
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pediatr., 09 December 2021
Sec. Pediatric Cardiology
Volume 9 - 2021 | https://doi.org/10.3389/fped.2021.764239
Along with inadequate access to high-quality care, competing health priorities, fragile health systems, and conflicts, there is an associated delay in evidence generation and research from LMICs. Lack of basic epidemiologic understanding of the disease burden in these regions poses a significant knowledge gap as solutions can only be developed and sustained if the scope of the problem is accurately defined. Congenital heart disease (CHD), for example, is the most common birth defect in children. The prevalence of CHD from 1990 to 2017 has progressively increased by 18.7% and more than 90% of children with CHD are born in Low and Middle-Income Countries (LMICs). If diagnosed and managed in a timely manner, as in high-income countries (HICs), most children lead a healthy life and achieve adulthood. However, children with CHD in LMICs have limited care available with subsequent impact on survival. The large disparity in global health research focus on this complex disease makes it a solid paradigm to shape the debate. Despite many challenges, an essential aspect of improving research in LMICs is the realization and ownership of the problem around paucity of local evidence by patients, health care providers, academic centers, and governments in these countries. We have created a theory of change model to address these challenges at a micro- (individual patient or physician or institutions delivering health care) and a macro- (government and health ministries) level, presenting suggested solutions for these complex problems. All stakeholders in the society, from government bodies, health ministries, and systems, to frontline healthcare workers and patients, need to be invested in addressing the local health problems and significantly increase data to define and improve the gaps in care in LMICs. Moreover, interventions can be designed for a more collaborative and effective HIC-LMIC and LMIC-LMIC partnership to increase resources, capacity building, and representation for long-term productivity.
Low- and middle-income countries (LMICs) encompass 92% of the global disease burden (1), and yet are severely lacking in resources to manage complex care. The disparity of disease burden to resources available is daunting, i.e., HIC has over 100 times more cardiac surgeons than LMIC, and while there are more than 4,000 cardiac centers worldwide, only one center per 10 million population exists in LMIC (2). Despite these differences, there is a lack of basic epidemiologic understanding of the disease burden in these regions. A large knowledge gap is a hindrance toward identifying appropriate solutions to multifaceted healthcare challenges. This stark contrast in disease burden vs. research disparity has important implications toward complex diseases as they remain low on healthcare improvement agendas (3). We use the example of pediatric heart disease to highlight this disparity, the challenges associated with contextual evidence generation and propose some solutions to overcome these barriers.
Congenital heart disease (CHD) is the most common birth defect in children. The prevalence of CHD from 1990 to 2017 has progressively increased by 18.7% (4), and more than 90% of children with CHD are born in LMICs (5). The spectrum of CHD ranges from simple defects managed through conservative observation vs. complex conditions requiring surgical or catheter based intervention for survival. If managed in a timely manner, as in high-income countries (HICs), the majority of children lead a healthy life (5). However, children with CHD in LMICs have limited care available (6). According to Global burden of Disease (GBD) 2017, there are 261,247 deaths due to CHD annually with more than 21 million years of life (YLL) and a similarly high number of disability-adjusted life years (DALYs) lost (4). Since 1990 CHD mortality for infants has declined by only 6% in the low sociodemographic index (SDI) subgroup compared with a decline of more than 50% in the middle to high SDI subgroups (4). Additional to the disease burden, patients in LMICs have a distinct disease spectrum, often present late and with serious comorbidities (e.g., malnutrition, blood stream and lung infections) or complications (7). Inadequate quality of health care, poor infrastructure, cost of care, dearth of equipment, expertise and skills, are some of the problems unique to LMICs adding to the complexity of managing these patients (8). Due to these factors there is significantly higher pre-operative and operative mortality among these patients in LMICs when compared with HICs (9). These patients also have more pre- and post-operative morbidities significantly affecting their outcomes. More than half of pre-operative patients with CHD in LMICs had severe malnutrition (10). Compared to HIC, major infections contributed toward increased post-operative mortality and morbidity (increased ventilation time and ICU stays) in LMICs (11).
The scenario is not much different for acquired heart diseases. There has been a marked decline in the prevalence of rheumatic heart disease (RHD) in developed countries (12). However, in LMICs, RHD continues to pose a significant public health issue. A meta-analysis conducted in 2019 reported an RHD prevalence of 8.2–31.0 per 1,000 in low income countries and 5.5–13.5 per 1,000 in LMICs (13). In 2015, just five countries (India, China, Pakistan, Indonesia, and the Democratic Republic of the Congo) accounted for 73% of the global cases of RHD. Like CHD, patients with RHD also present with significantly advanced disease. Most RHD patients usually present with moderate-to-severe valvular heart disease associated with pulmonary hypertension and up to a quarter of patients present with left ventricular dysfunction, reflecting delayed referral patterns to tertiary care centers (14).
Due to the unique disease spectrum of patients with heart disease in LMICs, management needs to be tailored and demands a thoughtful and cautious approach. Late presenting left to right CHD shunt lesions are rarely seen in HICs, while it is a common occurrence in LMICs. Data around the management of such patients is scarce and leads to variation in practices (15–17). Cyanosis, coagulopathies, diastolic dysfunction, and hyper-viscosity increase post-operative morbidity and mortality in cyanotic heart lesions like the tetralogy of Fallot (7). Similarly, management of critical CHD conditions like transposition of great vessels is remarkably different in LMICs i.e., 2/3rd of patients, in a large global registry of CHD patients from LMICs, presented late and underwent a primary arterial switch operation beyond 4 weeks of age while 20% cases had a two-staged arterial switch (18).
Lack of contextually applicable guidelines leads to the use of HIC guidelines which can be impractical or even impossible and may lead to heterogeneity in care pathways and variability in outcomes (19, 20). LMICs account for 90% of the world's CHD population, yet there is an extreme dearth of data and health care research originating from these countries. The Global Burden of Disease 2017 study on CHD reports little to no direct data on CHD outcomes from a majority of the LMICs (4).
Despite realization of the importance of contextual research and evidence generation for local, cost-effective solutions in the LMIC, progress has been sluggish and uncoordinated. Therefore, it is imperative to recognize the barriers to conducting research and availability of data in LMICs so that systemic solutions can be designed to address them. These barriers can be broadly classified as following:
External funding comprising international donors, grants, and research collaborators encompass 90% of the resources for research in LMICs (21). However, the primary agenda for the largest funding health agencies in LMICs like the USAID, UKAID, Welcome Trust, WHO, UNICEF, Bill and Melinda Gates Foundation, and The Global Fund, continues to be infectious diseases, vaccines, and nutritional disorders. These diseases remain the focus in global health like Sustainable Development Goals indicators, driven mainly by donor interest. From 1990 to 2015, in LMICs, infant mortality rates dropped by more than 50% for infectious diseases and protein malnutrition while the death rate from CHD persisted, springing it up to the 5th leading cause of death (3). Despite this changing landscape of disease spectrum, minimum funds are allocated for evidence generation and research around CHD in LMICs. Policy Cures Research, a global health body for research and data collection and analysis, reported that 70% of the total neglected disease investment focused on HIV/AIDS, tuberculosis, and malaria in 2018, while CHD did not fall in their neglected diseases panel, and acute rheumatic fever received only 0.1% of this funding (22). Deep-seated disparities in the process of fund allocation are further highlighted by a survey conducted in 2010 where 85% of grant reviewers felt inadequately trained in grant review (23).
Many academic medical centers or health care delivery institutes in LMICs fail to adequately recognize their role in developing a clear vision around contextual evidence generation and research to identify more cost-effective measures of health care delivery. Frequently health care “leaders” in LMICs are unable to understand that contextual data and research is probably the most effective way of improving the health care outcomes in their countries (19). Apart from a few large tertiary care centers in LMICs, most local organizations do not invest in or support research conferences, workshops, or reward research productivity as a part of their academic objectives (24). Centers also provide more financial incentives for extra clinical productivity rather than for research work, thus making research work a lesser priority. Moreover, LMICs have a lower patient to physician ratios resulting in competing clinical demands, thus leaving inadequate time for quality research for the inundated physicians working in academic settings. For example, CHD prevalence in Pakistan is comparable to the United States, yet compared to the US there are < 5% pediatric cardiac care centers and < 1% of the pediatric cardiologists present in the country to manage overwhelming burden of disease (17, 25). Similar trends are seen in many other LMICs i.e., 0.04 adult cardiac surgeons and 0.03 pediatric cardiac surgeons per million population, compared with 7.15 adult cardiac surgeons and 1.67 pediatric cardiac surgeons in high-income countries (26). There is a lack of tenured or ring-fenced positions in institutions to incentivize the return of those clinician-scientists who have acquired post-graduate research outside their countries (27).
While the challenge of resources and funds can be addressed through different means (e.g., philanthropy), mentoring local researchers to pursue careers in clinical research is another tremendous challenge. These challenges are mainly due to a scarcity of infrastructure with very few research laboratories, proper equipment, organized national databases and skilled human resources (analysts, masters level, and Ph.D.- level graduates, statisticians, data scientists, etc.) (28). A lack of vision and incentivization to generate local evidence also transcribes into limited mentorship of young physicians and emerging health leaders in the importance of local data and contextual research. Additionally, weak governance and accountability around mentorship lead to inconsistent and ineffective coaching of the early career physicians. Few health care providers with limited research skills enter the workforce not adding much to the national capacity to do high quality research or evidence generation (24).
Poor representation of researchers from LMICs (29), a hierarchal relation between HIC and LMIC research collaborators (30) and a significant publication bias (31) against data from LMIC points toward a disparity between the research/researchers from the HICs vs. those in LMICs especially within the arena of global health (32). Such a disparity is yet another reason for lack of evidence from LMICs.
Global health forums address issues of insufficient resources, funding, and capacity in LMICs. However, for over a decade the Global South (predominantly LMICs) continue to receive sparse representation on leading global health boards and executive councils. It is estimated that 85% of the global health organization headquarters are in the Global North (29). These organizations have established advocacy pathways and funding resources to address global health problems in the Global South which may not be contextually relevant (29). The Consortium of Universities for Global Health (CUGH) is one of the world's largest academic-based organizationss established in 2007 to support academic institutions in addressing global health challenges. In 2016, only two out of the 16-member Board of Directors of CUGH were from the Global South (31). This inequity steers most of the capacity building and training for academic programs in global health toward the Global North while the disease burden exists in the South. Similarly, commissioners from HICs led 72% of the Global Surgery and 73% of the Global Health Lancet commissions (31). Due to the massive underrepresentation of LMIC attendees in global health conferences, the issues facing them are not brought to the forefront. Barely 4% of academic conferences are held in low-income countries, representing <40% of delegates from LMICs (29). This poor representation is due to financial constraints, visa restrictions, political barriers, and failure to actively seek and identify expertise from LMICs (29). Even amongst the low numbers of attendees from LMICs, those that attend have minimal active speaking opportunities, research presentations, and support, thus making their voices left unheard (29). When clinicians and researchers have no opportunities to participate in decision-making panels, it hampers their research interests as their efforts seem to add no significant value to impact clinical guidelines.
In research collaborations between HIC and LMICs, when decisions and conversations partake, the voices and concerns of these researchers in LMIC (usually taking role of “gatekeepers)” remain typically absent. As a result, plans made through such “collaborations” do not align with the academic programs, timetables and teaching systems of centers in the south. This uni-directional “gatekeeping” attitude to “facilitate” scholars from the Global North consumes more resources, time, energy, with little to no emphasis on improvement opportunities (30). An additional challenge is that funding is directed usually toward institutions in the Global North and researchers in Global South being dependent on the funds tend to not speak about these issues and may continue to suffer in silence. The dependence on such funds is also a reason for institutions in LMICs to not support their researchers when they share their concern about such hierarchy in collaborations (33, 34).
Publications in reputable global health journals allows dissemination of identified local challenges and contextual sustainable solutions and thus can direct future policymaking. The power imbalances within global surgery academia are at risk of being transferred to national policy and adversely affecting resource allocation (35). In an ideal situation, even if LMIC researchers tackle all the major gaps in resources and representation to conduct locally relevant research, publication bias still limits their credibility and voice. Bibliometric studies of global health publications have reported inequalities in global health research overall, with marginalization and disempowerment of LMIC authors (35). HICs publish almost all global health journals with very few editor-in-chiefs from LMICs and low representation of editors or editorial board members from LMICs (32). Top 12 health journals have 33% LMIC representation amongst editors and editorial board members (31). Amongst 27 specialty global health journals, 68% of editors and 73% of editors-in-chief came from HICs (36). Consequently, there is an increased preference for publishing research conducted by the developed countries of the Global North (31). This disparity was further aggravated amongst leading female roles from LMICs, encompassing only 4% of leading female editors belonging from LMICs amongst the top 12 health journals (31). LMIC female first authors had manuscripts published in journals with impact factors ~ 14 points lower than papers from their HIC counterparts (36). A systematic analysis of current trends in authorship demographics for global surgery publications reported 51% of authors affiliated only with HICs with over two-thirds of first and last authors were affiliated with at least one HIC institution (35). Elite journals frequently reject papers from LMICs with the presumption of it being of questionable data quality, not of interest to their reader or findings not being generalizable to HICs. Subsequently, authors are advised to submit their articles to local low-impact journals resulting in lower reach and hence limited citations. Unaffordable publication costs of other impactful global health journals add to this engrained disparity and further restrict southern data from being published in reputable journals (37).
The most essential aspect of improving research in LMIC is the realization and ownership of the problem around paucity of local evidence by patients, health care providers, academic centers and governments in these countries. In 2015 alone, poor quality health care in LMIC led to ~ USD six trillion in economic losses (38). Thus, all stakeholders must understand that valuing and investing into data/evidence generation is imperative for their own benefit. Nationally tailored plans increase ownership of problems and even though they might not be perfect, they are likely to have a greater impact (19). Moreover, Southern voices can only demand inclusivity in global health forums if they strengthen their systems, collaborate, come forward as a unified representation to add value to global data. Thus, LMICs must identify the challenges (as described above) and create a clear theory of change (ToC) to address these challenges at a micro- (individual patient or physician or institutions delivering health care) and a macro- (government and health ministries) level. ToC has been widely used and it is considered to be the basis of monitoring and (39) theory-based evaluations (40–42). According to Weiss, a ToC is, “a theory of how and why an initiative works (43).” A robust ToC includes several components that makes the model more systematic (39) i.e., preconditions, interventions, outcome and goal. Preconditions are a necessary requirement, condition or element that should be present for achieving the desired outcome while to fulfill these preconditions it is important to have efficient interventions that helps achieve the outcomes and impact of interest (39). Such ToCs are complex and need a very thoughtful approach. We propose a ToC, its pre-conditions, interventions and metrics (process and outcome) to measure the effectiveness of the intervention to enhance research output from LMICs (Figure 1, Table 1).
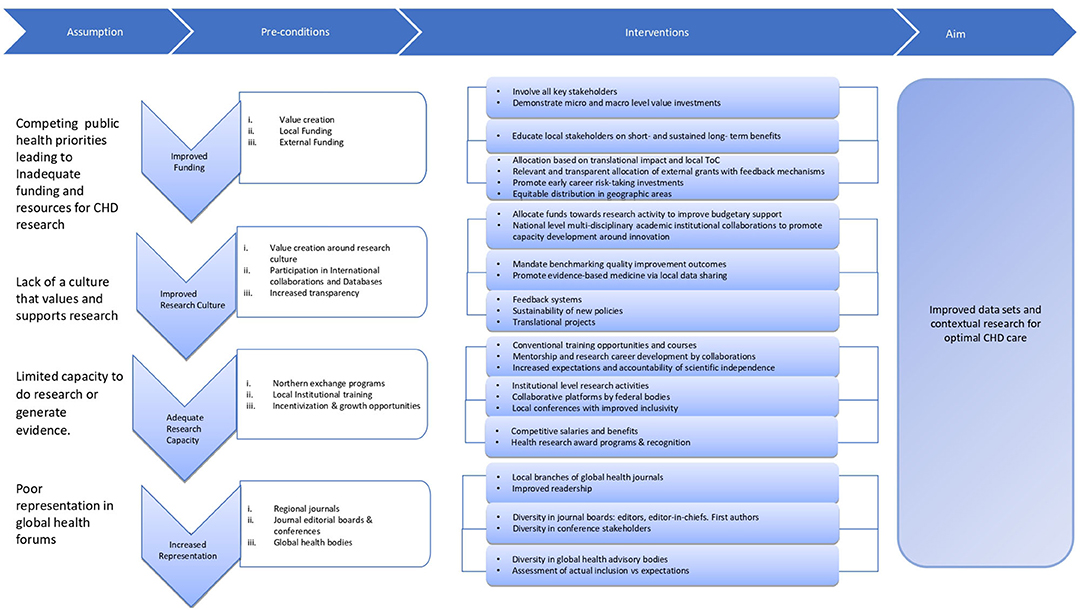
Figure 1. Theory of change around increasing data generation and contextual research output in LMICs.
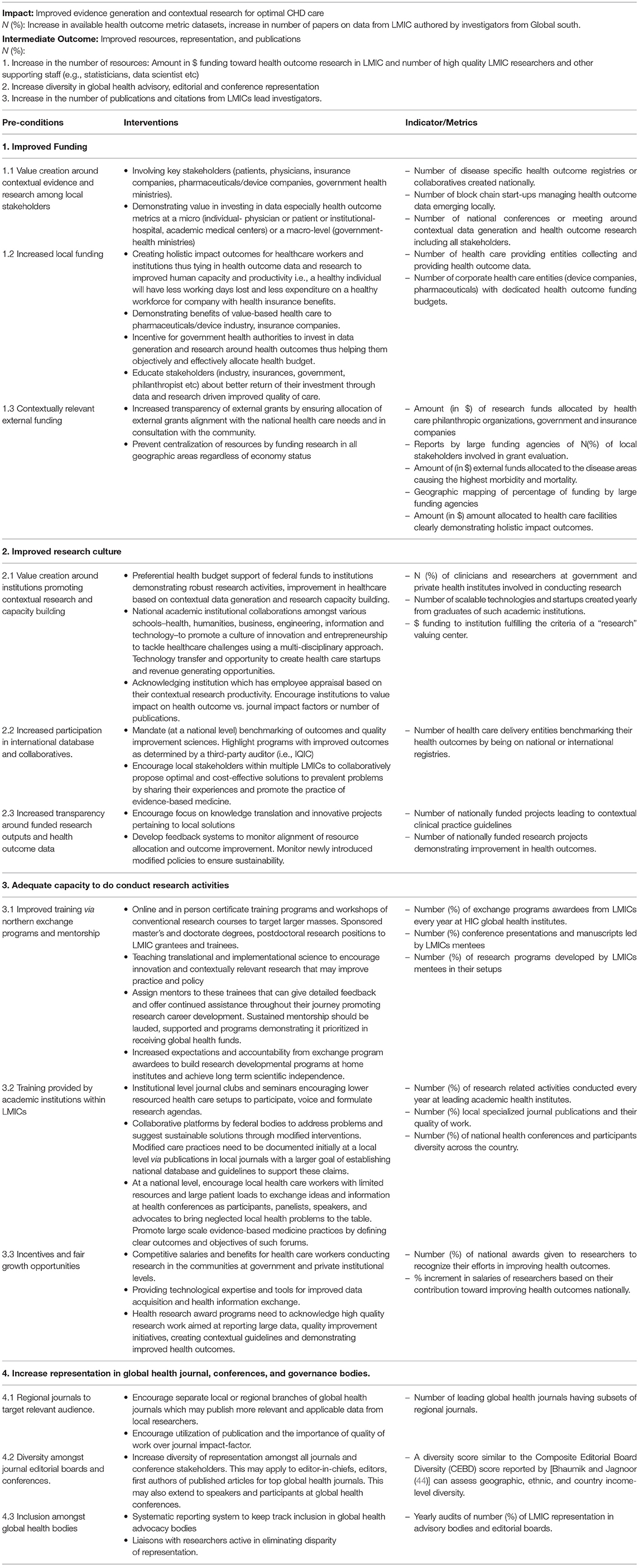
Table 1. A theory of change model to increase data generation and contextual research in low- to middle- income countries.
It is essential that funds are available to generate evidence and conduct contextually relevant research. Without funds, any effort toward creating value around evidence generation and research activities is not sustainable. The value of having high quality holistic outcome data and contextual research especially around quality improvement must be recognized by all stakeholders i.e., patients, health care providers, health care delivery facilities and institutions, insurance companies, health care industry and health ministries/government. Holistic health outcome data (encompassing the patients mental, physical and social wellbeing) (Figure 2), contextual research around service delivery (to improve attainment of healthy state, degree of health recovery and sustainability of health- the three tier of value-based health care outcomes) and innovation can benefit the health care ecosystem at a micro- (individual and institutional stakeholders) and a macro-level (government and health ministries). If and when this buy-in from local stakeholders comes, funds provided by them will be more readily and sustainably available. This realization among stakeholders and subsequent value around contextual evidence generation and research can be created through several ways:
It is a win-win situation where all the stakeholders can benefit if they invest in local evidence generation and contextual research.
Patient/Family Unit as a Stakeholder. Access to data will give patients the autonomy to select the best value for their care. Patients can save more money as poor quality of care is more costly and also leads to more mistrust in the health system which makes them doctor “shop” (38).
Physician and Health Care Delivery Institution. Ability to measure and share their outcomes can give these stakeholders an opportunity to increase their marketability of service, referral, and remuneration from insurance companies, pharmaceutical companies, and the healthcare industry. For example, in Pakistan, atherosclerotic cardiovascular disease (ASCVD) is one of the leading causes of early mortality in young adults with no subsequent assessment of risk factors within the population. The first-ever largest longitudinal cohort study in Pakistan, PAK SEHAT, is being implemented in collaboration with a specialized heart hospital, Tabba Heart Institute and Getz Pharma (https://www.dawn.com/news/1659222) to formulate a comprehensive database on the native Pakistani population to assess the likelihood of developing ASCVD. This serves as a great example of local stakeholders contributing towards locally relevant research in ways that can also be beneficial for them.
Insurance Companies, Industry, and health care philanthropists. In many LMICs, the majority of the cost reimbursement is out of pocket payments (45). Limited health insurance, government subsidies and philanthropists bear the cost of care for patients who cannot afford treatment (the overwhelming majority in LMIC) (46). Having access to health outcome data and quality improvement research may be of interest to these stakeholders as it gives them an opportunity to effectively use their funds. Health care startups like block chain will also find space in such an environment and becomes another stream of value creation and incentives for people to invest in local research activities.
Government. Access to health outcome data and local research can be of value to governments. It helps them achieve good health indicator-based international ratings, which helps governments in LMICs procure more funds and aids from bodies like the International Monetary Fund (IMF) or the World Bank. It also helps them in effective and objective use of their health budgets. Increased medical tourism is another incentive for governments in LMIC to invest in contextual data generation and research. These incentives can make the stakeholders value data and research and thus invest in it as they see it as something that will give them a good return on their investment (ROI).
Demonstrating “ROI” as money saved by improving health outcomes through local innovations and contextually relevant management may help in more sustained local investment. A dialogue with all the stakeholders along these lines will help direct their focus on short- and long-term benefits of improved quality of care offered by investing in contextual research and data generation.
Research funding by external bodies should be allocated based on translational impact and according to the local ToC (47). This has to be mandated by governments in LMICs trickling down to academic and research organizations as this is in the best interest of their country's health needs. External funders will have to respect the needs of the local communities and must realize that their “best” intentions may not necessarily be in the “best” interest of the local communities. One way of assessing these needs would be to involve the local health care stakeholders in the grant evaluation and monitoring process. Feedback mechanisms should also be encouraged to assess long-term progress amongst funded projects and whether the funding indeed has impacted disease burden and local challenges. Transparency of the grant evaluation process needs immediate attention (23) and the concept of repeatedly funding the “safe” researcher (48, 49) needs to be abolished. Building local capacity will only happen by encouraging early career researchers by funding their work even if they seem to be a “risky investment.” Evaluation of investigators must move away from just publications to actual impact. The San Francisco Declaration on Research Assessment (DORA) points out that using the Journal Impact Factor as a proxy measure for the value or quality of specific research may be erroneous and one-dimensional and lead to a biased research assessment and perpetuates the emphasis on publication in certain high-tier journals without a more comprehensive assessment of impact. Another strategy would be to prevent centralization of resource allocation and promote equitable distribution in all geographic areas (50).
Waiting to develop a culture of research only once adequate resources are available, may never fill the research gaps. The approach should be to foster a culture of innovation that can encourage research activities within the available resources. A few successful innovations can subsequently drive more funds. This can be done through several different ways.
Academic medical centers should be encouraged to engage in data generation and research and should be incentivized by giving such centers better budgetary support. Private centers should allocate funds toward research activity, publication costs etc. for clinical research mandated by the government. A government-level oversight will ensure appropriate utilizations of the funds by these centers. Other institutions (business schools, health care leader mentorship programs etc.) should educate their students on the value of data and its objectivity and create data-centric systems to aid in decision-making. They can further educate other stakeholders in the society to propagate a culture of research and data-driven health care. Creating national level collaborations between engineering, business, social science and health care institutions will help develop capacity around innovative fields like data science, artificial intelligence, social innovation and entrepreneurship.
Participating in international databases and collaboratives is a good start to maintain data and build local biorepositories. The International Quality Improvement Collaborative (IQIC) for CHD surgery and catheterization, a web-based platform providing benchmark data on clinical outcomes and resource utilization metrics, is an exemplary template for the collaborations that need to be developed. IQIC allows all institutions, lacking a national database or sufficient resources, to benchmark outcomes and compare their analyses with other LMICs centers. In addition, it also encourages collaborative learning through free webinars and supports local efforts in research activities and manuscript writings. Such measures will allow routine interaction amongst global institutions to share practices and care protocols. It will facilitate conversations between participating hospitals with similar resource backgrounds, who desire to improve the quality of their care and optimize cost-effectiveness and share their experiences through publications (51).
Feedback systems with quarterly reports to assess the progress of research developments can monitor resource allocation and outcome improvement. Meanwhile, newly introduced evidence-based modified policies will have to be monitored regularly to ensure that change sought via these frameworks remains sustainable. Nevertheless, emphasis should be on translation projects pertaining to local issues rather than replicating work done in HICs in a controlled, artificial setup that often cannot be replicated.
Individuals or experts working in the global health arena should be encouraged to act as mentors to early career researchers or clinicians in LMICs. The performance of these experts should be appraised by their affiliated organizations based on the quality of the mentees produced. The long-term expectation should be for these mentees to be autonomous researchers who demonstrate improvement in health outcomes in their respective countries through their work. Such systematic approach of collaborative staff training and knowledge sharing can promote long-term scientific independence (28). A combination of training opportunities such as fellowships, certificate programs, master's and doctorate degrees, postdoctoral positions can be offered to LMIC researchers, especially by programs seeking funding within the global health space. A fixed percentage of grantees and trainees from LMICs should be sponsored to receive these certificate and degree programs with the expectations of building research infrastructures at various institutes in their home countries. While not everyone can be sponsored to train at reputable institutes, utilizing virtual platforms to hold essential research courses at low-to-no costs will allow training in remote areas. Apart from the conventional research courses i.e., research design, grant writing, statistics, a special focus should be given in teaching translational and implementation science so that investigators are primed to be innovative and help catalyze uptake of evidence into policy and practice (52). Use of high quality implementation science has been proposed to understand the contextual factors associated with the scaling of surgical systems in LMICs (53). Such quality evidence around implementation may also help HIC adopt such robustly tested processes in LMICs. These efforts will hone research career development amongst young researchers and improve the workforce. Research collaborations between HIC and LMIC must include capacity building of the LMIC investigators to lead manuscripts and present at various conferences and forums. The accountability of these deliverables has to be monitored by both the collaborating institutions in HIC and LMICs. One initiative in this regard, focusing on growing and disseminating talent in medicine is the Women as One Talent Directory (https://womenasone.org/register/sign-up/) is a robust and sortable online database of talented women in medicine to facilitate professional opportunities and to allow for networking.
At a micro level, large academic institutes should take the initiative to increase the pool of local researchers. The handful of institutions in LMICs which are adequately resourced should champion the cause and hold mandatory institutional level journal clubs and seminars, which could be linked to units without these remotely. This would promote partnerships with lower resourced health care setups to discuss and formulate contextual research agendas. The performance and subsequent funding of such institutions when linked to improvement in health outcomes, will lead to a more concerted and sustained effort by these institutions to promote a culture of contextually relevant research.
At a macro level, federal bodies should develop collaborative platforms to promote translational research and sharing of resources. These platforms can be utilized to address health inequities and encourage innovative sustainable solutions at the local level. There is a need for the establishment of local health journals where articles addressing community problems and data deficits can be published and disseminated. It will help develop modified interventions targeting the local population at-need and encourage discourse on outcomes of modified care practices. If care protocols are accurately reported and accessible to neighborhood local regions, similar setups can adopt successful strategies, thereby causing a bigger impact. Consistent documentation of these practices in multiple centers enables a wider outreach and acceptance. Furthermore, local health journals should invite small rural health care setups to publish data on their patients. Encouraging data from multiple small centers can help form a larger cumulative database in the future and impactful local guidelines (54). The student workforce at teaching hospitals can be given opportunities to participate in data collection to set up these large databases or do mini-research projects. They can use incentives of developing their resume and research mentorship from faculty to participate in these studies.
National governance bodies need to establish health conferences at a national level as an accessible medium for exchanging ideas and information. Such conferences need to have clear objectives and defined outcomes (i.e., establishing of local databases, exchanging ideas, creating research consortiums, tracking progress in heath outcome metrics, etc.). Such a focused approach will help reap the maximum benefit of such gatherings as such conferences are costly and require intense organizing efforts. Health care workers with limited access and large patient loads should be given special attention. They should be encouraged to participate as panelists, speakers, and advocates. It will encourage local stakeholders to gather data through mutual collaboration which can result in comprehensive solutions to prevalent problems and promote the practice of evidence-based medicine.
Once local partnerships are forged and local centers are driven toward establishing national healthcare policies through research and development, partnerships with programs in other LMICs with shared problems and challenges can be considered.
In LMICs where financial benefits lie within extra clinical hours, competitive salaries and benefits for contextual research can incentivize most physicians to begin investing their time toward contextual research. Such an initiative should be encouraged at both, government, and private institutional levels. Recognizing efforts and giving due credit through health research awards and national recognition can further enhance their interests.
While global health journals may find it impossible to be completely inclusive of all regions around the globe, regional subsets of journals may provide more relevant data grouping and improved readership while being cognizant of diversity and inclusivity from all ethnic and income-level backgrounds. This will increase prospects of publication, consolidate ethnic or regional groups of similar data and appeal to a more relevant readership. It will also increase the editorial board members in numbers and provide an incentive toward larger membership and improved diversity on decision making panels. Moreover, an important aspect that stakeholders (e.g., academic institutions) need to consider is the utilization of the publication. Value has to be placed on the quality of work and its significance toward achieving the outcomes rather than the impact-factor of the journal it gets into (37).
Global health journals can support reduction of data disparity by ensuring greater diversity of their editorial boards ranging from directors to editors to promote a balanced and fair perspective. Such an effort can provide a larger number of peer-reviewers and encourage submission from studies conducted in more diverse backgrounds, thus providing a better global health perspective. A commitment for blinded reviews from high-tier journals would be welcomed to preserve the quality and integrity of research reviews (55). Composite Editorial Board Diversity Score (CEBDS) (44) evaluated the diversity in top 27 global health journals amongst gender, geographic and income level regions serving as a great example to quantify actual diversity. A detailed assessment based on a globally approved scoring system may help target the gaps in inclusivity amongst editorial bodies. Furthermore, an assessment of first authors of all publications and their citations can help reflect upon the diversity of acceptance from research conducted in different backgrounds. The data can also be used by institutions in LMIC to advocate for more equitable partnerships. Thus, more inclusion will allow addressing problems in acquiring data at a global level. It will help devise global initiatives to assist in building capacity and funding projects to collect data from LMIC.
A goal for the pediatric heart disease researchers should be to have a unified voice in global forums like the Sustainable Developmental Goals (SDGs) to ensure the disease remains a national priority. This makes the case significantly easier with governments. Moreover, global health advocacy boards and global health journals should also work to increase representation from LMICs through ensuring accountability. Devising a systematic reporting system to keep track of “expected” vs. “real” inclusion of LMICs in global health will be fruitful. Measures should include mandatory yearly audits, reporting the percentage of LMIC representation in advisory bodies and editorial boards. These yearly audits will give concrete numbers to point to the reality of inclusivity and depict trends of improvement overtime. Liaison with groups of researchers active in the space of addressing the disparity in representation in global health will help magnify the efforts toward meaningful inclusivity of LMIC stakeholders in global health (56).
There are significant global inequities amongst the division of global health burden of pediatric cardiac disease and allocation of resources. These barriers are well known in LMIC countries, but it is essential to enumerate them to set the scene for the much less known systemic solutions to address them. Along with inadequate access to high-quality care, competing health priorities, fragile health systems, and conflicts, there is an increased lag of evidence generation and research from LMICs. Lack of basic epidemiologic understanding of the disease burden in these regions poses a significant knowledge gap as solutions can only be developed and sustained if the scope of the problem is accurately defined. Unaligned funding, poor representation in global health advocacy bodies, limited opportunities to publish in global health journals, and limited opportunities to participate and speak at global health conferences hinder progress. While global health bodies advocate for these problems, they tend to take control over local health challenges without focusing on the necessary investment they require. We have designed the solutions using a 'Theory of Change (ToC)' approach to understand how and why certain pathways work. As highlighted in the ToCs, the path begins with national ownership at the top leading to empowerment of the LMIC clinician to publish, especially around clinical outcome data. The proposed strategies have been designed in the best interest of all the stakeholders ensuring sustained engagement of the process. LMICs need to take it upon themselves to improve the health of their populations with the intent to gradually decrease dependence on external funds. The realization must be that an external can only help if “we” put in the effort to resolve our complex healthcare challenges. All stakeholders in society from government bodies, health ministries and systems, to frontline healthcare workers and patients need to be invested to address the local health problems. Moreover, interventions can be designed for a more collaborative and effective HIC-LMIC and LMIC-LMIC partnership directed at increasing resources, capacity building and representation for long-term productivity. We believe that given the strength of the ToC model, it can be very well used by HIC centers too. As a next step, the authors will conduct workshops with a group of practitioner clinicians, hospitals, programs, and other relevant stakeholders to refine the ToC and its associated process/outcome metrics. The listed fundamental outcome metrics of each of the strategies in the ToC table will be the desired goal/outcome for the next set of workshops we have with the relevant stakeholders; for example, the first metric is the number of national disease-specific collaboratives or registries created. In our workshop, we will start with this as the outcome and then map the process with the key stakeholders, including physicians, insurance companies, pharmaceuticals/device companies, and government health ministries listed in the second column. The results of these associated process/outcome metrics will need at least 5 years for a measurable change and are beyond this paper's scope.
BH: conception and design, formulation of ToC, drafted, critically reviewed, and revised the manuscript. MR: conception and design, critically reviewed, and revised the manuscript specifically the theory of change. AW: formulation of ToC, drafted, reviewed, and revised the manuscript. RK and LZ: conception and design, reviewed and revised the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Franzen SR, Chandler C, Lang T. Health research capacity development in low and middle income countries: reality or rhetoric? A systematic meta-narrative review of the qualitative literature. BMJ Open. (2017) 7:E012332. doi: 10.1136/bmjopen-2016-012332
2. Vervoort D, Swain JD, Pezzella AT, Kpodonu J. Cardiac surgery in low- and middle-income countries: a state-of-the-art review. Ann Thorac Surg. (2021) 111:1394–400. doi: 10.1016/j.athoracsur.2020.05.181
3. Zimmerman M, Smith A, Sable CA, Echko M, Naghavi M, Hugo-Hamman C, et al. Relative impact of congenital heart disease on morbidity and mortality in infancy around the globe: the global burden of disease study. Circulation. (2017) 136:A14666–A. doi: 10.1161/circ.136.suppl_1.14666
4. Global regional and and national burden of congenital heart disease 1990–2017: 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet Child Adolesc Health. (2020) 4:185–200. doi: 10.1016/S2352-4642-x
5. Liu Y, Chen S, Zuhlke L, Black GC, Choy MK, Li N, et al. Global birth prevalence of congenital heart defects 1970-2017: updated systematic review and meta-analysis of 260 studies. Int J Epidemiol. (2019) 48:455–63. doi: 10.1093/ije/dyz009
6. Tchervenkov CI, Jacobs JP, Bernier PL, Stellin G, Kurosawa H, Mavroudis C, et al. The improvement of care for paediatric and congenital cardiac disease across the world: a challenge for the world society for pediatric and congenital heart surgery. Cardiol Young. (2008) 18 Suppl 2:63–9. doi: 10.1017/S1047951108002801
7. Iyer PU, Moreno GE, Fernando Caneo L, Faiz T, Shekerdemian LS, Iyer KS. Management of late presentation congenital heart disease. Cardiol Young. (2017) 27:S31–S9. doi: 10.1017/S1047951117002591
8. Zuhlke L, Lawrenson J, Comitis G, De Decker R, Brooks A, Fourie B, et al. Congenital heart disease in low- and lower-middle-income countries: current status and new opportunities. Curr Cardiol Rep. (2019) 21:163. doi: 10.1007/s11886-019-1248-z
9. Webb G, Mulder BJ, Aboulhosn J, Daniels CJ, Elizari MA, Hong G, et al. The care of adults with congenital heart disease across the globe: current assessment and future perspective: a position statement from the international society for adult congenital heart disease (ISACHD). Int J Cardiol. (2015) 195:326–33. doi: 10.1016/j.ijcard.2015.04.230
10. Khan A, Abdullah A, Ahmad H, Rizvi A, Batool S, Jenkins KJ, et al. Impact of international quality improvement collaborative on congenital heart surgery in Pakistan. Heart. (2017) 103:1680–6. doi: 10.1136/heartjnl-2016-310533
11. Sen AC, Morrow DF, Balachandran R, Du X, Gauvreau K, Jagannath BR, et al. Postoperative infection in developing world congenital heart surgery programs: data from the international quality improvement collaborative. Circ Cardiovasc Qual Outcomes. (2017) 10:e002935. doi: 10.1161/CIRCOUTCOMES.116.002935
12. Ordunez P, Martinez R, Soliz P, Giraldo G, Mujica OJ, Nordet P. Rheumatic heart disease burden, trends, and inequalities in the Americas, 1990–2017: a population-based study. Lancet Glob Health. (2019) 7:E1388–E97. doi: 10.1016/S2214-109X(19)30360-2
13. Noubiap JJ, Agbor VN, Bigna JJ, Kaze AD, Nyaga UF, Mayosi BM. Prevalence and progression of rheumatic heart disease: a global systematic review and meta-analysis of population-based echocardiographic studies. Sci Rep. (2019) 9:17022. doi: 10.1038/s41598-019-53540-4
14. Zuhlke L, Engel ME, Karthikeyan G, Rangarajan S, Mackie P, Cupido B, et al. Characteristics, complications, and gaps in evidence-based interventions in rheumatic heart disease: the global rheumatic heart disease registry (the REMEDY Study). Eur Heart J. (2015) 36:1115–22a. doi: 10.1093/EURHEARTJ/EHU449
15. Hasan B, Hansmann G, Budts W, Heath A, Hoodbhoy Z, Jing ZC, et al. Challenges and special aspects of pulmonary hypertension in middle- to low-income regions: JACC state-of-the-art review. J Am Coll Cardiol. (2020) 75:2463–77. doi: 10.1016/j.jacc.2020.03.047
16. Hansmann G, Koestenberger M, Alastalo TP, Apitz C, Austin ED, Bonnet D, et al. 2019 updated consensus statement on the diagnosis treatment of pediatric pulmonary hypertension: the European pediatric pulmonary vascular disease network (EPPVDN), endorsed by AEPC, ESPR ISHLT. J Heart Lung Transplant. (2019) 38:879–901. doi: 10.1016/j.healun.2019.06.022
17. Active Physicians by Age Specialty. (2019). Available online at: https://www.Aamc.org/Data-Reports/Workforce/Interactive-Data/Active-Physicians-Age-and-Specialty-2019 (accessed June 6, 2021).
18. Schidlow DN, Jenkins KJ, Gauvreau K, Croti UA, Giang DTC, Konda RK, et al. Transposition of the great arteries in the developing world: surgery and outcomes. J Am Coll Cardiol. (2017) 69:43–51. doi: 10.1016/j.jacc.2016.10.051
19. Bhakuni H, Abimbola S. Epistemic injustice in academic global health. Lancet Glob Health. (2021) 9:e1465–e1470. doi: 10.1016/S2214-109X(21)00301-6
20. Acharya KP, Pathak S. Applied research in low-income countries: why and how? Front Res Metr Anal. (2019) 4:3. doi: 10.3389/frma.2019.00003
21. Cordero C, Delino R, Jeyaseelan L, Lansang MA, Lozano JM, Kumar S, et al. Funding agencies in low- and middle-income countries: support for knowledge translation. Bull World Health Organ. (2008) 86:524–34. doi: 10.2471/BLT.07.040386
22. World Health Organization. R&D funding flows for neglected diseases (G-FINDER), by disease, year and funding category (2020). Available online at: https://www.who.int/research-observatory/monitoring/inputs/neglected_diseases/en/ (accessed April 24, 2020).
23. Schroter S, Groves T, Hojgaard L. Surveys of current status in biomedical science grant review: funding organisations' and grant reviewers' perspectives. BMC Med. (2010) 8:62. doi: 10.1186/1741-7015-8-62
24. Harris E. Building scientific capacity in developing countries. EMBO Rep. (2004) 5:7–11. doi: 10.1038/sj.embor.7400058
25. Khokhar RA, Gowa MA, Bangash SK, Tahir A. The spectrum of pediatric cardiac procedures and their outcomes: a six-month report from the largest cardiac facility in Sindh, Pakistan. Cureus. (2019) 11:e5339. doi: 10.7759/cureus.5339
26. Vervoort D, Meuris B, Meyns B, Verbrugghe P. Global cardiac surgery: access to cardiac surgical care around the world. J Thorac Cardiovasc Surg. (2020) 159:987–96.e6. doi: 10.1016/j.jtcvs.2019.04.039
27. Ahmed A, Daily JP, Lescano AG, Golightly LM, Fasina A. Challenges and strategies for biomedical researchers returning to low- and middle-income countries after training. Am J Trop Med Hyg. (2020) 102:494–6. doi: 10.4269/ajtmh.19-0674
28. Malekzadeh A, Michels K, Wolfman C, Anand N, Sturke R. Strengthening research capacity in LMICs to address the global NCD burden. Glob Health Action. (2020) 13:1846904. doi: 10.1080/16549716.2020.1846904
29. Velin L, Lartigue JW, Johnson SA, Zorigtbaatar A, Kanmounye US, Truche P, et al. Conference equity in global health: a systematic review of factors impacting LMIC representation at global health conferences. BMJ Glob Health. (2021) 6:e003455. doi: 10.1136/bmjgh-2020-003455
30. Tilley E, Kalina M. “My flight arrives at 5 am, can you pick me up?”: the gatekeeping burden of the African academic. J African Cult Stud. (2021) 33:1–11. doi: 10.1080/13696815.2021.1884972
31. Sheikh K, Bennett SC, El Jardali F, Gotsadze G. Privilege and inclusivity in shaping global health agendas. Health Policy Plan. (2017) 32:303–4. doi: 10.1093/heapol/czw146
32. Nafade V, Sen P, Pai M. Global health journals need to address equity, diversity and inclusion. BMJ Glob Health. (2019) 4:E002018. doi: 10.1136/bmjgh-2019-002018
33. O'Donovan J, Hamala R, Namanda AS, Musoke D, Ssemugabo C, Winters N. 'We are the people whose opinions don't matter'. A photovoice study exploring challenges faced by community health workers in Uganda. Glob Public Health. (2020) 15:384–401. doi: 10.1080/17441692.2019.1663233
34. Kalinga C. Caught between a rock and a hard place: navigating global research partnerships in the global south as an indigenous researcher. J African Cult Stud. (2019) 31:270–2. doi: 10.1080/13696815.2019.1630261
35. Ravi K, Bentounsi Z, Tariq A, Brazeal A, Daudu D, Back F, et al. Systematic analysis of authorship demographics in global surgery. BMJ Glob Health. (2021) 6:E006672. doi: 10.1136/bmjgh-2021-006672
36. Merriman R, Galizia I, Tanaka S, Sheffel A, Buse K, Hawkes S. The gender and geography of publishing: a review of sex/gender reporting and author representation in leading general medical and global health journals. BMJ Glob Health. (2021) 6:E005672. doi: 10.1136/bmjgh-2021-005672
37. Gupta A, Reddy V, Solanki H. Cost in high impact journals: the problem for researchers from low-and middle-income countries. Int J Public Health Res. (2018) 5:45–9. doi: 10.17511/ijphr.2018.i1.06
38. Kruk ME, Gage AD, Arsenault C, Jordan K, Leslie HH, Roder-DeWan S, et al. High-quality health systems in the sustainable development goals era: time for a revolution. Lancet Glob Health. (2018) 6:E1196–E252. doi: 10.1016/S2214-109X(18)30386-3
39. Mayne J. Theory of change analysis: building robust theories of change. Can J Program Eval. (2017) 32:155–173. doi: 10.3138/cjpe.31122
40. Coryn CL, Noakes LA, Westine CD, Schröter DC. A systematic review of theory-driven evaluation practice from 1990 to 2009. Am J Eval. (2011) 32:199–226. doi: 10.1177/1098214010389321
41. Donaldson SI. Program Theory-Driven Evaluation Science: Strategies and Applications. New York: Psychology Press (2007).
42. Funnell SC, Rogers PJ. Purposeful Program Theory: Effective use of Theories of Change and Logic Models. John Wiley & Sons, San Francisco: Jossey-Bass (2011).
43. Weiss CH. Theory-based evaluation: past, present, and future. New Dir Eval. (1997) 1997:41–55. doi: 10.1002/ev.1086
44. Bhaumik S, Jagnoor J. Diversity in the editorial boards of global health journals. BMJ Glob Health. (2019) 4:E001909. doi: 10.1136/bmjgh-2019-001909
45. Qin VM, Hone T, Millett C, Moreno-Serra R, McPake B, Atun R, et al. The impact of user charges on health outcomes in low-income and middle-income countries: a systematic review. BMJ Glob Health. (2018) 3(Suppl 3):E001087. doi: 10.1136/bmjgh-2018-001087
46. Chang AY, Cowling K, Micah AE, Chapin A, Chen CS, Ikilezi G, et al. Past, present, and future of global health financing: a review of development assistance, government, out-of-pocket, and other private spending on health for 195 countries, 1995–2050. The Lancet. (2019) 393:2233–60. doi: 10.1016/S0140-6736(19)30841-4
47. Breedt DS, Odland ML, Bakanisi B, Clune E, Makgasa M, Tarpley J, et al. Identifying knowledge needed to improve surgical care in Southern Africa using a theory of change approach. BMJ Glob Health. (2021) 6:E005629. doi: 10.1136/bmjgh-2021-005629
48. Gyorffy B, Herman P, Szabó I. Research funding: past performance is a stronger predictor of future scientific output than reviewer scores. J Informetr. (2020) 14:101050. doi: 10.1016/j.joi.2020.101050
49. Murray DL, Morris D, Lavoie C, Leavitt PR, MacIsaac H, Masson ME, et al. Bias in research grant evaluation has dire consequences for small universities. PLoS ONE. (2016) 11:E0155876. doi: 10.1371/journal.pone.0155876
51. Hickey PA, Connor JA, Cherian KM, Jenkins K, Doherty K, Zhang H, et al. International quality improvement initiatives. Cardiol Young. (2017) 27:S61–S8. doi: 10.1017/S1047951117002633
52. Eboreime EA, Olawepo JO, Banke-Thomas A, Abejirinde IO, Abimbola S. Appraising and addressing design and implementation failure in global health: a pragmatic framework. Glob Public Health. (2021) 16:1122–30. doi: 10.1080/17441692.2020.1814379
53. Jumbam DT, Durnwald L, Munabi N, Ayala R. Implementation science: a missing link in global surgery. ECAJS. (2021) 26. doi: 10.4314/ecajs.v26i2.1
54. Kumar RK. Delivering pediatric cardiac care with limited resources. Ann Pediatr Cardiol. (2014) 7:163–6. doi: 10.4103/0974-2069.140825
55. Manchikanti L, Kaye AD, Boswell MV, Hirsch JA. Medical journal peer review: process and bias. Pain Physician. (2015) 18:E1–14. doi: 10.36076/ppj/2015.18.E1
Keywords: complex care, contextual clinical research, global health, global health inequity, research disparity, theory of change
Citation: Hasan BS, Rasheed MA, Wahid A, Kumar RK and Zuhlke L (2021) Generating Evidence From Contextual Clinical Research in Low- to Middle Income Countries: A Roadmap Based on Theory of Change. Front. Pediatr. 9:764239. doi: 10.3389/fped.2021.764239
Received: 25 August 2021; Accepted: 18 November 2021;
Published: 09 December 2021.
Edited by:
Hannes Sallmon, Deutsches Herzzentrum Berlin, GermanyReviewed by:
Krittika Joshi, Children's Hospital of San Antonio, United StatesCopyright © 2021 Hasan, Rasheed, Wahid, Kumar and Zuhlke. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liesl Zuhlke, bGllc2wuenVobGtlQHVjdC5hYy56YQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.