- 1Division of Pediatric Critical Care, Department of Pediatric, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand
- 2Division of Cardiothoracic Surgery, Department of Surgery, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand
Introduction: Extracorporeal Membrane Oxygenation (ECMO) is a lifesaving procedure for patients with refractory cardiac or respiratory failure. The indications for ECMO are growing, and it is increasingly being used to support cardiopulmonary failure in children. However, the risks and benefits of ECMO should be weighed before deploying it on the patients. The objectives of this study were to identify the mortality risk factors and to determine the ECMO outcomes.
Methods: The retrospective chart reviews were done for all patients aged 1 day−20 years old receiving ECMO between January 2010 and December 2020.
Results: Seventy patients were enrolled in the study. The median age was 31.3 months. The incidence of VA and VV ECMO was 85.7 and 14.3%, respectively. The most common indication for ECMO was the failure to wean off cardiopulmonary bypass after cardiac surgery. Pre-existing acute kidney injury (OR 4.23; 95% CI 1.34–13.32, p = 0.014) and delayed enteral feeding (OR 3.85, 95% CI 1.23–12.02, p = 0.020), and coagulopathy (OD 12.64; 95% CI 1.13–141.13, p = 0.039) were associated with the higher rate of mortality. The rates of ECMO survival and survival to discharge were 70 and 50%, respectively.
Conclusion: ECMO is the lifesaving tool for critically ill pediatric patients. Pre-existing acute kidney injury, delayed enteral feeding, and coagulopathy were the potential risk factors associated with poor outcomes in children receiving ECMO. However, ECMO setup can be done successfully in a developing country.
Introduction
Extracorporeal Membrane Oxygenation (ECMO) is a life-saving procedure that is used to treat patients who have failed to respond to the conventional treatments for cardiac or respiratory failure (1–3). According to the Extracorporeal Life Support Organization (ELSO) report 2016, 24% of all ECMO implants were performed on children, and newborns accounted for 47% of the total cohort (4). Prior to ECMO initiation, the patient's risks and benefits should be outlined. Hemorrhagic and thrombotic complications are among the most common complications associated with ECMO. Nosocomial infections are also common in patients receiving ECMO and are associated with poor outcomes, especially in the neonatal population. Bartlett et al. performed the first neonatal ECMO in 1975, saving the life of a 1-day-old neonate with severe meconium aspiration syndrome (5). Following that, ECMO had been evolved in terms of equipment and management. Furthermore, this sophisticated technology had been successfully used to perform emergency cardiopulmonary resuscitation (E-CPR) (1, 6, 7). The Extracorporeal Life Support Organization (ELSO) gathered data and reported on the global growth, outcomes, and complications of the ECMO technology. In neonatal and pediatric patients, two primary ECMO circuits are used: venovenous (VV) and venoarterial (VA) ECMO. Vascular access is determined by the patient's condition and the surgeon's experience. While VA ECMO maintains both the hemodynamic and the respiratory function, VV ECMO only supports the respiratory function. ECMO is never recommended in the presence of lethal chromosomal or syndromic abnormalities, as well as severe irreversible brain or multiorgan failure.
The most common short-term complications in patients receiving ECMO were hemorrhagic and thrombotic events, cardiac depression, seizures, and acute kidney injury (AKI) (8–10). Long-term complications included hearing loss, delayed development, and decreased lung volume, particularly in patients with respiratory failure (10–13). Our hospital has been developing an ECMO program since 2003. Our center is the tertiary referral center in Bangkok, Thailand. Many patients have multiple conditions, including those who have undergone cardiac surgery, severe acute respiratory distress syndrome, solid organ transplantation, and heart transplantation. Each year, the number of pediatric patients receiving ECMO at our center increases. The objective of this study was to assess the outcomes of ECMO and to identify the risk factors for mortality in neonatal and pediatric patients receiving ECMO.
Materials and Methods
This study was performed in a tertiary care referral center. We retrospectively analyzed the medical records of children aged 1 day−20 years who received ECMO from January 2010 to December 2020. This study was approved by the Institutional Review Board. Incomplete medical records were excluded. The demographic and baseline characteristics of the patients were collected, including their age, gender, body weight, immunological status, underlying disease, The Pediatric Risk of Mortality III, length of intensive care unit stays, and in-hospital mortality. Additionally, the characteristics of ECMO were reviewed, including indication, type of ECMO, duration of cannulation, and complications.
Initially the ECMO program was run by an individual attending cardiothoracic surgeon who reviewed the ELSO guideline and literature. Since 2015, our center has established an ECMO committee which comprised of pediatric intensivists, cardiothoracic surgeons, anesthesiologists, perfusionists, and certified ECMO nurses to assess the risks and benefits of ECMO in each patient. A cardiothoracic surgeon was initially ECMO program director with responsibility for overall operation. Two to three medical directors were responsible for locating an ECMO fund, establishing ECMO facilities and equipment, training in ECMO education, and preparing an annual ECMO summary. As the financial restrictions, the selected ECMO patients were required to undergo ECMO under the supervision of two of three ECMO committee members other than the patient's attending staff. These patients would be waived the ECMO expense from our center. Additionally, ECMO patients would have shortened the duration of the initial ECMO. As the lack of perfusionists, our center had only four to seven perfusionists who were responsible for cardiopulmonary bypass (CPB) and ECMO machine in both adults and children. Occasionally, we had three ECMO patients in the same period. There were insufficient perfusionists to cover all ECMO patients during 24-h on-call period. We had initiated a local basic and advanced ECMO course to train ICU nurses to become certified ECMO nurses capable of caring for the ECMO machine in place of the perfusionist. We had annually local ECMO meeting and workshop to keep updated ECMO knowledge and skills to the certified ECMO physicians and nurses. The indications of ECMO were patients who had the potentially reversible causes of severe cardiopulmonary failure such as pediatric acute respiratory distress syndrome with oxygen index > 30 for 6 h or >40 for 2 h, acute fulminant myocarditis, failure to weaning from CPB, progressive ventricular failure, or severe pulmonary hypertensive crisis. The location of ECMO cannulation was specified as an operating room or pediatric intensive care unit (PICU). Cannulation configurations were classified as central (sternotomy) for patients undergoing cardiopulmonary surgery or peripheral vessel for patients undergoing medical cardiopulmonary failure. We had the initial standing order in place for the ECMO-selected patients. The ECMO circuits were primed with packed red blood cells if the patient's weight was <10 kg. Heparin 100 unit/kg was given as a bolus infusion with a goal of an activated clotting time of 300 s. The initial pump flow rate was 20–30 mL/kg/min and increased gradually to the target flow of 2.4–3 L/m2/min. Typically, the initial sweep gas flow rate was equal to the blood flow rate (1:1), and the sweep flow was adjusted according to the PaCO2 level of each patient. Adjustment of pump flow was used to ensure adequate systemic perfusion (central venous oxygen saturation > 70%, normal arterial lactate, and adequate mean arterial blood pressure). The anticoagulant was using unfractionated heparin with monitoring via activated clotting time, activated partial thromboplastin time, and antiXa level. We had the multidisciplinary team including ICU physicians, ICU nurses, certified ECMO nurses, perfusionists, pediatric nutritionists, pediatric hematologists, clinical pharmacists. Weaning from ECMO was initiated when the following criteria were met: improvement in clinical course, recovery of end-organ function, and stable respiratory and hemodynamic status. Weaning from the VA ECMO was accomplished by decreasing the ECMO flow to 30 mL/kg/min, bridging ECMO for 1 h, and monitored the hemodynamic status and central venous oxygen saturation. If successful bridging was achieved, the decannulation of ECMO was performed. The protocol for weaning from VV ECMO was to decrease the FiO2 in the ECMO to 0.21 and weaned the sweep gas to zero. We then monitored the oxygen saturation and the arterial blood gases. If the oxygen saturation was >92% and the arterial blood gases were within the normal range, the VV ECMO could be then decannulated.
Definitions
Successful ECMO weaning was defined as the patients who survived for more than 72 h after successful cessation of ECMO support (14).
Hospital survival was defined as the patients who were successfully weaned off from ECMO and continued to survived until hospital discharge.
Early enteral feeding was defined as enteral feeding within 48 h after ECMO cannulation, whereas delayed enteral feeding was defined as enteral feeding after 48 h of ECMO cannulation.
The definition of acute kidney injury (AKI) was defined according to the Kidney Disease Improving Global Outcome (KDIGO) (15). The pre-existing AKI was defined as patients who had developed AKI within 48 h prior to initiate ECMO or after 48 h of ECMO initiation.
Statistical Methods
Data were analyzed using PASW Statistics, version 21 (SPSS Inc., Chicago, IL, USA). Descriptive statistics were presented as frequency, distribution, and percentage. Differences in the frequencies of discrete variables were tested using Pearson's Chi-square test or Fisher's exact test. Logistic regression was used to measure the association between the clinical variable and hospital mortality. Risk factors determined to be clinically significant a priori on the bivariable analyses were identified as candidate variables for the multivariable model. A log-rank test was used to analyze the survival of patients who received ECMO. A p-value of <0.05 was considered statistically significant.
Results
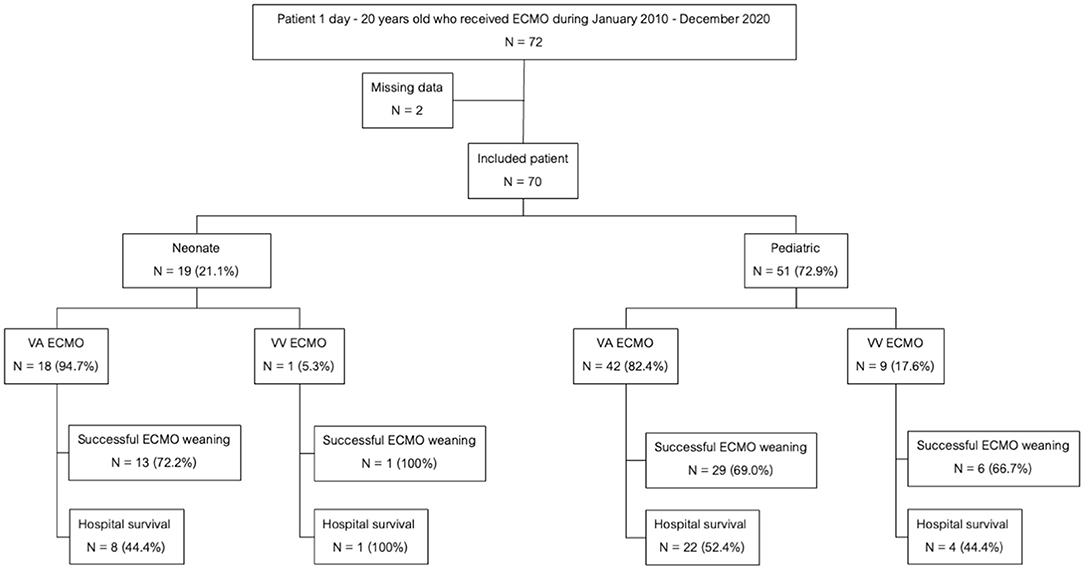
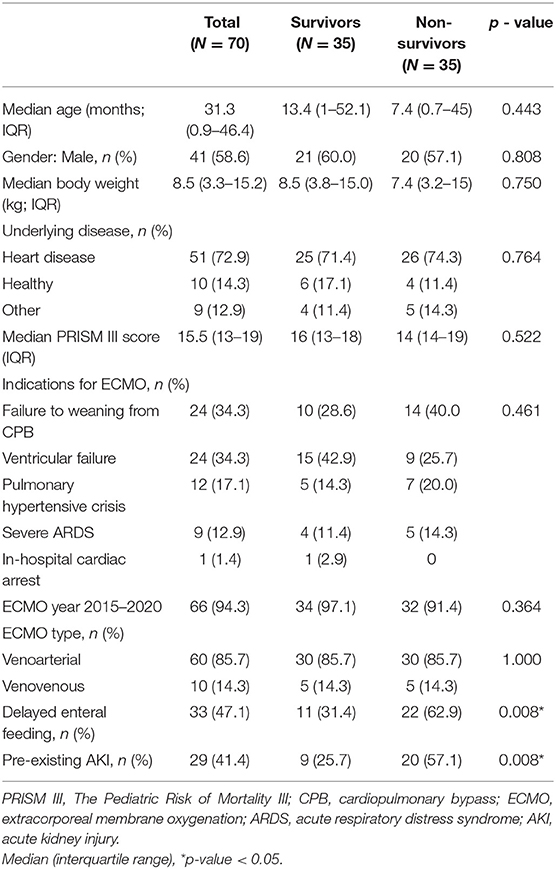
A total of 70 children underwent ECMO support during the study period. Fifty-one patients (72.8%) were among the pediatric group while the remaining 19 (27.2%) were in the neonatal group. There were 60 (85.7%) patients required VA ECMO and 10 patients (14.3%) required VV ECMO. The flow chart of all patients receiving ECMO support in this study was shown in Figure 1. There were 4 and 66 patients who received ECMO support during 2010–2015 and 2015–2020, respectively. The mortality rate was not significantly different between two periods (75 vs. 48.5%, p = 0.326). The baseline characteristics were shown in Table 1. Central cannulation was the most frequently used site for cannulation in neonatal and children. Central cannulation was performed in 71% of cases. The mean age was 31.3 (0.9–46.4) months, and 58.6% of participants were male. Forty-nine (70%) patients were successfully weaned from ECMO. Overall hospital mortality was 50%. Among the pediatric group, the mortality rate was 25 of 51(52.6%) patients while the neonatal group suffered a mortality rate of 10 of 19 (49%) patients. The mortality rate of VA ECMO and VV ECMO were 50 and 50%, respectively. The most common comorbidity was congenital heart disease. The three major indications for ECMO support the failure to wean off cardiopulmonary bypass (CPB) (34.3%), ventricular failure (34.3%), and pulmonary hypertensive crisis (17.1%). Twenty-nine patients (41.1%) had pre-existing AKI stage 2 or above. Two patients who required ECMO for bridging to heart transplantation and both of them survived.
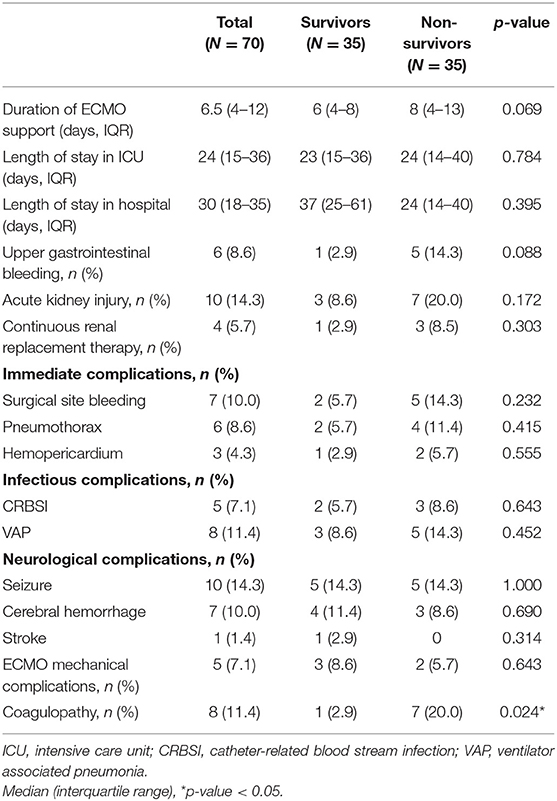
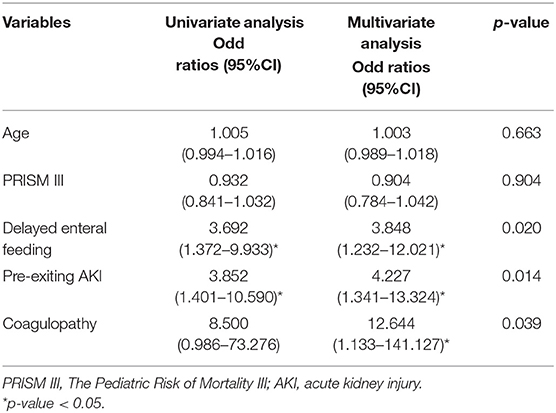
The median duration of ECMO support was 8.3 days (range 3 h−27 days). The median length of stay in ICU was 29.8 days (range 3–134 days). The most frequently encountered complications were hematological (28.6%) and neurological in nature (28.6%). Table 2 summarized the outcomes and associated complications. The delayed enteral feeding was found to be higher among the non-survivors than survivors (62.9 vs. 31.4%, p = 0.008). Coagulopathy was found to be more prevalent in the non-survivors than the survivors (20 vs. 2.9%, p = 0.024). Only those who died in the hospital had disseminated intravascular coagulation (DIC). There were 5 patients with thrombocytopenia, 2 patients with heparin-induced thrombocytopenia (HIT) and only 1 patient with heparin resistance. Multiple logistic regression analysis to adjust for the clinical variables significantly associated with hospital mortality (age, PRISM III, delay enteral feeding, pre-existing AKI, and coagulopathy) confirmed that delayed enteral feeding [Odds ratio (OR) 3.85; 95% confidence interval (CI) 1.23–12.02; p = 0.020], pre-existing AKI [OR 4.23; 95% CI 1.34–13.32, p = 0.014], and coagulopathy [OD 12.64; 95% CI 1.13–141.13, p = 0.039] were potential risk factors for increasing mortality (Table 3).
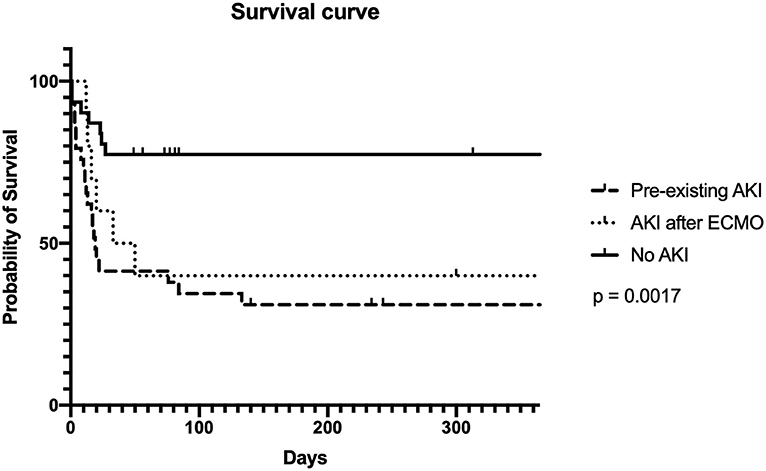
Fifty percent of the neonatal and pediatric patients on ECMO support survived until discharge. The leading cause of in-hospital death was septicemia (54.3%). Other causes of death included heart failure (28.6%), brain death (5.7%), and renal failure (1.4%). The overall 1-year survival rate of our patients was 47.8%. Survival rates were 31.0, 30, and 73.3%, respectively, in the pre-existing AKI, AKI after ECMO, and the non-AKI group (p = 0.050) (Figure 2).
Discussion
In this study, the distribution of age group among patients requiring ECMO varied similarly to the previously reported studies by ELSO, 85.7% in pediatric age group and 14.3% in neonates. This study found that the neonates with congenital heart disease had a higher rate of requiring VA ECMO implantation than the general population, which was consistent with the previous research (13). The most common indication for ECMO within this cohort was the failure to wean off cardiopulmonary bypass following cardiac surgery. The number of patients who received ECMO support climbed rapidly after the ECMO committee established. The mortality rate was slightly decreased but no significant difference in two period due to the very low number of ECMO patients in pre-ECMO committee program period. The ECMO survival rate and survival rate to discharge were 70 and 50%, respectively, which were comparable to the previous studies (12, 13, 16, 17). This study found the ECMO survival rate to discharge in VA and VV were 50%, while the ELSO registry summarized that the mortality rate of VA ECMO was 51.9% and VV ECMO was 61.8% in Asia pacific registration (18). Our ECMO program used unfractionated heparin as the routine anticoagulant. Our study found ECMO patients had HIT 2.8% and heparin resistance 1.4%. An overall incidence of immune-mediated HIT with unfractionated heparin was 2.6% by a meta-analysis (19). Bivalirudin is an alternative anticoagulant in ECMO for patients with HIT or heparin resistance (20). To our knowledge, this study was the first study to report the clinical characteristics, risk factors, and outcomes in pediatric patients with ECMO in Thailand.
The incidences of AKI in neonatal and pediatric ECMO patients were estimated to be between 50 and 62% in a multicenter retrospective observational cohort study (21). Additionally, patients with AKI were shown to require a longer duration of ECMO support, longer ventilator days, and had a higher mortality rate (17, 21, 22). A recent meta-analysis study in 3,523 pediatric patients on ECMO demonstrated that AKI was significantly associated with reduced survival outcome (23). AKI was the most frequently encountered complication in this study and was associated with a significant increase in mortality rate, particularly in those with pre-existing AKI. Additionally, we also found that the non-AKI group had a higher 1-year survival rate than the AKI group (31 vs. 73%, p = 0.050).
Providing optimal nutrition to critically ill children is also an important aspect of a favorable outcome. There were few nutritional support studies in pediatric patients who received ECMO even though they were nutritionally vulnerable (24, 25). A prior retrospective study showed that early enteral feeding was associated with a lower hospital mortality rate (25). Our study found that late enteral feeding after 48 h was associated with an increased risk of mortality.
Septicemia was the leading cause of death in our patients. The most prevalent pathogen was bacteria, particularly those with extensive drug resistance, which was consistent with other studies (26, 27).
Our study had several strengths. We reported the first clinical characteristics and outcomes of pediatric ECMO in Thailand. This study demonstrated a successful ECMO program that developing countries might use to drive the establishment of their own programs and improve patient outcomes. This program utilized an established ECMO committee to select ECMO patients, create certified ECMO nurses as ECMO specialists, establish ECMO training and continue annual ECMO training.
This study had some limitations. First, this was a retrospective study with some variables having missing data. Second, because this study was conducted in a single center with a small sample size, it cannot be generalized. Our center, on the other hand, was a large referral center that may represent the region. Finally, the federal government imposes spending limits on ECMO patients. ECMO may be initiated late in the course of some patients' illnesses. However, our center utilized an ECMO committee to determine which patients would receive ECMO, which likely shortened the time required to initiate ECMO. Additionally, this financial crisis may be comparable to that of other developing countries. Additional collaborative and prospective research are required to validate risk factors and outcomes in developing countries.
Conclusion
ECMO is the lifesaving treatment for rescuing neonatal and pediatric patients with refractory cardiopulmonary failure. Pre-existing acute kidney injury and delayed enteral feeding were associated with an increased mortality rate. Nosocomial infection was the leading cause of death in hospitals. An ECMO program could be done successfully with a multidisciplinary team in developing country.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by Human Research Ethics Committee, Faculty of Medicine Ramathibodi Hospital, Mahidol University. Written informed consent from the participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author Contributions
WI and NA contributed to design of the study, data collection, data analysis, and manuscript drafting. PS and RL contributed to design of the study. NA critically revised it for important intellectual content. All authors gave final approval of the version to be published.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to acknowledge the pediatric intensivists, cardiothoracic surgeon, perfusionist and nurses at Ramathibodi Hospital, Mahidol University for taking care of critically ill children and providing the data in this project. We would like to express our thankfulness to Mrs. Umaporn Udomsubpayakul for providing advice on statistical analysis. We would like to thank Mr. Stephen Pinder and Dr. Chanapai Chaiyakulsil for comprehensive English language review. Furthermore, our special thanks to all patients who were recruited into this study.
References
1. Morris MC, Wernovsky G, Nadkarni VM. Survival outcomes after extracorporeal cardiopulmonary resuscitation instituted during active chest compressions following refractory in - hospital pediatric cardiac arrest. Pediatr Crit Care Med. (2004) 5:440–6. doi: 10.1097/00130478-200409000-00040
2. Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): multicenter randomized controlled trial. Lancet. (2009) 374:1351–63. doi: 10.1016/S0140-6736(09)61069-2
3. Unosawa S, Sezai A, Hata M, Nakata K, Yoshitake I, Wakui S, et al. Long-term outcome of patients undergoing extracorporeal membrane oxygenation for refractory postcardiotomy cardiogenic shock. Surg Today. (2013) 43:264–70. doi: 10.1007/s00595-012-0322-6
4. Thiagarajan RR, Barbaro RP, Rycus PT, Mcmullan DM, Conrad SA, Fortenberry JD, et al. Extracorporeal life support organization registry international report 2016. ASAIO J. (2017) 63:60–7. doi: 10.1097/MAT.0000000000000475
5. Bartlett RH, Gazzaniga AB, Jefferies MR, Huxtable RF, Haiduc NJ, Fong SW. Extracorporeal membrane oxygenation (ECMO) cardiopulmonary support in infancy. Trans Am Soc Artif Intern Organs. (1976) 22:80–93.
6. Thiagarajan RR, Laussen PC, Rycus PT, Bartlett RH, Bratton SL. Extracorporeal membrane oxygenation to aid cardiopulmonary resuscitation in infants and children. Circulation. (2007) 116:1693–700. doi: 10.1161/CIRCULATIONAHA.106.680678
7. Shin TG, Choi JH, Jo IJ, Sim MS, Song HG, Jeong YK, et al. Extracorporeal cardiopulmonary resuscitation in patients with inhospital cardiac arrest: a comparison with conventional cardiopulmonary resuscitation. Crit Care Med. (2011) 39:1–7. doi: 10.1097/CCM.0b013e3181feb339
8. Dalton HJ, Reeder R, Garcia-Filion P, Holubkov R, Berg RA, Zuppa A, et al. Factors associated with bleeding and thrombosis in children receiving extracorporeal membrane oxygenation. Am J Respir Crit Care Med. (2017) 196:762–71. doi: 10.1164/rccm.201609-1945OC
9. Dalton HJ, Garcia-Filion P, Holubkov R, Moler FW, Shanley T, Heidemann S, et al. Association of bleeding and thrombosis with outcome in extracorporeal life support. Pediatr Crit Care Med. (2015) 16:167–74. doi: 10.1097/PCC.0000000000000317
10. Polito A, Barrett CS, Wypij D, Rycus PT, Netto R, Cogo PE, et al. Neurologic complications in neonates supported with extracorporeal membrane oxygenation. An analysis of ELSO registry data. Intensive Care Med. (2013) 39:1594–601. doi: 10.1007/s00134-013-2985-x
11. Hanekamp MN, Mazer P, van der Cammen-van Zijp MH, van Kessel-Feddema BJ, Nijhuis-van der Sanden MW, Knuijt S, et al. Follow-up of newborns treated with extracorporeal membrane oxygenation: a nationwide evaluation at 5 years of age. Crit Care. (2006) 10:R127. doi: 10.1186/cc5039
12. Roeleveld PP, Mendonca M. Neonatal cardiac ECMO in 2019 and beyond. Front Pediatr. (2019) 7:327. doi: 10.3389/fped.2019.00327
13. Amodeo I, Di Nardo M, Raffaeli G, Kamel S, Macchini F, Amodeo A, et al. Neonatal respiratory and cardiac ECMO in Europe. Eur J Pediatr. (2021) 180:1675–92. doi: 10.1007/s00431-020-03898-9
14. Lin YJ, Chung SY, Liang CD, Kuo HC, Huang CF, Chien SJ, et al. Impact of extracorporeal membrane oxygenation support on clinical outcome of pediatric patients with acute cardiopulmonary failure: a single-center experience. Biomed J. (2013) 36:28–34. doi: 10.4103/2319-4170.107161
15. Kellum JA, Lameire N, Aspelin P, Barsoum RS, Burdmann EA, Goldstein SL, et al. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. (2012) KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl. (2012) 2:1–138. doi: 10.1038/kisup.2012.1
16. Carpenter JL, Yu YR, Cass DL, Olutoye OO, Thomas JA, Burgman C, et al. Use of venovenous ECMO for neonatal and pediatric ECMO: a decade of experience at a tertiary children's hospital. Pediatr Surg Int. (2018) 34:263–8. doi: 10.1007/s00383-018-4225-5
17. Kattan J, González Á, Castillo A, Caneo LF. Neonatal and pediatric extracorporeal membrane oxygenation in developing Latin American countries. J Pediatr. (2017) 93:120–9. doi: 10.1016/j.jped.2016.10.004
18. ELSO Registry Report Registry Dashboard. Available online at: https://www.elso.org/Registry/ELSOLiveRegistryDashboard.aspx (accessed September 2, 2021).
19. Martel N, Lee J, Wells PS. Risk for heparin-induced thrombocytopenia with unfractionated and low-molecular-weight heparin thromboprophylaxis: a meta-analysis. Blood. (2005) 106:2710–5. doi: 10.1182/blood-2005-04-1546
20. Sanfilippo F, Asmussen S, Maybauer DM, Santonocito C, Fraser JF, Erdoes G, et al. Bivalirudin for alternative anticoagulation in extracorporeal membrane oxygenation: a systematic review. J Intensive Care Med. (2017) 32:312–9. doi: 10.1177/0885066616656333
21. Fleming GM, Sahay R, Zappitelli M, King E, Askenazi DJ, Bridges BC, et al. The incidence of acute kidney injury and its effect on neonatal and pediatric extracorporeal membrane oxygenation outcomes: a multicenter report from the kidney intervention during extracorporeal membrane oxygenation study group. Pediatr Crit Care Med. (2016) 17:1157–69. doi: 10.1097/PCC.0000000000000970
22. Elella RA, Habib E, Mokrusova P, Joseph P, Aldalaty H, Ahmadi MA, et al. Incidence and outcome of acute kidney injury by the pRIFLE criteria for children receiving extracorporeal membrane oxygenation after heart surgery. Ann Saudi Med. (2017) 37:201–6. doi: 10.5144/0256-4947.2017.201
23. Hansrivijit P, Lertjitbanjong P, Thongprayoon C, Cheungpasitporn W, Aeddula NR, Salim SA, et al. Acute kidney injury in pediatric patients on extracorporeal membrane oxygenation: a systematic review and meta-analysis. Medicines. (2019) 6:109. doi: 10.3390/medicines6040109
24. Farr BJ, Rice-Townsend SE, Mehta NM. Nutrition support during pediatric extracorporeal membrane oxygenation. Nutr Clin Pract. (2018) 33:747–53. doi: 10.1002/ncp.10212
25. Ong C, Mok YH, Tan ZH, Lim CYS, Ang B, Tan TH, et al. Nutritional practices and adequacy in children supported on extracorporeal membrane oxygenation. Clin Nutr ESPEN. (2018) 26:21–6. doi: 10.1016/j.clnesp.2018.05.005
26. Ayyildiz P, Kasar T, Ozturk E, Yildiz O, Ozturk S, Ergul Y, et al. The evaluation of nosocomial infections in pediatric patients with extracorporeal membrane oxygenation support. Braz J Cardiovasc Surg. (2017) 32:468–74. doi: 10.21470/1678-9741-2017-0072
Keywords: extracorporeal membrane oxygenation, pediatric, risk factors, outcome, neonatal
Citation: Iamwat W, Samankatiwat P, Lertbunrian R and Anantasit N (2021) Clinical Characteristics and Outcomes of Children With Extracorporeal Membrane Oxygenation in a Developing Country: An 11-Year Single-Center Experience. Front. Pediatr. 9:753708. doi: 10.3389/fped.2021.753708
Received: 05 August 2021; Accepted: 11 October 2021;
Published: 16 November 2021.
Edited by:
Krishan Chugh, Fortis Healthcare, IndiaReviewed by:
Ahmed S. Said, Washington University in St. Louis, United StatesNikoleta S. Kolovos, Washington University School of Medicine in St. Louis, United States
Copyright © 2021 Iamwat, Samankatiwat, Lertbunrian and Anantasit. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nattachai Anantasit, bmF0dGFjaGFpMDMyQGhvdG1haWwuY29t
 Wirapatra Iamwat
Wirapatra Iamwat Piya Samankatiwat
Piya Samankatiwat Rojjanee Lertbunrian1
Rojjanee Lertbunrian1 Nattachai Anantasit
Nattachai Anantasit