- Section of Human Anatomy, Department of Neuroscience, University of Padova, Padova, Italy
In this report, we describe an autopsy case of a child affected by acute lymphoblastic leukemia and opportunistic pulmonary aspergillosis. The patient died because of a full-thickness tracheal wall ulceration with right inferior thyroid artery lesion and sudden hemorrhage, likely ascribable to undiagnosed invasive Aspergillus laryngotracheitis. Aspergillus infection, particularly in immunocompromised patients, should be considered an urgent risk factor to manage as it may lead to sudden fatal events in absence of evident critical symptoms.
Introduction
Aspergillus species (spp.) are responsible for opportunistic infections inducing a wide spectrum of diseases (sinusitis, bronchitis, allergic bronchopulmonary aspergillosis, aspergilloma, invasive aspergillosis) whose severity is related to the host's immunity (1, 2). Specifically, among immunocompromised patients, invasive pulmonary aspergillosis is the most common clinical manifestation, leading to severe life-threatening conditions, high morbidity/mortality and healthcare costs (3–5). The infection is typically associated with pulmonary parenchymal invasion, inflammation, and possible hematogenous spread (1). The risk for its occurrence increases along with the extent/duration of neutropenia, coexistence of other pathological conditions (i.e., solid/hematological malignancies, human immunodeficiency virus (HIV) infection, graft-versus-host disease), or specific therapies/clinical approaches (i.e., corticosteroid or immunosuppressive agents, chemotherapy, hematopoietic stem cell or solid organ transplantation) (6–9). Prompt initiation of systemic/local antifungal therapy and restoration of host defenses are fundamental to improve outcomes and patient survival. In fact, once the respiratory failure occurs, the prognosis is poor (10–13).
An uncommon variant of invasive pulmonary aspergillosis is Aspergillus laryngotracheobronchitis (1, 14–16), which, in turn, can be subdivided into three types: obstructive, showing massive intraluminal growth of Aspergillus ssp. with the presence of thick mucus plugs; pseudomembranous necrotizing, showing formation of whitish pseudomembranes characterized by the presence of hyphae, fibrin, and necrotic debris; and ulcerative, characterized by focal lesions penetrating the tracheobronchial wall with possible formation of bronchoesophageal or bronchoarterial fistulas (14, 17, 18). These three variants may coexist in different portions of the laryngo-tracheobronchial tract or may represent different stages of disease development (18). Moreover, their diagnosis may be difficult due to non-specific clinical manifestation (especially in the early stages) and lacking in typical radiographic findings (9). Direct bronchoscopy for airway visualization combined with bronchial biopsy is reported to be the gold standard for early identification of the infection. Unfortunately, the procedure is not free from risks because of possible hemorrhage associated with the removal of the infected material (6, 12, 19). Death is usually due to multiple organ failure or airway obstruction and acute respiratory distress syndrome (ARDS) (6).
In this scenario, larynx aspergillosis is an extremely rare condition being usually an expression of a widespread infection rather than an isolated process (20). It occurs as dissemination from lower airways (21), secondary to pulmonary involvement (22), invasive aspergillosis of the tracheobronchial tree (23) and bronchopulmonary disease (24, 25).
To the best of our knowledge, we here report the first autopsy case of a pediatric immunocompromised patient likely affected by invasive Aspergillus laryngotracheitis, who suddenly died of hemorrhage caused by full-thickness ulceration of the tracheal wall with injury of the right inferior thyroid artery.
Case Description
A child of age below 6 years old was diagnosed for pre-B acute lymphoblastic leukemia, rearranged TEL/AML = t (12; 21), SNC1; hence, chemotherapy started according to the international collaborative treatment protocol AIEOP2017. At day 52 after the start of chemotherapy, fever occurred (38.6°C) (also accompanied by a 20-day catarrhal cough), and it was managed with Piperacillin-Tazobactam therapy. Diagnostic insights also revealed positivity only for Rhinovirus in the superficial respiratory secretions. At day 63, because of good general conditions, the patient was discharged; however, at day 66, the child was accompanied to the emergency department for fever recurrence. Bone marrow aplasia with very severe leukopenia (0.3 × 103/μL), thrombocytopenia (7 × 103/μL), and anemia (hemoglobin: 7.0 g/dL) were diagnosed, requiring hospitalization in pediatric onco-hematology. Positivity for Aspergillus and pan-fungal antigens (i.e., galatomannan and beta-D-glucan, respectively) was detected (Table 1); mycete culture in nasopharyngeal aspirate and peripheral blood were negative. Cytomegalovirus (CMV) presence was also shown. Additionally, in a clinical setting characterized by the persistence of medullary aplasia, a computed tomography (CT) scan showed the presence of a worsening interstitial pneumonia. Therapy consisted of antibiotics (Piperacillin-Tazobactam), antivirals (Foscavir), and antifungals (Amphotericin B); transfusions of both platelets and concentrated red blood cells were also performed, together with granulocyte colony-stimulating factor administration. Specifically, as regards antifungal therapy, Amphotericin B dosage was adjusted over time (Table 1): from 3 mg/kg/day (days 69–76) to 5 mg/kg/day (days 77–82). At day 83, the child was transferred to the pediatric intensive care unit; positivity for aspergillary and pan-fungal antigens together with the presence of CMV-DNA persisted. The dosage of Amphotericin B therapy was increased to 6.25 mg/kg/day. At day 90, responsiveness to antibiotic/antiviral/antifungal treatments led to a significant improvement of the clinical picture; however, 3 days later, following a sudden cough, the child presented a very abundant bleeding from the oral cavity initially supposed to be of gastric origin. The hemorrhage required resuscitation with ventilation, tracheal intubation, and external cardiac massage for the onset of extreme bradycardia followed by asystole. Despite the resuscitation maneuvers, the infusion of fluids, adrenaline, and bicarbonate, the child died (Table 1).
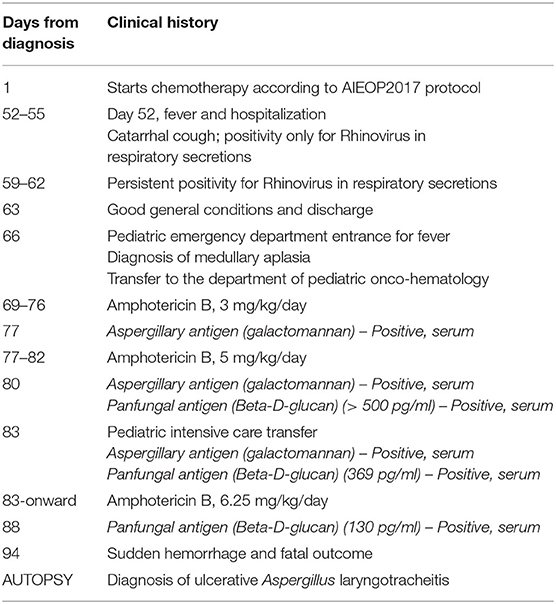
At autopsy, blood presence was observed in the lumen of the upper tracts of the alimentary canal (i.e., the oral cavity, pharynx, esophagus, stomach, and duodenum) in the absence of wall injury (Figures 1A–C).
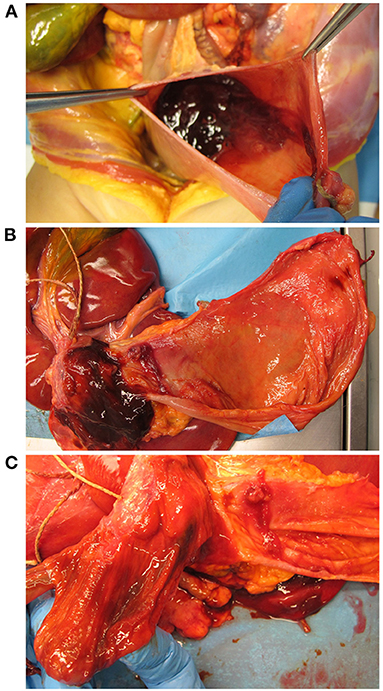
Figure 1. Macroscopic examination of the gastric, pyloric, and duodenal mucosa. Opening of the stomach showing presence of a brownish gelatinous material inside, (A) which, once removed, revealed an intact mucosa without signs of lesions (B). Presence of brownish gelatinous material in the duodenum (B) with no sign of ulcerative alteration of the pyloric and duodenal mucosa (C).
Considering the laryngotracheal tract, three ulcerative lesions with destruction of the cartilage components were observed; necrotic pseudomembranous features were partly also recognized (Figures 2A–D). Specifically, the ulcers were located at the anterior aspect of the cricoid cartilage (circular appearance, 1 cm in diameter); at the left antero-lateral aspect of the first tracheal ring (circular appearance, 1 cm in diameter); at the right antero-lateral side of the trachea along the first tracheal rings (oriented according to the tracheal axis, 1 × 4.5 cm) (Figures 2A–C). Death occurred as a consequence of right inferior thyroid artery lesion due to full-thickness ulcerative perforation of the tracheal wall. An artery protruded at the cranial end of the right anterolateral tracheal ulcer (Figure 2C, insert).
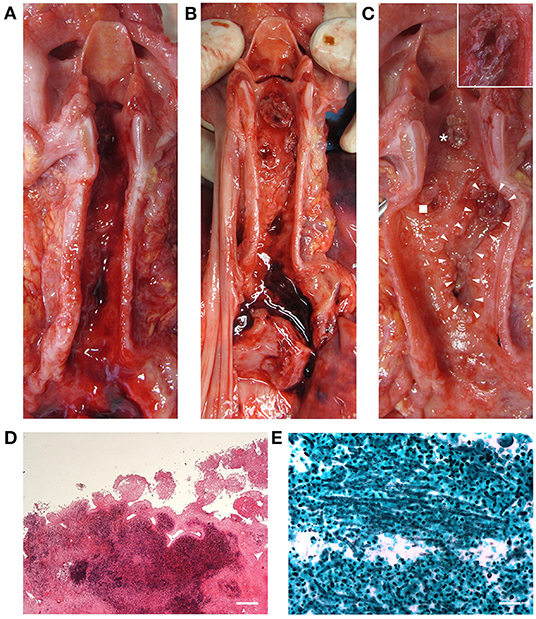
Figure 2. Macroscopic examination of the laryngeal cavity and tracheal lumen. Opening of the larynx, trachea, and bronchi with evidence of blood (A). Laryngotracheal ulcerative lesions with presence of some necrotic pseudomembranous-like features (asterisk, cricoid lesion; square, left anterolateral tracheal lesion; triangles, right anterolateral tracheal lesion) consistent with invasive Aspergillus laryngotracheitis (B,C); the insert in (C) shows a magnification of the site corresponding to tracheal wall perforation and thyroid artery damage. Histopathologic examination by hematoxylin and eosin of the laryngeal mucosa at the site of ulceration (transversal section), showing inflammatory infiltration and signs of tissue necrosis (Scale bar: 400 μm) (D). Grocott–Gomori's methenamine silver stain showing Aspergillus branching hyphae (black) infiltrating the laryngotracheal wall in a necrotic ground (E) (Scale bar: 50 μm).
Macroscopic examination of the lungs showed patchy black/red areas of hemorrhagic invasion of airspaces (Figure 3A).
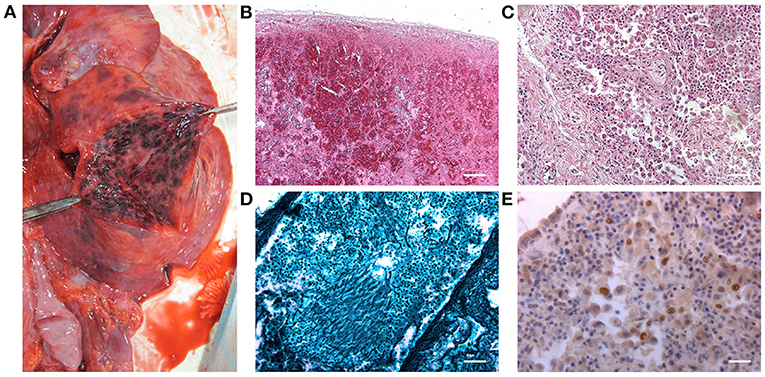
Figure 3. Macroscopic appearance of the inferior lobe of the right lung after surface incision (A). Histopathological characterization by hematoxylin and eosin stain of the right lung parenchyma: hemorrhagic invasion of the air spaces (B) and the typical appearance of the tissue in interstitial pneumonia (C) were detected [Scale bars: 200 μm (B); 50 μm (C)]. Grocott–Gomori's methenamine silver stain showing Aspergillus branching hyphae (black) infiltrating the lung parenchyma (D) (Scale bar: 50 μm). Immunohistochemical staining for CMV, highlighting the presence of immunoreactive cells (E) (Scale bar: 25 μm).
The laryngo-tracheal lesions and the lungs were sampled, fixed in 10% buffered formalin, and processed for histopathological analyses. Specifically, all tissues underwent hematoxylin and eosin staining (Figures 2D, 3B,C, respectively) and the presence of hyphae was detected by Grocott–Gomori's methenamine silver (GMS) staining (Figures 2E, 3D, respectively). In parallel, immunoperoxidase staining was performed on a Dako EnVision Autostainer according to manufacturer recommendations; antibody for E-13 CMV (monoclonal mouse anti-human, aurogene, code number: 11-003) was used to detect presence of eventual positive cellular elements. Regarding the lung tissue, focal areas of necrosis, likely associated to Aspergillus infection (as confirmed by hyphae presence) and interstitial pneumonia evidence, consistent with CMV etiopathogenesis, were observed; hemorrhagic invasion of airspaces was also found (Figures 3B–E).
Discussion
Invasive Aspergillus laryngotracheitis is a rare clinical variant of invasive pulmonary aspergillosis, characterized by local invasion of the laryngotracheal wall by Aspergillus spp. As it typically affects immunocompromised patients, prolonged neutropenia is recognized among the main risk factors for its onset (18, 26–28). Considering the typical clinical manifestations associated with invasive laryngotracheal aspergillosis (obstructive/pseudomembranous/ulcerative elements), their coexistence may occur in the laryngo-tracheobronchial tract, thus leading to a complex clinical picture (18).
Revising the literature, ulcerative disruption of respiratory tract, descending from invasive Aspergillus laryngotracheal infection, are reported in adults [e.g., (9, 10, 14, 20, 25, 29–46)] and in adolescents (15 and 17 years old) (47, 48) but not in pediatric patients. According to our knowledge, only Barnes et al. (49) and Athanassiadou et al. (50) described Aspergillus laryngotracheobronchitis in children (6 and 2 years old) affected by acute lymphocytic leukemia; however, in these cases, the characterizing elements included plaques and necrotic cells, whereas ulcerative lesions were not reported. Moreover, the infection positively resolved with proper antifungal treatment without recurrence.
Here, we describe for the first time to our knowledge, a fatal hemorrhagic event caused by a previously undiagnosed ulcerative Aspergillus laryngotracheitis in an immunocompromised child. On examination, the presence of ulcerative lesions, also displaying necrotic tissue evidence, were recognized in the larynx and trachea. Specifically, the histopathological analysis showed compromised cartilage integrity and, more surprisingly, a full thickness perforation of the tracheal wall. This event led to right inferior thyroid artery damage followed by sudden hemorrhage and death.
Revising the literature, complete tracheal wall perforation by Aspergillus was described by Gonzalez et al. (48) reporting about an immunocompromised adolescent (recurrent precursor B-cell acute lymphoblastic leukemia) showing a 15 ×15 mm large defect of the right-sided distal tracheal wall on the membranous pars; communication with the right pleural cavity was established. In that case, the perforation was successfully repaired and resolved using a pedicle muscular flap (latissimus dorsi) and temporary airway stenting. Additionally, Swiss et al. (44) reported about an invasive Aspergillus laryngopharyngitis affecting an immunocompromised woman (history of myelodysplastic syndrome and acute myelogenous leukemia) and leading to autolaryngectomy: laryngeal destruction up to complete laryngotracheal separation were observed. The patient survived by aggressive antifungal therapy and surgical debridement. Only fatal hemorrhage amenable to necrotizing tracheobronchial aspergillosis was referred by Berlinger and Freeman (10). A 21-year-old immunocompromised man (acute lymphoblastic leukemia) died of a profuse and uncontrollable bleeding due to a fistula in the bronchus intermedius, which, in turn, compromised the right pulmonary artery. To our knowledge, the other case reports describing ulcers in Aspergillus laryngo-tracheobronchitis were mainly all positively managed with disease resolution. As for the fatal cases, death occurred for exacerbation of the concomitant disease (20, 36, 39) or complications secondary to its therapy (i.e., bone marrow transplantation) (47), respiratory failure, ARDS, and pulmonary disease (9, 10, 38, 41–43) up to cardiopulmonary arrest (29).
The specific elements (pediatric age) and the clinical findings (larynx involvement; full-thickness tracheal perforation; thyroid artery damage; death for sudden hemorrhage) here reported confirm the unicity of this autopsy case showing features descending from fungal infection/invasion (as confirmed by GMS staining) and that may be likely ascribed to ulcerative Aspergillus laryngotracheitis. The most frequent complaints associated to Aspergillus laryngotracheobronchitis include productive cough, fever, dyspnea, chest pain, and hemoptysis (9, 33, 51); it is likely that both the cough and recurrent vomiting played a fundamental role in worsening the severity of the laryngotracheal lesions, thus inducing the fatal bleeding event. Additionally, the severe thrombocytopenia, characterizing the bone marrow aplasia, furtherly aggravated the hemorrhagic syndrome as consequence of hemostasis alteration. In this context, the severity of the clinical conditions described may also be further worsened by CMV interstitial pneumonia whose presence is prevalent in immunocompromised hosts; according to the literature, immunocompromised children are expected to develop a pneumonic process in about 80% of the cases (52). Specifically, clinical presentation of CMV interstitial pneumonia includes cough, increased work of breathing, hypoxemia, diffuse adventitious lung sounds and persistent fever too (53, 54).
Although Aspergillus was probably involved in the present case, other thin septate fungi could also be considered in differential diagnosis, such as Penicillium and Talaromyces. For instance, Talaromyces marneffei (formerly Penicillium marneffei), despite mainly affecting HIV-positive patients, triggers symptoms similar to that here described (fever, cough, and dyspnea) in HIV-negative patients. However, only a few cases were reported in presence of hematological malignancies in children (55). Additionally, this infection is typically associated to Southeast Asia regions (and not Europe) (56, 57).
In conclusion, invasive Aspergillus may trigger aggressive pathological conditions, especially in immunocompromised patients; multiple sites of infection may coexist, and they can be also characterized by different specific features. Apart from these physio-pathological and clinical considerations, it is also important to stress that in this case the ulcerative tracheobronchitis was only diagnosed at autopsy and the hemorrhagic event occurred in a quite surprising way for the physicians. Unfortunately, diagnosis of Aspergillus laryngotracheitis is particularly difficult due to its rarity and ambiguous symptoms presence, especially in pediatric patients. It descends that pediatric clinical practice on invasive Aspergillus ssp. related diseases largely derives from data gathered in adult subjects, itself suffering from significant gaps on prevention, diagnosis, and treatment (5).
Considering the clinical setting described, further elements have been provided for more consciousness on eventual fatal consequences ascribable to invasive aspergillosis. A high index of suspicion should be adopted in case of Aspergillus opportunistic infection in particularly weak patients. In fact, early infection identification and rapid therapy initiation undoubtedly represent the keystones to avoid unexpected ominous outcomes with safe resolution (58). Additionally, multiple diagnostic approaches should be considered due to possible different sensitivities of culture and antigen tests (59). In the present case, for instance, mycete cultures were negative, although in the presence of several antigen tests positivities (i.e., galatomannan and beta-D-glucan, respectively). In accordance with Kuo et al. (20), bronchoscopy should be recommended in immunocompromised patients showing signs of airway hoarseness, unexplained sore throat, or obstruction to guarantee adequate clinical management and avoid poor prognosis: reestablishment of immune function is mandatory for good outcomes. Physicians should be better aware about the risk of ulcerative Aspergillus laryngotracheitis in immunocompromised patients and the possibility of rapidly fatal hemorrhagic evolution although rare.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author Contributions
AP and RDC performed the autopsy, identified the case, and sampled the tissues. AE performed the histological and immunohistochemical analyses. RDC, AP, and VM were responsible of data interpretation, clinical correlations, and performed the final supervision of the manuscript. AP and ES conducted the literature search. ES prepared the first draft of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Barnes PD, Marr KA. Aspergillosis: spectrum of disease, diagnosis, and treatment. Infect Dis Clin North Am. (2006) 20:545–61. doi: 10.1016/j.idc.2006.06.001
2. Chouksey S, Thulasidas PA. Rare Case of Vocal Cord Aspergillosis. Indian J Otolaryngol Head Neck Surg. (2019) 71:868–71. doi: 10.1007/s12070-019-01626-w
3. Brissaud O, Guichoux J, Harambat J, Tandonnet O, Zaoutis T. Invasive fungal disease in PICU: epidemiology and risk factors. Ann Intensive Care. (2012) 2:6. doi: 10.1186/2110-5820-2-6
4. Sheth MC, Paul RR, Mathews SS, Albert RR. Isolated aspergillus laryngitis: spectrum, management, and review of literature. J Voice. (2020) 28:S0892-1997(20)30345-3. doi: 10.1016/j.jvoice.2020.09.009
5. Otto WR, Green AM. Fungal infections in children with haematologic malignancies and stem cell transplant recipients. Br J Haematol. (2020) 189:607–24. doi: 10.1111/bjh.16452
6. Antonogiannaki EM, Proklou A, Tamiolakis D, Vassalou E, Kondili E. Invasive Aspergillus tracheobronchitis in a patient with hairy cell leukemia and previous Plasmodium falciparum infection. Monaldi Arch Chest Dis. (2019) 89:2329. doi: 10.4081/monaldi.2019.1055
7. Barberán J, García-Pérez FJ, Villena V, Fernández-Villar A, Malmierca E, Salas C, et al. Development of Aspergillosis in a cohort of non-neutropenic, non-transplant patients colonised by Aspergillus spp. BMC Infect Dis. (2017) 17:34. doi: 10.1186/s12879-016-2143-5
8. Warris A, Lehrnbecher T, Roilides E, Castagnola E, Brüggemann RJM, Groll AH, et al. guideline: diagnosis and management of invasive aspergillosis in neonates and children. Clin Microbiol Infect. (2019) 25:1096–113. doi: 10.1016/j.cmi.2019.05.019
9. Fernández-Ruiz M, Silva JT, San-Juan R, de Dios B, García-Luján R, López-Medrano F, et al. Aspergillus tracheobronchitis: report of 8 cases and review of the literature. Medicine (Baltimore). (2012) 91:261–73. doi: 10.1097/MD.0b013e31826c2ccf
10. Berlinger NT, Freeman TJ. Acute airway obstruction due to necrotizing tracheobronchial aspergillosis in immunocompromised patients: a new clinical entity. Ann Otol Rhinol Laryngol. (1989) 98:718–20. doi: 10.1177/000348948909800911
11. Baggott C, Sharp C, Bhatt N, Plummeridge M, Adamali H. Lesson of the month 1: a cough that doesn't fit the mould. Clin Med (Lond). (2015) 15:492–4. doi: 10.7861/clinmedicine.15-5-492
12. Gauguet S, Madden K, Wu J, Duncan C, Lee GS, Miller T, et al. Case report of a child after hematopoietic cell transplantation with acute aspergillus tracheobronchitis as a cause for respiratory failure. Case Rep Pediatr. (2016) 2016:9676234. doi: 10.1155/2016/9676234
13. Hope WW, Walsh TJ, Denning DW. Laboratory diagnosis of invasive aspergillosis. Lancet Infect Dis. (2005) 5:609–22. doi: 10.1016/S1473-3099(05)70238-3
14. Barry ME, Thomas WW, Song B, Mirza N. Invasive aspergillus laryngotracheobronchitis in an adult with primary CNS Lymphoma. Mycopathologia. (2017) 182:733–7. doi: 10.1007/s11046-017-0123-y
15. Walsh TJ, Anaissie EJ, Denning DW, Herbrecht R, Kontoyiannis DP, Marr KA, et al. Infectious Diseases Society of America. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. (2008) 46:327–60. doi: 10.1086/525258
16. Kim DS, Jeong JS, Kim SR, Jin GY, Chung MJ, Lee YC, et al. Case report of mass-forming aspergillus tracheobronchitis successfully treated with voriconazole. Medicine (Baltimore). (2015) 94:e1434. doi: 10.1097/MD.0000000000001434
17. Denning DW. Commentary: unusual manifestations of aspergillosis. Thorax. (1995) 50:812–3. doi: 10.1136/thx.50.7.812
18. Casal RF, Adachi R, Jimenez CA, Sarkiss M, Morice RC, Eapen GA. Diagnosis of invasive aspergillus tracheobronchitis facilitated by endobronchial ultrasound-guided transbronchial needle aspiration: a case report. J Med Case Rep. (2009) 3:9290. doi: 10.1186/1752-1947-3-9290
19. He R, Hu C, Niu R. Analysis of the clinical features of tracheobronchial fungal infections with tumor-like lesions. Respiration. (2019) 98:157–64. doi: 10.1159/000496979
20. Kuo PH, Lee LN, Yang PC, Chen YC, Luh KT. Aspergillus laryngotracheobronchitis presenting as stridor in a patient with peripheral T cell lymphoma. Thorax. (1996) 51:869–70. doi: 10.1136/thx.51.8.869
21. Delbrouck C, Chantrain G, Kampouridis S, Petein M. Laryngeal aspergillosis in an acquired immunodeficiency syndrome patient. J Laryngol Otol. (1998) 112:488–90. doi: 10.1017/S0022215100140861
22. Gallo A, Manciocco V, Simonelli M, Minni A, De Vincentiis M. Clinical findings of laryngeal aspergillosis. Otolaryngol Head Neck Surg. (2000) 123:661–2. doi: 10.1067/mhn.2000.110106
23. Dutta M, Jotdar A, Kundu S, Ghosh B, Mukhopadhyay S. Primary laryngeal aspergillosis in the immunocompetent state: a clinical update. Braz J Otorhinolaryngol. (2017) 83:228–34. doi: 10.1016/j.bjorl.2015.06.002
24. Richardson BE, Morrison VA, Gapany M. Invasive aspergillosis of the larynx: case report and review of the literature. Otolaryngol Head Neck Surg. (1996) 114:471–3. doi: 10.1016/S0194-5998(96)70221-8
25. Ferlito A. Clinical records. Primary aspergillosis of the larynx. J Laryngol Otol. (1974) 88:1257–63. doi: 10.1017/S0022215100079986
26. Wu N, Huang Y, Li Q, Bai C, Huang HD, Yao XP. Isolated invasive Aspergillus tracheobronchitis: a clinical study of 19 cases. Clin Microbiol Infect. (2010) 16:689–95. doi: 10.1111/j.1469-0691.2009.02923.x
27. Ranford D, Kang C, Kelly M, Volpini L. Primary aspergillosis of the larynx causing acute airway distress. BMJ Case Rep. (2021) 14:e240434. doi: 10.1136/bcr-2020-240434
28. Dagenais TRT, Keller NP. Pathogenesis of Aspergillus fumigatus in invasive aspergillosis. Clin Microbiol Rev. (2009) 22:447–65. doi: 10.1128/CMR.00055-08
29. Hall J, Heimann P, Costas C. Airway obstruction caused by Aspergillus tracheobronchitis in an immunocompromised patient. Crit Care Med. (1990) 18:575–6. doi: 10.1097/00003246-199005000-00022
30. Kramer MR, Denning DW, Marshall SE, Ross DJ, Berry G, Lewiston NJ, et al. Ulcerative tracheobronchitis after lung transplantation. A new form of invasive aspergillosis. Am Rev Respir Dis. (1991) 144:552–6. doi: 10.1164/ajrccm/144.3_Pt_1.552
31. Kemper CA, Hostetler JS, Follansbee SE, Ruane P, Covington D, Leong SS, et al. Ulcerative and plaque-like tracheobronchitis due to infection with Aspergillus in patients with AIDS. Clin Infect Dis. (1993) 17:344–52. doi: 10.1093/clinids/17.3.344
32. Benson-Mitchell R, Tolley N, Croft CB, Gallimore A. Aspergillosis of the larynx. J Laryngol Otol. (1994) 108:883–5. doi: 10.1017/S0022215100128403
33. Sriskandabalan P, Roy RB. Aspergillus infection of the epiglottis in a HIV positive patient. Genitourin Med. (1996) 72:431–2. doi: 10.1136/sti.72.6.431
34. Fairfax AJ, David V, Douce G. Laryngeal aspergillosis following high dose inhaled fluticasone therapy for asthma. Thorax. (1999) 54:860–1. doi: 10.1136/thx.54.9.860
35. Boettcher H, Bewig B, Hirt SW, Möller F, Cremer J. Topical amphotericin B application in severe bronchial aspergillosis after lung transplantation: report of experiences in 3 cases. J Heart Lung Transplant. (2000) 19:1224–7. doi: 10.1016/S1053-2498(00)00154-6
36. Buchheidt D, Weiss A, Reiter S, Hartung G, Hehlmann R. Pseudomembranous tracheobronchial aspergillosis: a rare manifestation of invasive aspergillosis in a non-neutropenic patient with Hodgkin's disease. Mycoses. (2003) 46:51–5. doi: 10.1046/j.1439-0507.2003.00812.x
37. Nath A, Agarwal R, Singh A, Gupta R, Joshi K. An unusual cause of hoarseness in an elderly asthmatic. Mycoses. (2010) 53:544–6. doi: 10.1111/j.1439-0507.2009.01744.x
38. Gómez F, Tarín V, Cuadrado M, Vecilla F, Blanquer D, Costa-Bauzá A, et al. Tracheal oxalosis associated with Aspergillus niger tracheobronchitis. Eur Respir J. (2013) 41:995–7. doi: 10.1183/09031936.00185312
39. Argento AC, Wolfe CR, Wahidi MM, Shofer SL, Mahmood K. Bronchomediastinal fistula caused by endobronchial aspergilloma. Ann Am Thorac Soc. (2015) 12:91–5. doi: 10.1513/AnnalsATS.201406-247BC
40. Ohashi T, Mizuta K, Kuze B, Aoki M, Ito Y. Invasive epiglottic aspergillosis: a case report and literature review. Auris Nasus Larynx. (2015) 42:501–4. doi: 10.1016/j.anl.2015.05.001
41. Haidar G, Crespo M, Maximous S, Lendermon E, Clancy CJ, Nguyen MH. Invasive tracheobronchial aspergillosis in a lung transplant recipient receiving belatacept as salvage maintenance immunosuppression: a case report. Transplant Proc. (2016) 48:275–8. doi: 10.1016/j.transproceed.2016.01.003
42. Kushima H, Tokimatsu I, Ishii H, Kadota J. Invasive pulmonary aspergillosis presenting with tracheobronchial involvement. Intern Med. (2016) 55:1679. doi: 10.2169/internalmedicine.55.6822
43. Argüder E, Sentürk A, Hasanoglu HC, Hasanoglu I, Kanbay A, Dogan HT. Unique case of pseudomembranous aspergillus tracheobronchitis: tracheal perforation and horner's syndrome. Mycopathologia. (2016) 181:885–9. doi: 10.1007/s11046-016-0025-4
44. Swiss T, Cervantes SS, Hinni M, Lott DG. Invasive fungal laryngopharyngitis resulting in laryngeal destruction with complete laryngotracheal separation: report of a case. Ear Nose Throat J. (2017) 96:E33–6. doi: 10.1177/014556131709600105
45. Lee SY, Nguyen P, Chapman S. Invasive tracheal aspergillosis after chemoradiotherapy treatment. Respirol Case Rep. (2019) 7:e00473. doi: 10.1002/rcr2.473
46. Kanai T, Samejima Y, Noda Y, Kim SH, Tamura K, Umakoshi T, et al. Invasive tracheobronchial aspergillosis with bronchial ulcers complicated by nontuberculous mycobacterial disease. Intern Med. (2020) 59:1189–94. doi: 10.2169/internalmedicine.3827-19
47. Drury AE, Allan RA, Underhill H, Ball S, Joseph AE. Calcification in invasive tracheal aspergillosis demonstrated on ultrasound: a new finding. Br J Radiol. (2001) 74:955–8. doi: 10.1259/bjr.74.886.740955
48. Gonzalez M, Ma Tooh M, Krueger T, Ris HB, Letovanec I, Wang Y, et al. Repair of tracheal aspergillosis perforation causing tension pneumothorax. Ann Thorac Surg. (2013) 96:2256–8. doi: 10.1016/j.athoracsur.2013.06.064
49. Barnes C, Berkowitz R, Curtis N, Waters K. Aspergillus laryngotracheobronchial infection in a 6-year-old girl following bone marrow transplantation. Int J Pediatr Otorhinolaryngol. (2001) 59:59–62.5. doi: 10.1016/S0165-5876(01)00429-3
50. Athanassiadou F, Kourti M, Papageorgiou T, Danielidis J. Invasive aspergillosis of the larynx in a child with acute lymphoblastic leukemia. Pediatr Infect Dis J. (2005) 24:190–1. doi: 10.1097/01.inf.0000153171.16525.28
51. Edmonds LC, Prakash UB. Lymphoma, neutropenia, and wheezing in a 70-year-old man. Chest. (1993) 103:585–7. doi: 10.1378/chest.103.2.585
52. Furuya ME, Ramírez-Figueroa JL, Vargas MH, Bernáldez-Ríos R, Vázquez-Rosales JG, Rodríguez-Velasco A. Diagnoses unveiled by early bronchoscopy in children with leukemia and pulmonary infiltrates. J Pediatr Hematol Oncol. (2012) 34:596–600. doi: 10.1097/MPH.0b013e318240d54b
54. Restrepo-Gualteros SM, Gutierrez MJ, Villamil-Osorio M, Arroyo MA, Nino G. Challenges and clinical implications of the diagnosis of cytomegalovirus lung infection in children. Curr Infect Dis Rep. (2019) 21:24. doi: 10.1007/s11908-019-0681-x
55. Ding X, Huang H, Zhong L, Chen M, Peng F, Zhang B, et al. Disseminated talaromyces marneffei infection in a non-HIV infant with a homozygous private variant of RELB. Front Cell Infect Microbiol. (2021) 11:605589. doi: 10.3389/fcimb.2021.605589
56. Lee PP, Lao-Araya M, Yang J, Chan KW, Ma H, Pei LC, et al. Application of flow cytometry in the diagnostics pipeline of primary immunodeficiencies underlying disseminated talaromyces marneffei infection in HIV-negative children. Front Immunol. (2019) 10:2189. doi: 10.3389/fimmu.2019.02189
57. Chan JF, Lau SK, Yuen KY, Woo PC. Talaromyces (Penicillium) marneffei infection in non-HIV-infected patients. Emerg Microbes Infect. (2016) 5:e19. doi: 10.1038/emi.2016.18
58. Dornbusch HJ, Groll A, Walsh TJ. Diagnosis of invasive fungal infections in immunocompromised children. Clin Microbiol Infect. (2010) 16:1328–34. doi: 10.1111/j.1469-0691.2010.03336.x
Keywords: invasive aspergillosis, Aspergillus laryngotracheitis, laryngotracheal ulcers, inferior thyroid artery, hemoptysis, acute lymphoblastic leukemia
Citation: Porzionato A, Stocco E, Emmi A, Macchi V and De Caro R (2022) Case Report: Sudden Fatal Hemorrhage in Ulcerative Fungal Laryngotracheitis—A Pediatric Case Report. Front. Pediatr. 9:764027. doi: 10.3389/fped.2021.764027
Received: 22 September 2021; Accepted: 25 November 2021;
Published: 11 January 2022.
Edited by:
Bülent Taner Karadaǧ, Marmara University, TurkeyReviewed by:
Gönül Tanır, Dr Sami Ulus Child Health and Diseases Training and Research Hospital, TurkeyRekha Boloor, Father Muller Medical College, India
Copyright © 2022 Porzionato, Stocco, Emmi, Macchi and De Caro. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elena Stocco, ZWxlbmEuc3RvY2NvJiN4MDAwNDA7dW5pcGQuaXQ=
 Andrea Porzionato
Andrea Porzionato Elena Stocco
Elena Stocco Aron Emmi
Aron Emmi Veronica Macchi
Veronica Macchi Raffaele De Caro
Raffaele De Caro