
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Pediatr., 26 November 2021
Sec. Pediatric Urology
Volume 9 - 2021 | https://doi.org/10.3389/fped.2021.775468
 Hiroyuki Kitano1*
Hiroyuki Kitano1* Keisuke Hieda1
Keisuke Hieda1 Hiroki Kitagawa2
Hiroki Kitagawa2 Yusuke Nakaoka3
Yusuke Nakaoka3 Yumiko Koba3
Yumiko Koba3 Kohei Ota4
Kohei Ota4 Norifumi Shigemoto2
Norifumi Shigemoto2 Tetsutaro Hayashi1
Tetsutaro Hayashi1 Seiya Kashiyama2
Seiya Kashiyama2 Jun Teishima1
Jun Teishima1 Nobuaki Shime4
Nobuaki Shime4 Hiroki Ohge2
Hiroki Ohge2 Nobuyuki Hinata1
Nobuyuki Hinata1A 14-year-old girl noticed malodorous urine and experienced left flank pain. The patient was presented to our hospital with gradually increasing pain. She had no underlying disease but had a history of pain on micturition for several days. Hematologic examination indicated low white blood cell and platelet counts and a high serum lactate level. Computed tomography showed that a part of the parenchyma of the left kidney had poor contrast and was deteriorated, with fluid and gas retention from the perirenal region to the retroperitoneal cavity. A left hydroureter and large ureterocele were observed in the bladder. She was diagnosed with emphysematous pyelonephritis (EPN) with a giant congenital ureterocele. Vasopressors and blood transfusion failed to maintain normal circulatory dynamics, and an open left nephrectomy and transurethral ureterocele fenestration were performed. The excised outer portion of the left kidney was dissolved by the infection and replaced with blood clots and necrotic tissue. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry identified the inflammatory, gas-producing bacteria Actinotignum schaalii, Peptoniphilus asaccharolyticus, and Actinomyces odontolyticus. Meropenem was administered for 4 days postoperatively and then de-escalated to sulbactam/ampicillin for another 10 days. The patient was discharged on day 17 of hospitalization, and the postoperative course remained favorable. EPN is extremely rare in pediatric patients, and it is believed that nephrectomy is sometimes necessary if the patient does not have normal circulatory dynamics despite the use of catecholamines.
Emphysematous pyelonephritis (EPN) is an acute, severe necrotizing infection of the renal parenchyma, its surrounding tissue, and the urinary tract, and results in gas accumulation in the kidney or perinephric tissue (1, 2). EPN is a rare disease with a mortality rate of ~20% (3, 4). In adults, diabetes mellitus, urinary tract obstruction, and immune-incompetence predispose patients to EPN (2, 5), and Escherichia coli is the most common pathogenic organism, accounting for 43–70% of cases (2, 3). However, the pathogenesis of EPN in children remains unclear, partially due to a paucity of reports on EPN in children.
The clinical course of EPN can be severe and life-threatening without prompt diagnosis and treatment. Computed tomography helps prompt diagnosis by revealing renal gas accumulation, and surgical intervention and antibiotic therapy are the primary therapeutic options (3, 4). To date, very few cases of EPN have been reported in pediatric patients (6–12). Herein, we report a case of EPN in a child with a congenital giant ureterocele. Inflammatory bacteria, including Actinotignum schaalii, Peptoniphilus asaccharolyticus, and Actinomyces odontolyticus, were identified; to the best our knowledge, this is the first report of EPN caused by these bacteria in a child. A review of the relevant literature regarding pediatric EPN was also conducted.
A 14-year-old girl was presented to our hospital with a complaint of worsening left flank pain; she noticed malodorous urine 2–3 weeks prior and experienced left flank pain for several days before presentation. There was no personal or family history of underlying disease, but she was being treated for frequent by a local physician. The patient was admitted to the intensive care unit where she developed a fever (37.6°C) and tachycardia (heart rate 112 beats/min), with a blood pressure of 81/42 mmHg and respiratory rate of 20 breaths per min. A complete blood count revealed leukocytosis (1.57 × 109 cells/L), thrombocytopenia (73 × 109 cells/L), and anemia (hemoglobin 7.3 g/dL). Levels of C-reactive protein and procalcitonin were elevated (4.79 mg/dL and 294.64 ng/mL, respectively), and a prolonged prothrombin time was observed, with an internal normalized ratio of 2.28. Plasma D-dimer levels were elevated (286.4 μg/mL).
Acute kidney injury (serum creatinine, 2.06 mg/dL) was detected. Serum lactate level (4.8 mmol/L) was high. The sequential organ failure assessment (SOFA) scores and quick SOFA scores were 6 and 2, respectively. The Japan Coma Scale score was 1, and Glasgow Coma Scale score was 14 (eye opening, 4; verbal response, 4; motor response, 6). Contrast-enhanced computed tomography revealed destructive left renal parenchyma replacement with a poor-contrasted area (Figure 1A) and fluid and gas accumulation from the perirenal region to the retroperitoneal cavity (Figure 1B). A giant ureterocele was observed in the left side of the bladder (Figure 1C). The patient was diagnosed with left emphysematous pyelonephritis with a congenital giant ureterocele.
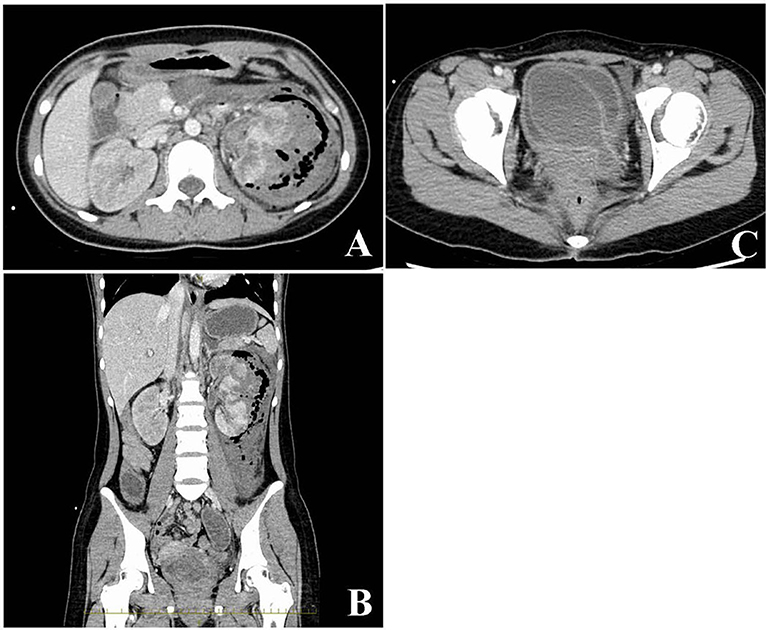
Figure 1. Radiological findings from an abdominal contrasted computed tomography scan. The damaged left kidney exhibits a poorly enhanced area and fluid and gas accumulation in the left retroperitoneal cavity (A,B). A giant ureterocele evident in the bladder (C).
The patient required noradrenalin administration (0.2 μg/kg/min). Her blood pressure remained normal, and surgical treatment, including transurethral ureterocele fenestration followed by urgent nephrectomy, was performed. Pus-containing urine spilled when the ureterocele was opened during fenestration, and a malodor was detected during transperitoneal open left nephrectomy. The excised outer portion of the left kidney had been dissolved by the infection and replaced with blood clots and necrotic tissue. The surgical specimen (weight, 354 g) consisted of the entire left kidney and ureter which was dissected near the bladder. The operative durations for the transurethral ureterocele fenestration and open left nephrectomy were 50 and 228 min, respectively. The estimated intraoperative blood loss was 1,424 mL, and no intraoperative complications occurred. Histopathological examination revealed neutrophils and bacteria in the perirenal fat, renal cortex, and medulla. Four bacterial species were detected in the urine culture, and three bacterial species were found in the blood culture before the administration of antibiotics. Bacteria detected in the urine sample were A. schaalii, Actinomyces turicensis, Prevotella bergensis, and Prevotella disiens.
Matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) identified A. schaalii, P. asaccharolyticus, and A. odontolyticus in the blood culture as the inflammation-causing bacteria. All bacteria detected in urine and blood samples, along with Clostridium perfringens, which was used as the control for detecting gas production, were cultured using a pre-reduced anaerobically sterilized medium (Kyokuto Pharmaceutical Industrial), and the medium was observed after 24 and 48 h (Figures 2A,B). A. schaalii and P. asaccharolyticus were identified as the gas-producing bacteria in the blood culture (Figures 2C,D). Meropenem (1 g/body every 8 h) was initiated on admission and switched to ampicillin-sulbactam (4.5 g/body every 8 h) on postoperative day 4. The total course of antibiotic administration was 14 days. She had experienced wound pain, which decreased in intensity after ~1 week and her general condition improved. She was discharged on day 17 of hospitalization. The patient had no recurrent urinary tract infections after discharge. No postoperative complications were noted 6 months after hospital discharge.
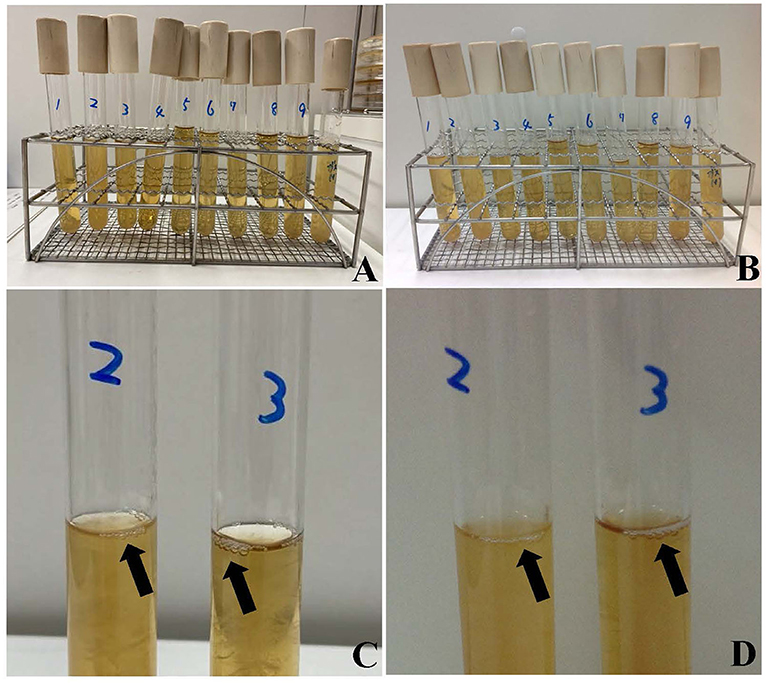
Figure 2. Bacteria detected in urine and blood samples were cultured in a pre-reduced anaerobically sterilized medium. Bacteria detected after 24 h (A) and after 48 h (B). Clostridium perfringens was cultured for comparison of gas production. Actinotignum schaalii and Peptoniphilus asaccharolyticus produced gas in the KM media for 24 h (C), 48 h (D). Bacterial cultures from left to right are Actinotignum schaalii (1), Actinotignum schaalii (2), Peptoniphilus asaccharolyticus (3), Actinomyces odontolyticus (4), Prevotella bergensis (5), Actinotignum schaalii (6), Actinomyces turicensis (7), Prevotella disiens (8), Actinomyces odontolyticus (9), and Clostridium perfringens. Cultures 1–4 and 9 are from blood samples, with 1 and 2, and 4 and 9, cultured from independent blood samples. Cultures 5–8 are from urine samples.
EPN is a relatively rare but rapidly progressive urinary tract infection characterized by gas accumulation in the renal parenchyma, collecting ducts, and perinephric tissue (1, 2). In particular, EPN is extremely rare in children (12). To the best of our knowledge, this is the first reported case of severe EPN with a ureterocele in a pediatric patient with A. schaalii, P. asaccharolyticus, and A. odontolyticus as the causative bacteria.
The first case of EPN in adults was reported in 1898 (13), and the first case of EPN in a pediatric patient was reported in 1985 (6). Several key factors related to the development of EPN have been identified, including high renal glucose levels, urinary tract obstruction, decreased immunity, and presence of gas-producing bacteria (4, 14). Approximately 90% of patients who develop EPN (especially female patients) have diabetes mellitus (2). Conversely, none of the reported cases of EPN in pediatric patients have indicated diabetes mellitus as a comorbidity (Table 1). Obstructive uropathy, neurogenic bladder, kidney stones, and impaired host immunity may be risk factors for pediatric EPN (12).
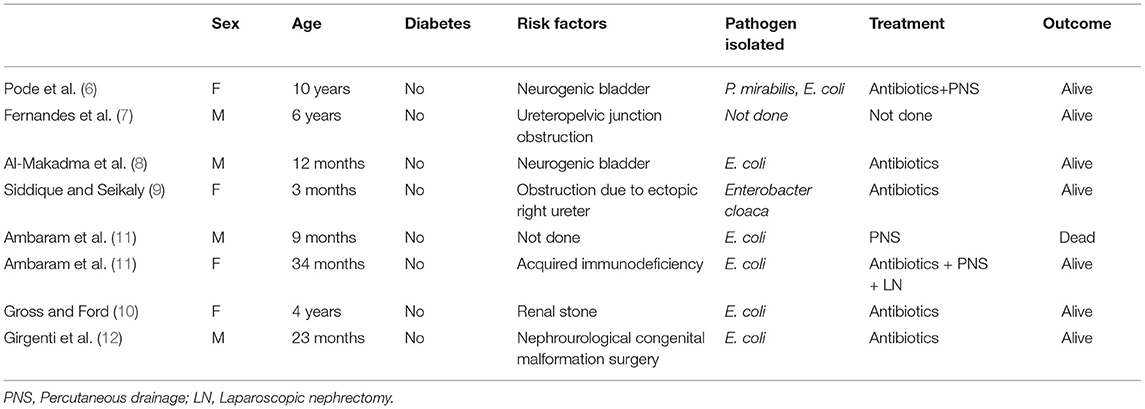
Table 1. Review of the literature regarding emphysematous pyelonephritis in the pediatric age group.
The most common EPN-causing pathogen is E. coli, accounting for ~70% of cases; other pathogens include Proteus mirabilis, Klebsiella pneumoniae, group D Streptococcus, coagulase-negative Staphylococcus, and Enterobacteriaceae (2, 15). The causative pathogens of pediatric EPN are largely unknown; previous reports have indicated that Enterobacteriaceae and E. coli are common (6–12). In our case, several causative pathogens were identified. It is rare for A. schaalii (formerly Actinobaculum schaalii) to cause urinary tract infections. A. schaalii is a facultative, anaerobic, gram-positive, rod-shaped bacterium. The requirement of blood agar media incubated for 48 h under 5% CO2, or in anaerobiosis, to detect A. schaalii in urine or blood samples may cause it to be under-detected because most clinical microbiological laboratories do not routinely culture urine samples under anaerobic conditions (16, 17).
A. schaalii has been reported in urine samples of children aged < 4 years and in older individuals aged > 60 years (18, 19) and is considered a part of the normal urogenital flora (18–20). We used MALDI-TOF MS analysis and performed 16S rRNA gene sequencing, which are reportedly more accurate than polymerase chain reaction in identifying A. schaalii (17). To the best of our knowledge, the ability of A. schaalii to produce gas has not been reported. We observed production of small quantities of gas by A. schaalii. In the patient in the present report, there was no history of diabetes mellitus, which supports the conclusion that the bacteria detected in the urine and blood samples produced the gas in the kidney.
In this case, A. schaalii was resistant to antibiotic therapy. A. schaalii is frequently resistant to trimethoprim–sulfamethoxazole and second-generation quinolones (norfloxacin and ciprofloxacin) (17), so careful selection of antibiotics is needed in urinary tract infections caused by gram-positive, rod-shaped bacteria including A. schaalii, with β-lactams as the first-line treatment.
Although EPN is a life-threatening illness with a mortality rate of 12.5–50% (3, 12), no guidelines have been established for the optimal management of patients with EPN. The main treatment options are percutaneous drainage (PCD) and medical management with or without stenting of the urinary tract (12). Medical management of EPN requires fluid and electrolyte replacement, correction of acid-base imbalances, and antibiotic therapy. Inotropes are also required in some patients (12). Urgent nephrectomy is the standard surgical treatment, though recent surgical strategies suggest that an initial nephron-sparing approach with PCD followed by elective nephrectomy at a later time is effective (4, 12). Nephrectomy has been described in only one pediatric patient (6–12). In this patient, urgent nephrectomy was recommended because of the poor general condition and lack of response to conservative treatment, and good results were obtained. Most cases of EPN in children are treated conservatively; however, given the young age of our patient, PCD may have been preferable to preserve renal function. However, the indications for nephrectomy for pediatric EPN require further study and accumulation of more cases.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Written informed consent was obtained from the minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Michaeli J, Mogle P, Perlberg S, Heiman S, Caine M. Emphysematous pyelonephritis. J Urol. (1984) 131:203–8. doi: 10.1016/S0022-5347(17)50309-2
2. Pontin AR, Barnes RD, Joffe J, Kahn D. Emphysematous pyelonephritis in diabetic patients. Br J Urol. (1995) 75:71–4. doi: 10.1111/j.1464-410X.1995.tb07237.x
3. Lu YC, Chiang BJ, Pong YH, Chen CH, Pu YS, Hsueh PR, et al. Emphysematous pyelonephritis: clinical characteristics and prognostic factors. Int J Urol. (2014) 21:277–82. doi: 10.1111/iju.12244
4. Huang JJ, Tseng CC. Emphysematous pyelonephritis: clinicoradiological classification, management, prognosis, and pathogenesis. Arch Intern Med. (2000) 160:797–805. doi: 10.1001/archinte.160.6.797
5. Nayeemuddin M, Wiseman OJ, Turner AG. Emphysematous pyelonephritis. Nat Clin Pract Urol. (2005) 2:108–12. doi: 10.1038/ncpuro0095
6. Pode D, Perlberg S, Fine H. Emphysematous renal and perirenal infection in nondiabetic patient. Urology. (1985) 26:313–5. doi: 10.1016/0090-4295(85)90139-6
7. Fernandes LP, Sajwany MJ, Derweesh A. Emphysematous pyelonephritis and cystitis associated with bilateral pelviureteric junction obstruction: a case report. J Pediatr Surg. (1998) 33:739–40. doi: 10.1016/S0022-3468(98)90203-2
8. Al-Makadma AS, Al-Akash SI. An unusual case of pyelonephritis in a pediatric renal transplant recipient. Pediatr Transplant. (2005) 9:258–60. doi: 10.1111/j.1399-3046.2004.00276.x
9. Siddique K, Seikaly MG. Emphysematous pyelonephritis in an infant. Pediatr Infect Dis J. (2013) 32:1157–8. doi: 10.1097/INF.0b013e31829aaae3
10. Gross IT Ford R. Emphysematous pyelonephritis in a child with nephrolithiasis. J Pediatr. (2016) 168:250.e1. doi: 10.1016/j.jpeds.2015.09.056
11. Ambaram PR, Kala UK, Petersen KL. Emphysematous pyelonephritis in children. Pediatr Infect Dis J. (2016) 35:1159–61. doi: 10.1097/INF.0000000000001254
12. Girgenti V, Pelizzo G, Amoroso S, Rosone G, Di Mitri M, Milazzo M, et al. Emphysematous pyelonephritis following ureterovesical reimplantation for congenital obstructive megaureter. Pediatric case report and review of the literature. Front Pediatr. (2019) 7:2. doi: 10.3389/fped.2019.00002
13. Kelly HA, MacCallum WG. Pneumaturia. JAMA. (1898) XXXI:375–81. doi: 10.1001/jama.1898.92450080001001
14. Fatima R, Jha R, Muthukrishnan J, Gude D, Nath V, Shekhar S, et al. Emphysematous pyelonephritis: a single center study. Indian J Nephrol. (2013) 23:119–24. doi: 10.4103/0971-4065.109418
15. Roberts JA. Management of pyelonephritis and upper urinary tract infections. Urol Clin North Am. (1999) 26:753–63. doi: 10.1016/S0094-0143(05)70216-0
16. Cattoir V. Actinobaculum schaalii: review of an emerging uropathogen. J Infect. (2012) 64:260–7. doi: 10.1016/j.jinf.2011.12.009
17. Lotte R, Lotte L, Ruimy R. Actinotignum schaalii (formerly Actinobaculum schaalii): a newly recognized pathogen-review of the literature. Clin Microbiol Infect. (2016) 22:28–36. doi: 10.1016/j.cmi.2015.10.038
18. Andersen LB, Bank S, Hertz B, Søby KM, Prag J. Actinobaculum schaalii, a cause of urinary tract infections in children? Acta Paediatr. (2012) 101:e232–4. doi: 10.1111/j.1651-2227.2011.02586.x
19. Bank S, Jensen A, Hansen TM, Søby KM, Prag J. Actinobaculum schaalii, a common uropathogen in elderly patients, Denmark. Emerg Infect Dis. (2010) 16:76–80. doi: 10.3201/eid1601.090761
Keywords: emphysematous pyelonephritis, congenital ureterocele, children, Actinotignum schaalii, Peptoniphilus asaccharolyticus
Citation: Kitano H, Hieda K, Kitagawa H, Nakaoka Y, Koba Y, Ota K, Shigemoto N, Hayashi T, Kashiyama S, Teishima J, Shime N, Ohge H and Hinata N (2021) Case Report: Emphysematous Pyelonephritis With a Congenital Giant Ureterocele. Front. Pediatr. 9:775468. doi: 10.3389/fped.2021.775468
Received: 14 September 2021; Accepted: 28 October 2021;
Published: 26 November 2021.
Edited by:
Pedro Lopez Pereira, University Hospital La Paz, SpainReviewed by:
Huixia Zhou, Bayi Children's Hospital, ChinaCopyright © 2021 Kitano, Hieda, Kitagawa, Nakaoka, Koba, Ota, Shigemoto, Hayashi, Kashiyama, Teishima, Shime, Ohge and Hinata. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hiroyuki Kitano, dGFub2tpbkBoaXJvc2hpbWEtdS5hYy5qcA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.