and in the left iliac region. An accessory port was then positioned in the epigastric region. No intraoperative complication occurred. The patient suffered from an anaphylactic shock on the first postoperative day that required adrenaline and corticosteroid administration. Further postoperative course was uneventful.
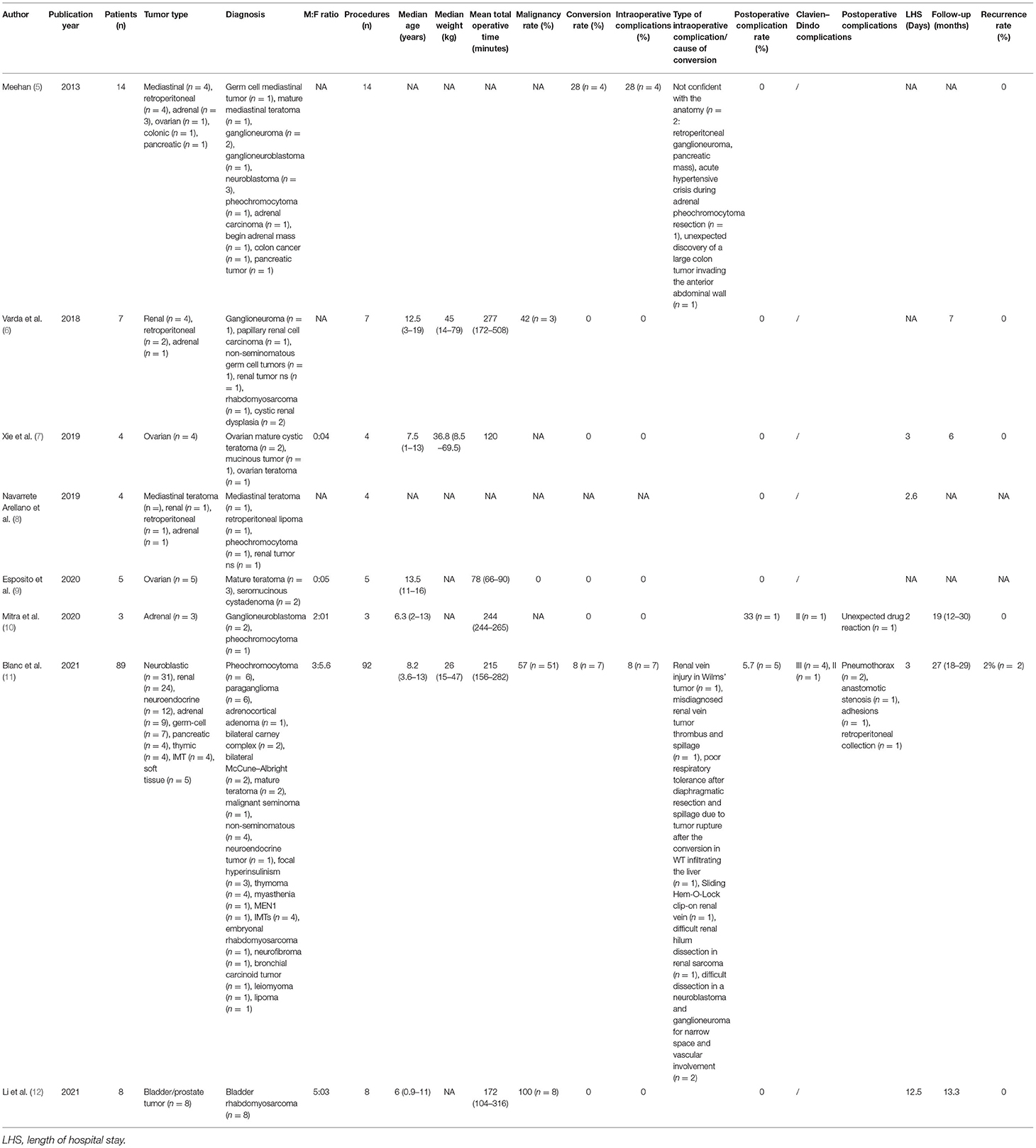
A systematic review was performed according to the PRISMA guidelines (Figure 2). Eight studies met the eligibility criteria, for a total of 137 procedures in 134 patients. Data are summarized in Table 2. The male-to-female (M:F) ratio was ~1:2, the median age was 9-years [0.9–19.0], and the median weight was 35 kg (when reported). Treated conditions were represented by a broad group of tumors, and the most common were adrenal. The malignancy rate was on average 65%. The median conversion to open surgery rate was 5%.
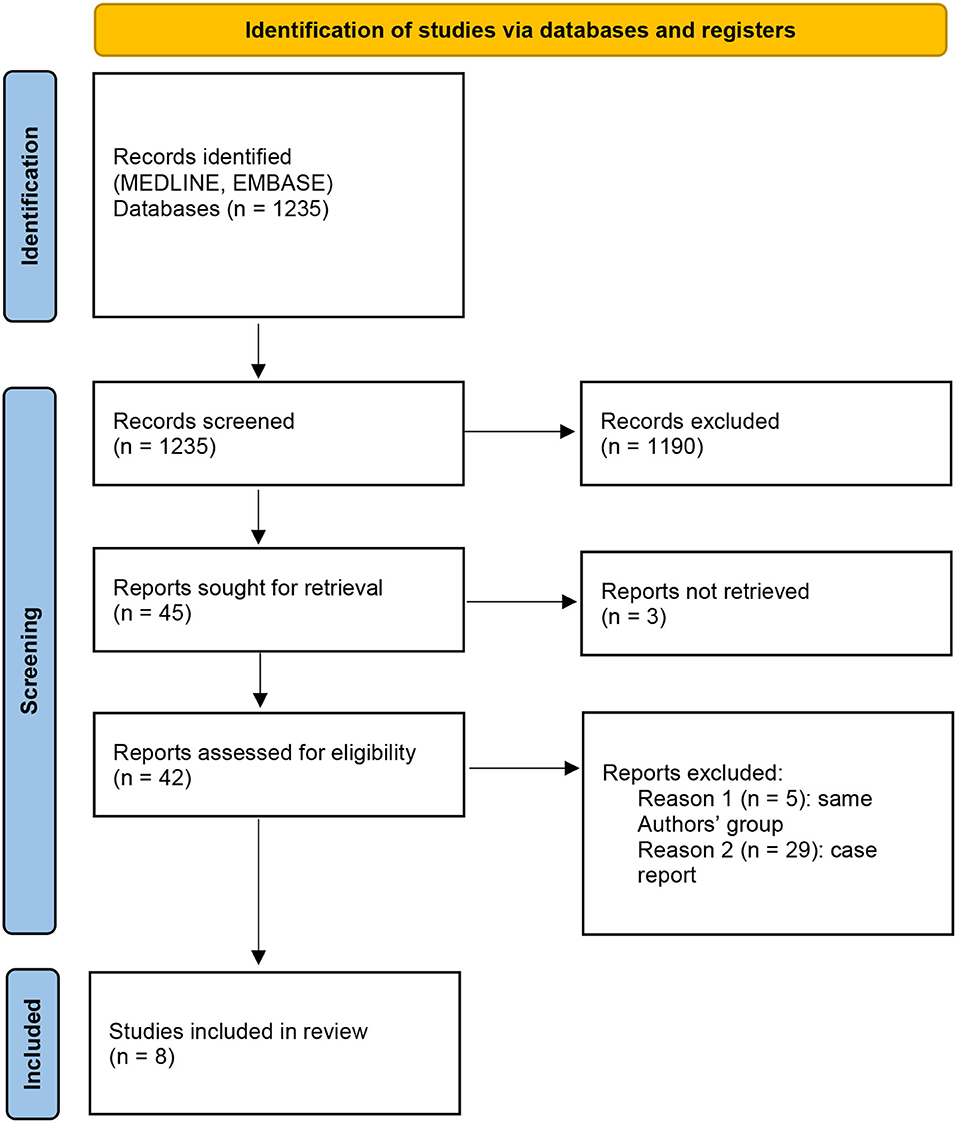
Figure 2. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 flow diagram.
The intraoperative complication rate ranged from 0 to 28%, and the main reported causes were difficult dissection and intraoperative discovery of more invading tumors than expected. Moreover, two conversions were performed due to a lack of confidence in the anatomy. No robotics-related complication was reported (e.g., injury to the patients due to robotic arms).
The median operative time, including docking, was 184 min. The postoperative complication rate accounted for 4% (most reported complications were pneumothorax, unexpected drug reaction, and adhesions). The median hospital stay was 4.6 days.
Follow-up, when stated, was carried out for a median of 14.4 months. The recurrence rate was 1.4%.
A comparison between our experience and the literature is reported in Table 3.
Discussion
The role of RAS is becoming progressively more important in every field, including pediatric oncology. In the last decade, several papers have been published on the subjects, even if the sample of the cohort is often small since most of the studies are case reports (5–12).
The still limited spread of this technique is due to both the concerns of the use of MIS for tumors and the well-known limitations of RAS in children (13, 14). Nevertheless, since the first cases reported by Meehan and Sandler (15), results have been encouraging.
To date, the published paper underlines the necessity to perform a strict selection of all children undergoing RAS, in order to adhere to oncological guidelines. The application of this technique requires an in-depth knowledge of pediatric oncology and the revision of each case by an ongoing multidisciplinary team, composed of medical oncologists, radiologists, anesthesiologists, and surgeons experienced in MIS and oncologic surgery (16).
Comparing our experience with the literature (Table 3), we found a different M:F ratio (0:1 vs. 1:2), probably due to our initial selection to perform surgery in adnexal lesions. In our practice, the first oncological procedures that were performed concerned adnexal lesions, as we believed that RAS is a perfect fit for these indications. Adult gynecology has already proven the feasibility of robotic procedures for both benign and malign pathologies (7, 17–20), and experience in pediatrics is growing (7, 9, 21). A robot allows a superb visualization of the pelvis, and in the majority of cases, port placement may be carried out easily, as most girls undergoing this kind of surgery are adolescents. Alongside, MIS offers good cosmetic results, which is an important factor, especially in this group of patients (22). Nevertheless, the relative simplicity of the surgical procedure must not let the surgeons underestimate the risk of spillage and/or rupture of ovarian lesions.
Although extremely rare, malignant ovarian neoplasm in children and adolescents may occur (23–27). If preoperative examinations point out the risk of malignancy, oophorectomy should be strongly considered, and, when performed, no salpingectomy is required, which is preferable in this age group (24, 25, 28, 29). Nevertheless, in pediatrics, there is an interest in preserving as much ovarian tissue as possible, to assure the development of normal puberty and future fertility (30). As many articles describe how laparoscopy may be safely applied to perform ovarian-sparing surgery in pediatrics, this topic is sometimes debated (31). In our opinion, the already cited technological advantages of the da Vinci system may further allow a surgeon to perform a safe excision minimizing the risk of spillage, as long as all oncological principles are followed (e.g., preoperative tumor markers, adequate imaging, and extraction of the mass using an Endobag).
Risks of tumor rupture and/or spillage, risk of incomplete resection, and risk of port-site recurrences count as the most cited problems for MIS/RAS.
In our experience, the complication rate was higher than in other series (14.2%, two spillages, vs. 4.5%). Spillage during RAS is reported in only one case by Blanc et al. (11), due to the leakage of a renal vein thrombus of a Wilms tumor, discovered after renal vein control. Overall, the spillage rate was 0.7%. Despite that the risk should not be underestimated, the use of MIS in malignancies where spillage or rupture is particularly dangerous has been accepted in selected cases. For example, in 2014, the Renal Tumor Strategy Group of the International Society of Paediatric Oncology (SIOP) published the largest cohort of laparoscopic excision of Wilms tumor (32). Moreover, in the same year, the SIOP Umbrella protocol (33, 34) proposed inclusion criteria to safely perform laparoscopic nephrectomy in Wilms tumors. Finally, in 2018, Bouty et al. showed, by performing a systematic review, that in highly selected cases, MIS in Wilms tumor did not worsen prognosis (35).
Although detractors of RAS are skeptical about its use due to the absence of haptic feedback, the technological advantages of the da Vinci system (3D vision, seven degrees of freedom, tremor filtration, and precise camera control) have expanded the possibilities of performing and reproducing difficult operations, especially when there is a deep and narrow field and when fine dissection is required for delicate tissue manipulation, as is the case in pediatric oncology surgery (16, 36).
Regarding the suspected incidence of port-site recurrences, a recent publication in adults shows equivalent outcomes between laparoscopic/robotic and open approaches (17, 37). In pediatrics, no recurrences have been cited so far.
In literature, the overall conversion rate to open surgery was about 5%, and difficult dissection or surgeon diffidence in continuing RAS were the most reported causes.
Conversion is required every time there is the possibility to upstage the tumor. Nevertheless, as the experience of the surgeon grows, a reduction in the rate of conversion is reported (11).
This is certainly due to improved confidence in RAS, associated with a better selection of patients addressed to this technique. Blanc et al. suggest beginning the experience with RAS with smaller tumors and converting in cases of difficult dissection, stressing that the main objective is to respect the oncological surgical principles (11).
For several authors, surgeons with or without previous laparoscopic or robotic experience could perform independently and properly robotic procedures (38, 39). In surgical oncology, the passage from open to laparoscopy or RAS is far from being easy. The approach to pediatric tumors needs an important surgical background that comes from open surgery. To apply RAS in tumor resection, it is not only necessary to improve personal learning curve, training, and exercising on virtual and animal models mastering basic and advanced robotic skills. For any surgeon, it is necessary to perform at least 250 procedures to consider himself/herself independent and a mentor in surgery.
The availability of senior surgeons with experience in both oncologic surgery and MIS provides valid support to the steep learning curve.
In our experience, the availability of a simulating station for the da Vinci system allowed us to perform specific personal training, both virtual and in vivo. The approach to pediatric tumors came after a consistent experience in other RAS and specific training of the whole surgical team. Thanks to the presence of 2 consoles, it was possible for younger surgeons to approach tumors, with senior surgeons mentoring live, even those with less experience in RAS.
An oncological procedure carried out with RAS, especially at the beginning, may require a long operative time. Installation of the patient requires meticulous attention. Comfortable positioning as well as the use of adequate padding and skin protection must always be verified (4, 40). It is important to avoid hyperextension or flexion in small children, as they are more pliable compared with adolescents and adults (4). Once the patient is correctly installed, the docking procedure needs to be correctly planned, especially in infants and toddlers, as the working space is limited. Particular attention is needed to avoid conflict between the robotic arms and, more importantly, between the robot and the patient. The key role in assuring and controlling potential harm to the child during surgery is played by the scrubbed nurse and the scrubbed assistant who need to control and verify patient safety throughout the intervention, alongside assisting the lead surgeon by passing needles, bandages, or other instruments through the assistant port (41).
In our experience, OT was comparable with that in the literature (166 vs. 184 min).
Anesthesiologists involved in RAS procedures must be familiar with the robot and its installation, as well as the degree of movements of the arms. All vascular accesses must be positioned before docking and arranged to minimize any possible conflicts with the robot. At the same time, robot installation must not prevent the work of the anesthesiology team during surgery.
In literature, concerning pediatric oncology, no case of robotics-related complication has been reported, in terms of injury to the patient due to a robotic arm, nor cases of robot malfunction. When operating with the da Vinci robot, especially in the case of delicate surgeries such as oncological procedures, all members of the surgical team have to keep in mind the possibility of malfunction and must be able to respond and properly provide assistance if necessary. In fact, during any robotics-assisted procedure, the role of the technical assistance team is crucial. Technical support should always be available and consists of in-person and phone support provided (42). Their help can solve most cases of dysfunction of the robot or any of its components. In our experience, we were assisted by a da Vinci specialist during the most complicated surgeries.
Limitations
The limitations of this study are represented by the retrospective nature of the analysis and the small cohort of patients (14) with a high prevalence of adnexal lesions. Since the application of RAS to pediatric oncology represents a new experience, even the systematic review is limited by a low number of papers with a small series.
Conclusions
RAS in pediatric oncology has proven to be feasible for different pathologies. Although optimistic reports have been published in the literature, the use of RAS should be limited to selected cases and performed by highly trained oncological surgeons. So far, the literature strongly recommends the presence of a multidisciplinary board of experts (surgeon, anesthesiologist, radiologist, and oncologist) to evaluate candidates to RAS. All procedures must be carried out while respecting oncological protocols. Preparation and patient positioning, alongside a correct port placement, are crucial to safely perform these surgeries.
Further studies are needed to assess the role of RAS in pediatric oncology, as well as to implement specific technical standards for each pediatric tumor.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
GR contributed to conception and design of the study. FV and MG organized the database and performed the statistical analysis. FV, MB, and AR wrote the first draft of the manuscript. FV, MB, and GR wrote sections of the manuscript. LA and RV reviewed the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Koukourikis P, Rha KH. Robotic surgical systems in urology: what is currently available? Investig Clin Urol. (2021) 62:14–22. doi: 10.4111/icu.20200387
2. Mizuno K, Kojima Y, Nishio H, Hoshi S, Sato Y, Hayashi Y. Robotic surgery in pediatric urology: current status. Asian J Endosc Surg. (2018) 11:308–17. doi: 10.1111/ases.12653
3. Bruns NE, Soldes OS, Ponsky TA. Robotic surgery may not “make the cut” in pediatrics. Front Pediatr. (2015) 3:10. doi: 10.3389/fped.2015.00010
4. Denning NL, Kallis MP, Prince JM. Pediatric robotic surgery. Surg Clin North Am. (2020) 100:431–43. doi: 10.1016/j.suc.2019.12.004
5. Meehan JJ. Robotic surgery for pediatric tumors. Cancer J. (2013) 19:183–8. doi: 10.1097/PPO.0b013e318289486c
6. Varda BK, Cho P, Wagner AA, Lee RS. Collaborating with our adult colleagues: a case series of robotic surgery for suspicious and cancerous lesions in children and young adults performed in a free-standing children's hospital. J Pediatr Urol. (2018) 14:182.e1-8. doi: 10.1016/j.jpurol.2018.01.003
7. Xie XX, Wang N, Wang ZH, Zhu YY, Wang JR, Wang XQ. Robotic-assisted resection of ovarian tumors in children: a case report and review of literature. World J Clin Cases. (2019) 7:2542–8. doi: 10.12998/wjcc.v7.i17.2542
8. Navarrete Arellano M, Garibay González F. Robot-assisted laparoscopic and thoracoscopic surgery: prospective series of 186 pediatric surgeries. Front Pediatr. (2019) 7:200. doi: 10.3389/fped.2019.00200
9. Esposito C, Settimi A, Del Conte F, Cerulo M, Coppola V, Farina A, et al. Image-guided pediatric surgery using Indocyanine Green (ICG) fluorescence in laparoscopic and robotic surgery. Front Pediatr. (2020) 8:314. doi: 10.3389/fped.2020.00314
10. Mitra AP, Vasquez E, Kokorowski P, Chang AY. Robotic adrenalectomy in the pediatric population: initial experience case series from a tertiary center. BMC Urol. (2020) 20:155. doi: 10.1186/s12894-020-00727-x
11. Blanc T, Meignan P, Vinit N, Ballouhey Q, Pio L, Capito C, et al. Robotic surgery in pediatric oncology: lessons learned from the first 100 tumors-a nationwide experience. Ann Surg Oncol. (2021) 28(Suppl 3):901. doi: 10.1245/s10434-021-10861-x
12. Li P, Zhou H, Cao H, Xu B, Guo T, Zhu W. Robotic-assisted Laparoscopic Management of Bladder/Prostate Rhabdomyosarcoma in Children: initial series and 1-yr outcomes. J Endourol. (2021) 35:1520–5. doi: 10.1089/end.2020.1238
13. Motterle G, Ahmed ME, Andrews JR, Moschini M, Kwon ED, Karnes J. Tumor seeding after robot-assisted radical prostatectomy: literature review and experience from a single institution. J Urol. (2020) 203:1141–6. doi: 10.1097/JU.0000000000000701
14. Cundy TP, Shetty K, Clark J, Chang TP, Sriskandarajah K, Gattas NE, et al. The first decade of robotic surgery in children. J Pediatr Surg. (2013) 48:858–65. doi: 10.1016/j.jpedsurg.2013.01.031
15. Meehan JJ, Sandler A. Pediatric robotic surgery: a single-institutional review of the first 100 consecutive cases. Surg Endosc. (2008) 22:177–82. doi: 10.1007/s00464-007-9418-2
16. Blanc T, Pio L, Clermidi P, Muller C, Orbach D, Minard-Colin V, et al. Robotic-assisted laparoscopic management of renal tumors in children: preliminary results. Pediatr Blood Cancer. (2019) 3:e27867. doi: 10.1002/pbc.27867
17. Qadan M, Kingham TP. Technical aspects of gallbladder cancer surgery. Surg Clin North Am. (2016) 96:229–45. doi: 10.1016/j.suc.2015.12.007
18. Zanagnolo V, Garbi A, Achilarre MT, Minig L. Robot-assisted surgery in gynecologic cancers. J Minim Invasive Gynecol. (2017) 24:379–96. doi: 10.1016/j.jmig.2017.01.006
19. Conrad LB, Ramirez PT, Burke W, Naumann RW, Ring KL, Munsell MF, et al. Role of minimally invasive surgery in gynecologic oncology: an updated survey of members of the society of gynecologic oncology. Int J Gynecol Cancer. (2015) 25:1121–7. doi: 10.1097/IGC.0000000000000450
20. Gala RB, Margulies R, Steinberg A, Murphy M, Lukban J, Jeppson P, et al. Systematic review of robotic surgery in gynecology: robotic techniques compared with laparoscopy and laparotomy. J Minim Invasive Gynecol. (2014) 21:353–61. doi: 10.1016/j.jmig.2013.11.010
21. Pelizzo G, Nakib G, Calcaterra V. Pediatric and adolescent gynecology: treatment perspectives in minimally invasive surgery. Pediatr Rep. (2019) 11:8029. doi: 10.4081/pr.2019.8029
22. Pelizzo G, Nakib G, Romano P, Avolio L, Mencherini S, Zambaiti E, et al. Five millimetre-instruments in paediatric robotic surgery: advantages and shortcomings. Minim Invasive Ther Allied Technol. (2015) 24:148–53. doi: 10.3109/13645706.2014.975135
23. Helmrath M, Shin C, Warner B. Ovarian cysts in the pediatric population. Semin Pediatr Surg. (1998) 7:19–28. doi: 10.1016/S1055-8586(98)70002-2
24. Cass D, Hawkins E, Brandt M, Chintagumpala M, Bloss RS, Milewicz AL, et al. Surgery for ovarian masses in infants, children, and adolescents: 102 consecutive patients treated in a 15-year period. J Pediatr Surg. (2001) 36:693–9. doi: 10.1053/jpsu.2001.22939
25. Hermans AJ, Kluivers KB, Wijen MH, Bulten J, Massuger LF, Coppus SF. Diagnosis and treatment of adnexal masses in children and adolescents. Obstet Gynecol. (2015) 125:611–15. doi: 10.1097/AOG.0000000000000665
26. Kelleher CM, Goldstein AM. Adnexal masses in children and adolescents. Clin Obstet Gynecol. (2015) 58:76–92. doi: 10.1097/GRF.0000000000000084
27. Strickland J. Ovarian cysts in neonates, children and adolescents. Curr Opin Obstet Gynecol. (2002) 14:459–65. doi: 10.1097/00001703-200210000-00004
28. Amies Oelschlager AM, Gow KW, Morse CB, Lara-Torre E. Management of large ovarian neoplasms in pediatric and adolescent females. J Pediatr Adolesc Gynecol. (2016) 29:88–94. doi: 10.1016/j.jpag.2014.07.018
29. ACOG Committee. Adnexal torsion in adolescents: ACOG Committee Opinion No, 783 Summary. Obst Gynecol. (2019) 134:435–6. doi: 10.1097/AOG.0000000000003376
30. Templeman CL, Fallat ME, Lam AM, Perlman SE, Hertweck SP, O'Connor DM. Managing mature cystic teratomas of the ovary. Obstet Gynecol Surv. (2000) 55:738–45. doi: 10.1097/00006254-200012000-00004
31. Guillén G, Martín-Giménez MP, López-Fernández S, Molino JA, López M. Results of ovarian sparing surgery in pediatric patients: is there a place for laparoscopy? J Laparoendosc Adv Surg Tech. (2020) 30:458–63. doi: 10.1089/lap.2019.0515
32. Warmann SW, Godzinski J, van Tinteren H, Heij H, Powis M, Sandstedt B, et al. Minimally invasive nephrectomy for Wilms tumors in children – data from SIOP 2001. J Pediatr Surg. (2014) 49: 1544–8. doi: 10.1016/j.jpedsurg.2014.06.005
33. Burnand K, Roberts A, Bouty A, Nightingale M, Campbell M, Heloury Y. Laparoscopic nephrectomy for Wilms. Pediatr Urol. (2018) 14: 253.e1-8. doi: 10.1016/j.jpurol.2018.01.005
34. Van den Heuvel-Eibrink MM, Hol JA, Pritchard-Jones K, van Tinteren H, Furtwängler R, Verschuur AC, et al. Position paper: rationale for the treatment of Wilms tumor in the UMBRELLA SIOP-RTSG 2016 protocol. Nat Rev Urol. (2017) 14: 743–52. doi: 10.1038/nrurol.2017.163
35. Bouty A, Burnand K, Nightingale M, Roberts A, Campbell M, O'Brien M, et al. What is the risk of local recurrence after laparoscopic transperitoneal radical nephrectomy in children with Wilms tumors? Analysis of a local series and review of the literature. J Pediatr Urol. (2018) 14: 327.e1-7. doi: 10.1016/j.jpurol.2018.03.016
36. Anderberg M, Backman T, Annerstedt M. Robot-assisted radical cystoprostatectomy in a small child with rhabdomyosarcoma: a case report. J Robotic Surg. (2008) 2:101–3. doi: 10.1007/s11701-008-0089-3
37. Mantica G, Smelzo S, Ambrosini F, Tappero S, Parodi S, Pacchetti A, et al. Port-site metastasis and atypical recurrences after robotic-assisted radical cystectomy (RARC): an updated comprehensive and systematic review of current evidences. J Robot Surg. (2020) 14:805–12. doi: 10.1007/s11701-020-01062-x
38. Pio L, Musleh L, Paraboschi I, Pistorio A, Mantica G, Clermidi P, et al. Learning curve for robotic surgery in children: a systematic review of outcomes and fellowship programs. J Robot Surg. (2020) 14:531–41. doi: 10.1007/s11701-019-01026-w
39. O'Brien ST, Shukla AR. Transition from open to robotic-assisted pediatric pyeloplasty: a feasibility and outcome study. J Pediatr Urol. (2012) 8:276–81. doi: 10.1016/j.jpurol.2011.04.005
40. Villanueva J, Killian M, Chaudhryn R. Robotic urologic surgery in the infant: a review. Curr Urol Rep. (2019) 20:35. doi: 10.1007/s11934-019-0902-8
41. Nayyar R, Yadav S, Singh P, Dogra PN. Impact of assistant surgeon on outcomes in robotic surgery. Indian J Urol. (2016) 32:204–9. doi: 10.4103/0970-1591.185095
42. Product Services DaVinci Surgical System. Intuitive (2021). Available online at: https://www.intuitive.com/en-us/products-and-services/da-vinci/services (accessed September 21, 2021).
Keywords: robotic-assisted surgery, oncology, pediatrics, children, mini-invasive surgery
Citation: Vatta F, Gazzaneo M, Bertozzi M, Raffaele A, Avolio L and Riccipetitoni G (2022) Robotics-Assisted Pediatric Oncology Surgery—A Preliminary Single-Center Report and a Systematic Review of Published Studies. Front. Pediatr. 9:780830. doi: 10.3389/fped.2021.780830
Received: 21 September 2021; Accepted: 18 November 2021;
Published: 18 January 2022.
Edited by:
Luca Pio, Giannina Gaslini Institute (IRCCS), ItalyReviewed by:
Paul D. Losty, University of Liverpool, United KingdomPaola Midrio, Ca' Foncello Hospital, Italy
Copyright © 2022 Vatta, Gazzaneo, Bertozzi, Raffaele, Avolio and Riccipetitoni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giovanna Riccipetitoni, g.riccipetitoni@gmail.com
 Fabrizio Vatta
Fabrizio Vatta