
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 02 December 2021
Sec. Children and Health
Volume 9 - 2021 | https://doi.org/10.3389/fped.2021.762684
This article is part of the Research Topic Children's Neurodevelopment in the Post-COVID Era: From Hospital to Community View all 5 articles
A correction has been applied to this article in:
Corrigendum: Impact of SARS-CoV-2 Infection During Pregnancy on Infant Neurobehavioral Development: A Case-Control Study
Background: Previous studies on the pneumonia outbreak caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have focused on the general population and pregnant women, while little is known about the effects of SARS-CoV-2 on retardation during and after pregnancy. The purpose of this study was to evaluate the potential influence of SARS-CoV-2 on infant neurobehavioral development.
Methods: A case-control study was conducted in Maternal and Child Health Hospital of Hubei Province. Nine pregnant women with SARS-CoV-2 infection and 9 controls matched by maternal age, parity, and status of chronic disease were included. Infantile neurobehavioral development was assessed through the Ages and Stages Questionnaires Edition 3 (ASQ-3).
Results: The majority of pregnant women with SARS-CoV-2 experienced cesarean section (7 of 9), which was higher than the control group (5 of 9). The throat swabs of all newborn were negative. We found that compared with the control group, neonates of mothers with SARS-CoV-2 infection during pregnancy had lower scores in communication, gross movement, fine movement, problem solving, and personal-social domains; but only fine movement domain yielded statistical significance (P = 0.031).
Conclusion: Infection with SARS-CoV-2 during pregnancy may have a certain impact on infant neurobehavioral development. Further studies with larger sample size are warranted for validation.
The current pneumonia outbreak of coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is a highly infectious disease. Importantly, on March 11, 2020, World Health Organization (WHO) announced that the ongoing outbreak of COVID-19 virus had become a global pandemic (1, 2). However, nobody can reliably predict how long the COVID-19 pandemic will last.
Pregnant women are a special group and are at higher risk for acquiring infection and dying compared with nonpregnant women (3, 4). Because of the immune and physiological changes that accompany pregnancy, pregnant women are more likely to encounter a series of negative emotions during the pandemic (5). In addition, the measures to deal with COVID-19 (such as isolation, physical distance, home isolation, and remote consultation with health professionals, etc.) may result in the inability to obtain the expected level of support and care before delivery, which may increase the psychological pressure of pregnant women (6, 7). Furthermore, studies have found that SARS-CoV-2 has direct neuroinvasion ability (8), which may attack the human central nervous system and have a potential impact on the survivors' cognitive and neuropsychological functions (9, 10). Mother and baby are closely linked during pregnancy, it is widely believed that the maternal psychological stress, as a teratogen, will continuously transmit painful signals to the unborn child, which may cause adverse effects on the fetus (11). Moreover, early mother-infant separation due to mandatory or voluntary isolation after childbirth may also have a negative impact on infant feeding and thereby early development (12–14). At present, there is little research evidence showing the long-term effects of SARS-CoV-2 infection on pregnant women and their babies. Research is needed to evaluate the burden of SARS-CoV-2 infection during pregnancy in children's personality formation and neurobehavioral development.
Above all, this study aims to evaluate the effect of maternal SARS-CoV-2 infection on the neurobehavioral development of their babies, so as to improve the preventive behavior and health care strategies for pregnant women, new mothers and babies during the COVID-19 pandemic.
A total of 60 patients delivered at the Maternal and Child Health Hospital of Hubei province from January 24 to March 14, 2020, and were confirmed to have COVID-19 by laboratory and clinical diagnosis. The maternal and fetal characteristics of the 60 patients in this study were shown in Supplementary Table 1. Later, 51 patients who refused to finish the Ages and Stages Questionnaires Edition 3 (ASQ-3) questionnaire were excluded. Finally, a total of 9 patients were included in the present analysis. Subsequently, we matched 9 pregnant women without SARS-CoV-2 infection and delivered at the same period at a ratio of 1:1 according to maternal age (within 3 years), parity, and status of chronic disease (including diabetes, hypertension, hypothyroidism, and hepatic disease). All research subjects were recruited in the third trimester. Sociodemographic information and blood samples were collected upon admission. The flowchart of the exclusion and inclusion process of our study population is presented in Figure 1. This study was reviewed and approved by the Ethics Committee of Maternal and Child Health Hospital of Hubei Province (No. 2021IECLW013).
ASQ-3 is a parent-rated screening tool for the delay of child early development at different ages and stages. ASQ-3 contains 21 questionnaire series, covering all children from 1 month to 5 and a half years old. The children in our study were between 8 and 10 months old when they were surveyed. The ASQ-3 subscale contains 30 items divided into five developmental domains (six items per domain): communication, gross movement, fine movement, problem solving, and personal-social. Each item is rated to a score of 0, 5, and 10, with a total score for each domain of ASQ-3 ranging from 0 to 60 scores (15). There is also a comprehensive question section, which is about the concerns that parents may have about their children. A higher score indicates a higher developmental level for children at each month, and “developmental delay” is defined as a child whose ASQ-3 score is lower than the critical point of their age in any of the five ASQ-3 areas (16). We contacted the parents of the children by phone, and explained the contents of the ASQ-3 questionnaire and the matters needing attention to fill in. An online questionnaire was completed by the child's guardian to obtain an ASQ-3 score.
The clinical symptoms and signs, laboratory findings and radiologic assessments were extracted from electronic medical records. Two experienced clinicians reviewed and abstracted the data. Maternal respiratory swabs were collected and tested for SARS-CoV-2 by reverse transcriptase–polymerase chain reaction (RT-PCR) assay (17). Positive confirmatory cases of SARS-COV-2 infection were defined as a case where any laboratory test results were positive.
Continuous and categorical variables were presented as mean ± standard deviation (SD), median [interquartile range (IQR)] and frequency (percentage) when suitable. Continuous variables were compared between two groups by using 2-tail Student's t-test or Mann-Whitney's U-test when appropriate, and categorical variables were compared by using chi-square test. All statistical analyses were performed using SAS version 9.4. A two-tailed P < 0.05 was considered as statistically significant.
The maternal characteristics of cases are shown in Table 1. COVID-19 pregnancies were 31.8 ± 4.6 years old (vs. 31.0 ± 4.9 years old in the control group), and their gestational age at delivery was 38.0 ± 1.1 weeks (vs. 38.9 ± 1.1 weeks in the control group). Besides, the median number of gravidity was 3 in pregnancies with COVID-19, significantly higher than the control group (P = 0.003).
All COVID-19 patients were detected with SARS-CoV-2 infection in the third trimester and presented with mild infection. None of the patients had suffered diabetes, chronic hypertension, or cardiovascular disease before pregnancy. Seven of the nine patients had cesarean section, more than the control group (five of nine). As shown in Table 2, all COVID-19 patients were in stable condition throughout the pregnancy, with body temperature fluctuating within a range of 37.0–38.6°C.
As shown in Table 1, all live birth newborn throat swabs in our study were negative. The proportion of premature birth and low birth weight among the newborns of pregnant women with COVID-19 were slightly higher than the control group. Meanwhile, the 1-min Apgar score of the affected group was 9.8 ± 0.4, which was lower than that of the control group (10.0 ± 0.0, P < 0.001).
We found that compared with normal pregnant women, neonates of pregnant women with SARS-CoV-2 had lower scores in communication, gross movement, fine movement, problem solving, and personal-social domains. However, only the difference of the fine movement domain reached statistically significant (P = 0.031, Table 3).
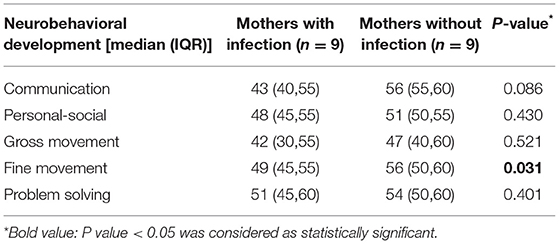
Table 3. The infantile ages and stages questionnaires edition 3 (ASQ-3) scores of mothers with and without SARS-CoV-2 infection.
This is a case-control study comparing the neurobehavioral development of neonates in pregnant women with and without COVID-19. It is worth noting that, compared with the control group, the newborns of COVID-19 patients have lower scores in the five developmental areas of ASQ-3, and the difference in fine movement domain is statistically significant.
Pregnant women with COVID-19 were more likely to have babies delivered preterm or with low birth weight, which were strongly associated with delayed cognitive and motor development in infants (18). Motor skills in early infancy can predict the development of infants' subsequent communication ability, and also reflect the strength of learning ability to a certain extent (19). Considering the plasticity of young brain, early detection and early intervention is more effective than post-remedies (20). Therefore, it is especially important to take effective early intervention to prevent and improve neurodevelopmental outcomes in children.
Pregnant women's mental health needs more attention during the pandemic. On the one hand, SARS-CoV-2 is a neuroinvasive virus that may have direct and indirect effects on the central nervous system (9), and may have long-term adverse consequences for the patients' cognitive and neuropsychological function (9, 21). On the other hand, fear and uncertainty about the short and long-term effects on themselves and their children may deteriorate mothers' depressive and anxiety symptoms (22). Ultimately, the mental health of pregnant women may adversely affect the neurobehavioral development of newborns (23).
Breast feeding is now recommended in asymptomatic or mild symptomatic women. Nevertheless, in the first line reaction to pandemic, breast-feeding rates are very low in the first few months of the fetus, due to fear of maternal-fetal transmission (24). This may have negative effects on infant brain development and mother-infant relationship (25). Studies have found that early childhood experiences and environmental influences leave a lasting mark on brain structure and long-term health (26–28). Furthermore, early childhood adversity is related to impairment of learning, behavior, and physical and mental health in the future (27). Therefore, we advocate active health education and guidance on breastfeeding and parenting behavior for affected pregnant women and their families during the pandemic for possible mother-to-child separation and early cessation of breastfeeding on infants. Also, timely monitoring of pregnant women's mental health, so as to effectively prevent and early detect psychological trauma.
At present, there is much controversy relating to the possibility of vertical mother-to-baby transmission of SARS-CoV-2. SARS-CoV-2 has been confirmed to have the ability of passing through placental barrier (29). In addition, severe SARS-CoV-2 placenta infection may trigger fetal inflammatory response, leading to organ damage and developmental deficiencies (30). However, vertical transmission seems to occur in a minority of cases of maternal COVID-19 infection in the third trimester (31, 32). Consistent with several studies (33–36), we found no evidence of vertical transmission. Thus, we believe that there is still no definite evidence of vertical transmission of SARS-CoV-2.
Our study has drawn a link between maternal COVID-19 and early infantile developmental delay in many domains, such as communication, gross movement, problem solving, personal-social, and social-emotional. Nevertheless, there are some limitations to our study that should be considered. First, the results of this study were limited by the small sample size, which may lead to unstable results. As the first batch of affected pregnancies in Wuhan, the mothers and their newborns underwent many examinations for both clinic and research purpose, therefore the participants' participation and compliance were less optimistic than expected. However, the maternal and fetal characteristics were comparable between included and overall affected population in the current study. Second, the ASQ-3 questionnaire survey in this study is conducted by phone and the Internet. Third, whether SARS-COV-2 can be propagated through vertical mother-to-baby transmission requires further research. In the future, it is necessary to carry out follow-up studies on COVID-19 pregnant women and their newborns to address these problems in order to make up for these shortcomings and gaps.
In summary, SARS-COV-2 infection during pregnancy may have an adverse effect on infant neurobehavioral development. We call for more research to estimate the burden of SARS-COV-2 infection in the formation of personality and neurobehavioral development of the children conceived in the era of the COVID-19 pandemic.
The data analyzed in this study is not readily available unless the application have been reviewed and approved by the Ethics Committee of Maternal and Child Health Hospital of Hubei Province. Please contact Yao Cheng (Y2hlbmd5YW8yMDE0QHNpbmEuY29t) if you have any requests.
JY and YC designed the study. HT and YX drafted the manuscript. JY and HT contributed in manuscript revision. JY and MY data analysis. MY and GS contributed in data collection. All authors have read and approved the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2021.762684/full#supplementary-material
1. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. (2020) 395:497–506. doi: 10.1016/S0140-6736(20)30183-5
2. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. (2020) 382:727–33. doi: 10.1056/NEJMoa2001017
3. Narang K, Enninga EAL, Gunaratne M, Ibirogba ER, Trad ATA, Elrefaei A, et al. SARS-CoV-2 infection and COVID-19 during pregnancy: a multidisciplinary review. Mayo Clin Proc. (2020) 95:1750–65. doi: 10.1016/j.mayocp.2020.05.011
4. Lam CM, Wong SF, Leung TN, Chow KM, Yu WC, Wong TY, et al. A case-controlled study comparing clinical course and outcomes of pregnant and non-pregnant women with severe acute respiratory syndrome. BJOG. (2004) 111:771–4. doi: 10.1111/j.1471-0528.2004.00199.x
5. Thapa SB, Mainali A, Schwank SE, Acharya G. Maternal mental health in the time of the COVID-19 pandemic. Acta Obstet Gynecol Scand. (2020) 99:817–8. doi: 10.1111/aogs.13894
6. Salehi L, Rahimzadeh M, Molaei E, Zaheri H, Esmaelzadeh-Saeieh S. The relationship among fear and anxiety of COVID-19, pregnancy experience, and mental health disorder in pregnant women: a structural equation model. Brain Behav. (2020) 10:e01835. doi: 10.1002/brb3.1835
7. Ghazanfarpour M, Bahrami F, Rashidi Fakari F, Ashrafinia F, Babakhanian M, Dordeh M, et al. Prevalence of anxiety and depression among pregnant women during the COVID-19 pandemic: a meta-analysis. J Psychosom Obstet Gynaecol. (2021) 2021:1–12. doi: 10.1080/0167482X.2021.1929162
8. Li Z, Liu T, Yang N, Han D, Mi X, Li Y, et al. Neurological manifestations of patients with COVID-19: potential routes of SARS-CoV-2 neuroinvasion from the periphery to the brain. Front Med. (2020) 14:533–41. doi: 10.1007/s11684-020-0786-5
9. Serrano-Castro PJ, Estivill-Torrus G, Cabezudo-Garcia P, Reyes-Bueno JA, Ciano Petersen N, Aguilar-Castillo MJ, et al. Impact of SARS-CoV-2 infection on neurodegenerative and neuropsychiatric diseases: a delayed pandemic? Neurologia (Engl Ed). (2020) 35:245–51. doi: 10.1016/j.nrleng.2020.04.002
10. Helms J, Kremer S, Merdji H, Clere-Jehl R, Schenck M, Kummerlen C, et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. (2020) 382:2268–70. doi: 10.1056/NEJMc2008597
11. Barker DJ. The fetal and infant origins of adult disease. BMJ. (1990) 301:1111. doi: 10.1136/bmj.301.6761.1111
12. Moore ER, Bergman N, Anderson GC, Medley N. Early skin-to-skin contact for mothers and their healthy newborn infants. Cochrane Database Syst Rev. (2016) 11:CD003519. doi: 10.1002/14651858.CD003519.pub4
13. Brahm P, Valdes V. [The benefits of breastfeeding and associated risks of replacement with baby formulas]. Rev Chil Pediatr. (2017) 88:7–14. doi: 10.4067/S0370-41062017000100001
14. Krol KM, Grossmann T. Psychological effects of breastfeeding on children and mothers. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. (2018) 61:977–85. doi: 10.1007/s00103-018-2769-0
15. Shrestha M, Schwinger C, Hysing M, Chandyo RK, Ulak M, Ranjitkar S, et al. Agreement between mothers and fieldworkers while assessing child development using ages and stages questionnaires, third edition in Nepal. Front Psychol. (2020) 11:579412. doi: 10.3389/fpsyg.2020.579412
16. Beswick J. The use of the ages and stages questionnaire 3 (ASQ-3). Community Pract. (2014) 87:14.
17. Centers for Disease Control and Prevention. Research Use Only 2019-Novel Coronavirus (2019-nCoV) Real-Time RT-PCR Primers and Probes. Available online at: https://www.cdc.gov/coronavirus/2019-ncov/lab/rt-pcr-panel-primer-probes.html.
18. Vohr BR, Wright LL, Dusick AM, Mele L, Verter J, Steichen JJ, et al. Neurodevelopmental and functional outcomes of extremely low birth weight infants in the National Institute of Child Health and Human Development Neonatal Research Network, 1993-1994. Pediatrics. (2000) 105:1216–26. doi: 10.1542/peds.105.6.1216
19. Valla L, Slinning K, Kalleson R, Wentzel-Larsen T, Riiser K. Motor skills and later communication development in early childhood: results from a population-based study. Child Care Health Dev. (2020) 46:407–13. doi: 10.1111/cch.12765
20. Atkins KL, Dolata JK, Blasco PM, Saxton SN, Duvall SW. Early intervention referral outcomes for children at increased risk of experiencing developmental delays. Matern Child Health J. (2020) 24:204–12. doi: 10.1007/s10995-019-02830-4
21. Heneka MT, Golenbock D, Latz E, Morgan D, Brown R. Immediate and long-term consequences of COVID-19 infections for the development of neurological disease. Alzheimers Res Ther. (2020) 12:69. doi: 10.1186/s13195-020-00640-3
22. Almeida M, Shrestha AD, Stojanac D, Miller LJ. The impact of the COVID-19 pandemic on women's mental health. Arch Womens Ment Health. (2020) 23:741–8. doi: 10.1007/s00737-020-01092-2
23. Glover V. Maternal depression, anxiety and stress during pregnancy and child outcome; what needs to be done. Best Pract Res Clin Obstet Gynaecol. (2014) 28:25–35. doi: 10.1016/j.bpobgyn.2013.08.017
24. Peng S, Zhu H, Yang L, Cao L, Huang X, Dynes M, et al. A study of breastfeeding practices, SARS-CoV-2 and its antibodies in the breast milk of mothers confirmed with COVID-19. Lancet Reg Health West Pac. (2020) 4:100045. doi: 10.1016/j.lanwpc.2020.100045
25. Dumpa V, Kamity R, Vinci AN, Noyola E, Noor A. Neonatal coronavirus 2019 (COVID-19) infection: a case report and review of literature. Cureus. (2020) 12:e8165. doi: 10.7759/cureus.8165
26. Berens AE, Jensen SKG, Nelson III CA. Biological embedding of childhood adversity: from physiological mechanisms to clinical implications. BMC Med. (2017) 15:135. doi: 10.1186/s12916-017-0895-4
27. Boyce WT. The lifelong effects of early childhood adversity and toxic stress. Pediatr Dent. (2014) 36:102–8.
28. Teicher MH. Childhood trauma and the enduring consequences of forcibly separating children from parents at the United States border. BMC Med. (2018) 16:146. doi: 10.1186/s12916-018-1147-y
29. Vivanti AJ, Vauloup-Fellous C, Prevot S, Zupan V, Suffee C, Do Cao J, et al. Transplacental transmission of SARS-CoV-2 infection. Nat Commun. (2020) 11:3572. doi: 10.1038/s41467-020-17436-6
30. Cardenas I, Means RE, Aldo P, Koga K, Lang SM, Booth CJ, et al. Viral infection of the placenta leads to fetal inflammation and sensitization to bacterial products predisposing to preterm labor. J Immunol. (2010) 185:1248–57. doi: 10.4049/jimmunol.1000289
31. Kotlyar AM, Grechukhina O, Chen A, Popkhadze S, Grimshaw A, Tal O, et al. Vertical transmission of coronavirus disease 2019: a systematic review and meta-analysis. Am J Obstet Gynecol. (2021) 224:35–53 e33. doi: 10.1016/j.ajog.2020.07.049
32. Cribiu FM, Erra R, Pugni L, Rubio-Perez C, Alonso L, Simonetti S, et al. Severe SARS-CoV-2 placenta infection can impact neonatal outcome in the absence of vertical transmission. J Clin Invest. (2021) 131:e145427. doi: 10.1172/JCI145427
33. Wang Y, Chen L, Wu T, Shi H, Li Q, Jiang H, et al. Impact of Covid-19 in pregnancy on mother's psychological status and infant's neurobehavioral development: a longitudinal cohort study in China. BMC Med. (2020) 18:347. doi: 10.1186/s12916-020-01825-1
34. Chen H, Guo J, Wang C, Luo F, Yu X, Zhang W, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. (2020) 395:809–15. doi: 10.1016/S0140-6736(20)30360-3
35. Karimi-Zarchi M, Neamatzadeh H, Dastgheib SA, Abbasi H, Mirjalili SR, Behforouz A, et al. Vertical transmission of coronavirus disease 19 (COVID-19) from infected pregnant mothers to neonates: a review. Fetal Pediatr Pathol. (2020) 39:246–50. doi: 10.1080/15513815.2020.1747120
Keywords: COVID-19, pregnancy, infant neurobehavioral development, ASQ-3, SARS-CoV-2
Citation: Cheng Y, Teng H, Xiao Y, Yao M, Yin J and Sun G (2021) Impact of SARS-CoV-2 Infection During Pregnancy on Infant Neurobehavioral Development: A Case-Control Study. Front. Pediatr. 9:762684. doi: 10.3389/fped.2021.762684
Received: 22 August 2021; Accepted: 05 November 2021;
Published: 02 December 2021.
Edited by:
Joav Merrick, Ministry of Social Affairs, IsraelReviewed by:
Niloofar Deravi, Shahid Beheshti University of Medical Sciences, IranCopyright © 2021 Cheng, Teng, Xiao, Yao, Yin and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guoqiang Sun, c3VuZ3VvcWlhbmdAaGJmeS5jb20=; Jieyun Yin, anl5aW5Ac3VkYS5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.