
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 18 October 2021
Sec. Pediatric Infectious Diseases
Volume 9 - 2021 | https://doi.org/10.3389/fped.2021.740785
 Susanne C. Diesner-Treiber1,2*†
Susanne C. Diesner-Treiber1,2*† Peter Voitl1,2,3†
Peter Voitl1,2,3† Julian J. M. Voitl1
Julian J. M. Voitl1 Klara Langer1
Klara Langer1 Ulrike Kuzio1
Ulrike Kuzio1 Angela Riepl1
Angela Riepl1 Pia Patel1
Pia Patel1 Alexandra Mühl-Riegler4
Alexandra Mühl-Riegler4 Bernhard Mühl4
Bernhard Mühl4Background: The Covid-19 pandemic compelled the implementation of measures to curb the SARS CoV-2 spread, such as social distancing, wearing FFP2 masks, and frequent hand hygiene. One anticipated ramification of these measures was the containment of other pathogens. This prospective, longitudinal study aimed to investigate the spread of 22 common seasonal non-SARS-CoV-2 pathogens, such as RSV and influenza, among children with an acute respiratory infection during a pandemic.
Methods: Three hundred ninety children (0-24 months) admitted to Vienna's largest pediatric center with acute respiratory infection (November 2020-April 2021) were included in this study. The researchers tested nasal swabs for 22 respiratory pathogens by Multiplex PCR, documented clinical features and treatment, and evaluated data for a potential connection with the lockdown measures then in force.
Results: The 448 smears revealed the most common pathogens to be rhino-/enterovirus (41.4%), adenovirus (2.2%), and coronavirus NL63 (13.6%). While the first two were active throughout the entire season, coronaviruses peaked in the first trimester of 2021 in conjunction with the lift of the lockdown period (OR 4.371, 95%CI 2.34-8.136, P < 0.001). RSV, metapneumovirus, and influenza were absent.
Conclusion: This prospective, longitudinal study shows that Covid-19 measures suppressed the seasonal activity of influenza, RSV, and metapneumovirus among very young children, but not of rhino-/enterovirus and adenovirus. The 0-24 month-olds are considered the lowest risk group and were only indirectly affected by the public health measures. Lockdowns were negatively associated with coronaviruses infections.
Respiratory infections are among the leading causes of children's admission to pediatric practices. Pathogens typically circulating in the winter can trigger similar symptoms in children (1) and, in severe cases, lead to hospitalization. The most commonly identified respiratory pathogens are rhinovirus/enterovirus, respiratory syncytial virus (RSV), adenovirus, influenza virus, parainfluenza viruses, and coronaviruses (2). While adenovirus occurs more often in children aged 3-6 years, RSV is more likely to affect infants and toddlers <3 years of age (3). Overall, infections with these pathogens thrive in winter, although seasonality may vary. While rhinovirus, adenovirus, and metapneumovirus are present throughout the year, influenza, RSV, and human coronaviruses (229E, HKU1, NL63, and OC43) have significantly higher communicability in winter (3, 4). In Austria, the RSV season ranges from October to the beginning of April, peaking around the 8th calendar week, while the influenza season usually falls in the first calendar weeks of the year, showing a distinct peak in the 5th calendar week of 2020 (5, 6).
Since the beginning of 2020, the world has been dealing with a new pandemic caused by SARS-CoV-2. At the time of our study, children typically had a milder clinical course, and their role in the transmission of the virus remained controversial (7). With all the numerous regulations worldwide, such as lockdowns, social distancing, hand hygiene, wearing mouth and nose protection (8, 9), the pandemic had a profound impact on public awareness of viral infections, and retrospective analyses on Covid-19 preventive measures showed a significant influence on the occurrence of co-infections. In the general population, Austria registered fewer rhinovirus, metapneumovirus, influenza, and RSV cases during the first lockdown in spring 2020 (10). In Italy, other infectious diseases such as varicella, pertussis, measles, and rubella were also less common (11).
Based on these observations, this longitudinal, prospective study aimed to investigate how the incidence of 22 seasonal non-Covid-19 respiratory pathogens developed in a representative Austrian pediatric cohort during a pandemic in a primary pediatric health care setting. Virological diagnostics specifically did not include SARS-CoV-2, as the health authorities are gathering data on the temporal course of its spread and connection with the current measures.
This prospective, longitudinal study was conducted at the “First Vienna Pediatric Medical Center” between November 2020 and April 2021, the first whole winter semester with the Covid-19 pandemic in Austria. The study center is Austria's largest pediatric outpatient practice, with more than 57.000 patient contacts per year, or over 16% of all outpatient contacts in Vienna (2015) (12, 13). Its broad range of medical specialties guarantees healthcare to patients coming from the whole region.
All families of children (0-24 months) meeting the inclusion criteria were asked to participate in this study. Eligible children presented with acute respiratory infection with at least one of the following symptoms: rhinitis, nasal congestion, cough, sore throat or fever of unknown origin (e.g., without gastroenteritis or urinary tract infection) with a maximum duration of one week. Preterm infants, children with severe congenital or chronic diseases (e.g., severe congenital heart malformations, severe pulmonary or neuromuscular diseases, severe immunodeficiency, and chromosomal aberration) were excluded.
The study was approved by the Ethics Committee of the Medical University of Vienna (EK-Nr. 1864/2020). Parents received extensive information on the objective and procedures of this study and provided written consent.
The case report form included the family socio-economic status (education of parents, housing situation, marital status, number of siblings), patient's general clinical data (weight, height, calculated BMI, chronic diseases), clinical features of the acute respiratory infection (rhinitis, nasal congestion, fever, fever duration, cough, pharyngitis), and treatment recommendations (decongestant nose spray, hypertonic saline nose spray, analgesics, antibiotics, inhalation therapy [salbutamol as short- acting beta mimetics (SABA), inhaled corticosteroids (ICS)], inhalation with sodium chloride), systemic corticosteroids, as well as indications for hospitalization if necessary.
We repeated nasal swabs in case of recurrent acute infection with healthy periods in-between. Socio-demographic and patient-specific data, however, were only collected at the first visit.
Nasal swab samples were collected by a trained study team from both nostrils and put into 0.9% sodium chloride until further analysis. The samples were screened using the Multiplex PCR respiratory panel (Biofire FilmArray Respiratory panel 2 plus kit) to detect adenovirus, coronaviruses (229E, HKU1, OC43, NL63), metapneumovirus, rhinovirus/enterovirus, influenza A (H1, H1-2009, H3), influenza B, parainfluenza 1-4, RSV, MERS-CoV, Chlamydia pneumoniae, Mycoplasma pneumoniae, Bordetella pertussis, Bordetella parapertussis, with high sensitivity and specificity for nasopharyngeal swabs (14). As recommended by the Ethics Committee, we performed the less invasive method of nasal swabs to reduce the discomfort of the procedure.
Samples were not tested for SARS-CoV-2 in this trial. However, in case of anamnestic or clinical signs of a possible SARS-CoV-2 infection, patients were referred to the official SARS-CoV-2 testing units.
Information on the Austrian Covid-19 regulations was constantly monitored on the homepage of the Federal Ministry of Social Affairs, Health, Care and Consumer Protection (9).
The numerous regulations to limit the circulation of the pathogen implicate physical distancing, temporary lockdowns, working from home, distance learning, kindergarten emergency assistance, the closure of shops not providing essential services, ban on travel, limitations to the foodservice industry, and curfews (8). Furthermore, it was mandatory to wear mouth and nose protection in certain public areas. In addition, the population was encouraged to disinfect their hands regularly (9).
In detail and for statistical analysis, lockdowns were either total lockdowns (calendar weeks 47-50, 53-6, and 13-16) which entailed a 24 h curfew and the cessation of cultural and leisure activities and events, the closure of hotels and hospitality facilities, or partial lockdowns (weeks 45-47, weeks 50-52) with a night-time curfew and the cessation of the above mentioned non-essential services but allowing retailers and service providers to remain open. During partial lockdowns, senior year students attended remote learning classes, which was then extended to all grades during total lockdowns. Attendance was not mandatory for kindergartens; however, childcare options were available for parents who could not look after their children at home. In-between lockdowns (calendar weeks 7-12), schools, shops, and museums were allowed to reopen, whereas the hospitality and food sectors remained closed.
From September 2020, mouth-nose protections and from January 2021 particle filtering half-masks (FFP2) were mandatory in public indoor places, such as shops, public transportation, indoor markets, gastronomy, and administrative authorities, for all people older than 12 years.
Raw data were sorted in Google Sheets and Microsoft Excel and analyzed by S.C.D.T. using IBM SPSS-Statistics version 27. The statistical significance level was set at p < 0.05.
All data were categorized into nominal variables except for the metric variables age, BMI, number of siblings, and fever duration. Age was categorized as follows: 0-6, 6-12, 12-18, and 18-24 months. For statistical analysis, detected pathogens were inventoried as a new variable “pathogen category”: rhino-/enterovirus, coronaviruses (coronaviruses 229E, HKU1, OC43, NL63), others (all single positive results without rhino-/enterovirus and coronaviruses), multiple infections (co-infections with more than one pathogen) or negative.
Descriptive statistics [median, interquartile range (IQR) Q1-Q3, mean, 95% confidence interval (CI)] were used to characterize the study population and recapitulate the number of weekly swabs. Shapiro-Wilk-Test was used to test for normal distribution. Weekly testing rates numbers in and out of lockdowns were compared using the unpaired Student's t-test. Analysis of qualitative values included absolute numbers and relative frequencies in percentage (%). Nominally scaled variables were tested using the Chi-Square test (X2). The association of two categorical variables was analyzed with the Cramér's V correlation coefficient, which was interpreted according to the Rea and Parker classification (r = 0.10-0.20 weak, r = 0.20-0.40 moderate, and r = 0.40-0.60 relatively strong association) (15). Multiple testing correction was done using the Bonferroni method, and adjusted p-values were calculated.
We performed multinomial logistic regression analysis to test the influence of diverse predictors (independent variables: age, sex, lockdown, siblings) on the occurrence of a specific pathogen—the nominal variable “pathogen category” being the dependent variable and the negative sample being the reference variable. Wald test was used to test for the significance of individual coefficients (16). Odds ratio (OR, 95% confidence interval and p-values were calculated.
During the November 2020-April 2021 winter season, 390 children (0-24 months, 59% male, 41% female) with an acute respiratory infection or fever of unknown origin were included in this study. A total of 448 nasal swabs were collected, once in 340 children, twice in 43, thrice in 6 children, and four times in one child. The median age was 12 months (Q1-Q3, 6-18 months). Table 1 summarizes socio-demographic and general clinical characteristics.
The main clinical manifestations (in detail, refer to Table 2) that emerged from all 448 swabs were rhinitis (70%), followed by cough (64%). Symptomatic treatment included decongestant nasal sprays (45.5%), analgesics (57%), and antibiotics (10.9%). Inhalation therapy or systemic corticosteroids to treat laryngitis or bronchitis were rarely prescribed. Hospitalization was indicated only in one case (Table 2).
The Multiplex PCR was negative in 166 out of the 448 samples (37.1%), co-infections emerged in 21 samples (4.68%). The typical seasonal RSV and metapneumovirus pathogens were not detected in any of the study subjects during the whole recruitment period. Influenza A (H3) and influenza B were found in only one patient who had been vaccinated with a live-attenuated nasal flu vaccine (Fluenz tetra®) 1 week prior to the study recruitment.
The most prevalent pathogen was rhinovirus/enterovirus (n = 184, 41.1%), followed by coronavirus NL63 (n = 61, 13.6%), adenovirus (n = 10, 2.2%), parainfluenza virus 3 (n = 3, 0.7%), and coronavirus OC4 (n = 2, 0.4%) (Figure 1). One patient tested positive for Bordetella parapertussis, and was immediately treated with antibiotics as symptoms persisted.
As expected, rhino-/enterovirus infections were associated with nasal symptoms and cough, while coronaviruses infections predominantly led to cough and, to a lesser extent, nasal symptoms. In children with fever we rarely detected a respiratory pathogen (Table 3).
The Chi-Square analysis determined a significant association between the pathogen groups and the observed symptoms for rhinitis (Cramér's V 0.462, p-adj. < 0.001), nasal congestion (Cramér's V 0.242, p-adj. < 0.001), fever (Cramér's V 0.315, p-adj. < 0.001), and cough (Cramér's V 0.358, p-adj. < 0.001).
Our longitudinal, prospective study design allowed the delineation of the weekly prevalence of respiratory pathogens in symptomatic children during a pandemic and the trends' correlation with the Covid-19 regulations.
Rhino-/enterovirus and adenovirus were detected throughout the winter months despite the pandemic regulations. Coronavirus NL63 peaked in the first trimester of 2021 (Figure 2 and Supplementary Figure 1), especially during the weeks without a lockdown. Other pathogens were found only sporadically. The weekly number of nasal swabs, an indicator of the infection rate in the pediatric cohort, plummeted in the calendar weeks 45 to 4 and increased during the first trimester of 2021. Lockdown intervals registered considerably fewer swabs per week (average weekly number of swabs during lockdowns: 15.79 swabs/week, 95%CI (13.08,18.50) compared to weeks without a lockdown [24.66 swabs/week, 95%CI (17.53,31.80)], [t(23) = 3.213, p = 0.004, d = 1.5].
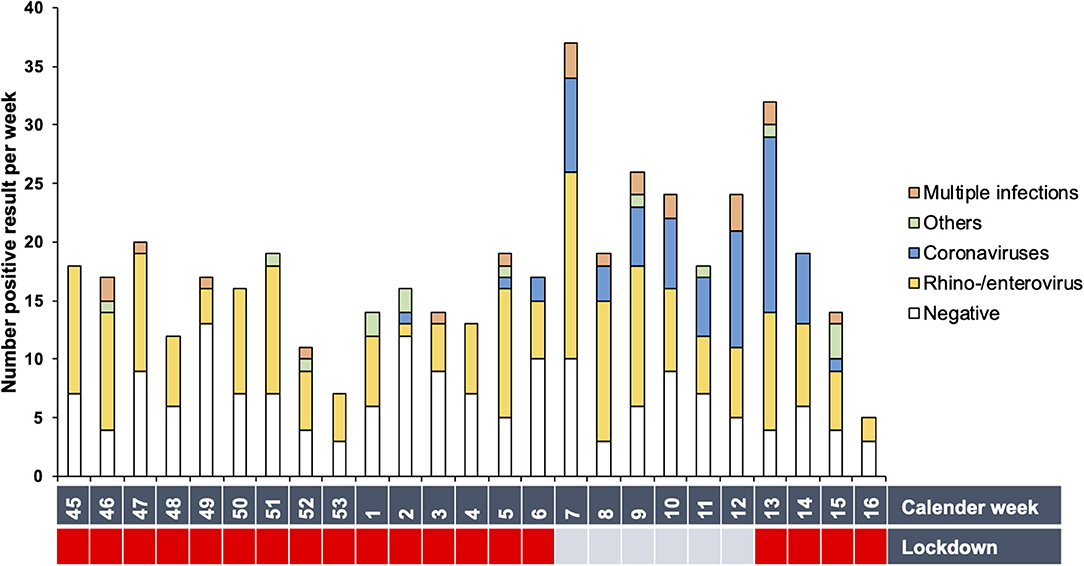
Figure 2. Prevalence of detected pathogens per calendar week. Total number of positive results per week are shown. White bars represent the number of negative results. The Covid-19 regulations are depicted as lockdown (red) or lockdown-free period (blue).
We explored the potential association between specific predictors such as age group, siblings, and lockdown and the prevalence of a pathogen category using multinominal logistic regression analysis. There was only a significant association of the lockdown with coronaviruses (OR 4.371, 95% CI 2.34-8.14, p-adj. < 0.001), indicating a four times higher prevalence during lockdown-free periods. Lockdowns did not significantly influence any other detected pathogens (Supplementary Table 1).
This prospective, longitudinal cohort study analyzed the epidemiological profile of winter respiratory pathogens in infants and toddlers during Covid-19 pandemic lockdowns. Children (0-24 months) visiting Vienna's largest pediatric primary health care center due to symptoms of acute respiratory tract infection or with unexplained fever were mainly positive for rhino-/enterovirus, adenovirus, and coronaviruses; however, they were spared from influenza, RSV, and metapneumovirus throughout the winter season. The only positive influenza sample was caused by viral shedding in the recipient of a nasal flu vaccination 1 week prior to testing. This finding is consistent with publications indicating that PCR assays can detect viral shedding weeks after vaccination (17). The lockdown free periods showed a significant association with a higher risk for coronaviruses.
Our study cohort consisted of otherwise healthy term infants and toddlers. We explicitly excluded children with severe chronic or congenital disorders as this category is more likely to develop severe respiratory infections, e.g., due to RSV (18). These patients are regularly admitted to the hospital and might have a different risk profile regarding the exposure to infectious diseases than healthy children. We have chosen the 0-24 months age group because typically, Austrian children start kindergarten at around 3 years (19). As kindergartners do not wear masks or abide by social distancing, other hygiene rules are applied. This circumstance might alter the risk of exposure to infections and would have biased our research on the viral activity in children during a lockdown. Therefore, we expected our study cohort to be only indirectly affected by non-pharmaceutical Covid-19 measures.
In addition, we chose this age group specifically as respiratory infections caused by RSV, adenovirus, rhino-/enterovirus, and parainfluenza virus are significantly more common in the first years of life (20). Unfortunately, we are unable to compare our results with those from pre-Covid-19 research, as there are no similar studies conducted on the same age group in an Austrian pediatric primary health care setting before. However, based on international data and national surveillance systems that contain adult and pediatric results, it is clear that rhinovirus, influenza, RSV, parainfluenza, metapneumovirus, and adenovirus accounted internationally for up to 80% of acute respiratory tract infections (2, 21, 22) and were the culprit of respiratory infections before the pandemic also in Austria (10).
Several retrospectively designed studies investigated the impact of Covid-19 regulations on the occurrence of non-SARS-CoV-2 respiratory pathogens using surveillance systems data (10, 23, 24). An Austrian sentinel surveillance data analysis in spring 2020, when the country adopted the first Covid-19 measures, reported a significant decrease in influenza, RSV, metapneumovirus, and rhinovirus 1 week after the start of a lockdown (10). However, those data do not reflect the children's specific situation and do not provide any information about the patients' individual symptoms. What emerged from our study was, in fact, the occurrence of rhino-/enterovirus infections in children aged 0-24 months throughout the winter independent of the Covid-19 measures. An Australian surveillance study described a significant decline in RSV and influenza coinciding with the Covid-19 pandemic, while rhinovirus infections were above average. The authors hypothesized that the Covid-19 screening of asymptomatic patients and the absence of competition from influenza and RSV might have led to the higher numbers of rhinovirus detections (23). Other studies reported analogous high rhinovirus positivity rates during the Covid-19 pandemic and a drastic decline of influenza virus, human metapneumovirus, parainfluenza viruses, RSV, and adenovirus in children (25, 26).
Besides the absence of RSV, influenza, and metapneumovirus, we observed an association between lockdown and coronaviruses infection rates. The coronavirus NL63 peak in our study cohort was concomitant with the lockdown-free period in the first trimester of 2021. Under normal circumstances, the seasonality of human coronavirus peaks in the winter and spring months (27). A Finnish register-based study did not find any direct impact of reopening of schools and day care centers on the incidences of respiratory pathogens; however, researchers did not investigate for coronaviruses infections (28).
We tried to contextualize our results in the frame of the national Covid-19 mitigation measures because they substantially affected the social life of the whole family. However, infants and toddlers were only indirectly affected by these non-pharmaceutical measures, but might suffer the consequences of social isolation. In addition, their older siblings, who are often the source for respiratory infections for young children, might have brought less infections to their homes (29). Our data might corroborate this hypothesis as we did not observe a significant association between having siblings and the onset of specific respiratory or multiple infections. It might be speculated that children per se were less often ill during the pandemic or stayed at home more often with mild disease. Considering our results in relation to the Covid-19 lockdowns, the limited number of nasal swab samples collected during lockdown periods might point to the effects Covid-19 regulations could have on the respiratory infection rates in the pediatric cohort. Other studies confirm this hypothesis as acute otitis media, gastroenteritis, common cold, and bronchiolitis have also decreased among other pediatric populations (30, 31). The Covid-19 mitigation measures changed not only the circulation patterns of respiratory pathogens but seemed to affect the hospitalization rate. Only one of our study subjects was referred to the hospital. Analogous records on hospitalizations for acute bronchiolitis come from international studies (32, 33). RSV is usually the pathogen with the highest severity of infection and the primary cause of hospitalization and PICU admissions in young children (34, 35). Lockdown and school closures significantly correlated with reduced hospitalizations and overall pediatric visits rates in other countries (33, 35–38).
Ultimately, the question remains, why is one pathogen more affected than the other? One hypothesis focuses on the effects Covid-19 measures have on the specific transmission routes. While RSV, influenza, and coronaviruses are mainly airborne, adenoviruses and rhino-/enteroviruses can also be transmitted by direct contact (20, 39). In addition to that, other factors, such as travel restrictions and a higher vaccination coverage for influenza, might have inhibited the transmission during the study period (40). However, these assumptions require further investigation.
Although our practice regularly received patients with respiratory infections throughout the pandemic winter season, many families avoided in-person visits either because of quarantine or fear of Covid-19, which is confirmed by significant lower numbers of patient visits in the recruitment months 2020 compared to previous years (21.000 vs. 27.000 (November 2018– April 2019)). Most of the families were willing to take part in this study, but we did not analyse the rate of refusal to participate in detail. It is therefore impossible to ascertain a significant effect on single pathogens. It is plausible that some of our patients had a SARS-CoV-2 infection or co-infection when the swab was collected (41).
During the study period, a separate system other than the primary health care centers was in charge of testing all patients with typical Covid-19 symptoms for SARS-CoV-2 in Austria. Therefore, the SARS-CoV-2 analysis would have led to a biased underrepresentation of this coronavirus in our study cohort. In addition, since the detection of SARS-CoV-2 was not the focus of this study and considering Covid-19 rarely occurred among young children (7, 38), who are often asymptomatic or mildly affected, we consider this bias a minor limitation in our study (42).
Another limiting factor could be the single-center design of our study. Since our health care facility is Austria's largest primary pediatric practice, and our conclusions support the national surveillance data (5, 6), it can be assumed that our data is fairly representative.
Based on our clinical results fever is most often found in children with negative swab samples implying that this parameter is not a precise inclusion criterion for children with airway infections. Other viral pathogens, such as HHV6, are quite common to cause fever in this age group but was not included in our panel analysis.
We chose nasal instead of nasopharyngeal swabs for a less invasive approach that yields satisfactory results, as other studies suggest (43–45). However, we cannot exclude that our sampling technique led to lower detection rates. Using this non-invasive technique in future winter seasons will provide more information on the typical distribution patterns of respiratory viruses in our study cohort, as the lack of comparability might constitute a limitation in the current study.
Further information on the socio-demographic background (e.g., attending childcare or other family members with respiratory tract infections) might glean valuable insight into influencing factors, and should be considered in future studies on this topic.
We are aware that our study has some limitations but we see these data as an important starting point for future research, which might give more insights in the upcoming years.
Our prospective longitudinal study shows that during the winter season, Covid-19 measures suppressed the seasonal activity of influenza, RSV, and metapneumovirus, but not of rhino-/enteroviruses among 0-2-year-old children in a pediatric primary health care setting. The lockdown measures were negatively associated with coronaviruses infections. We support the conclusions from retrospective surveillance studies about the influence of Covid-19 mitigation measures on the occurrence of other respiratory pathogens in children. However, further research is required to clarify the exact influence a particular measure has on a specific pathogen. This effort could be a crucial advancement in public health responses to future viral outbreaks.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Committee of the Medical University of Vienna (EK-Nr. 1864/2020). Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
SD-T conceptualized and designed the study, supervised data collection, carried out data analysis, drafted the initial manuscript, and reviewed and revised the manuscript. PV conceptualized and designed the study, supervised data collection, and reviewed and revised the manuscript. JV, KL, AR, UK, and PP recruited patients, collected data, and critically reviewed and revised the manuscript. AM-R and BM were responsible for supervision and interpretation of PCR analyses, and critically reviewed and revised the final manuscript. All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work.
This work was supported by MSD's Merck investigator Studies Program [MISP 60230]. The funders had no role in study design, data collection and analysis, decision to publish, or manuscript preparation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2021.740785/full#supplementary-material
Supplementary Figure 1. Detailed prevalence of detected pathogens per calendar week. Total number of all positive results per week are shown without pathogen grouping. White bars represent the number of negative results. The Covid-19 regulations are depicted as lockdown (red) or lockdown-free period (blue).
1. Bourgeois FT, Valim C, Wei JC, McAdam AJ, Mandl KD. Influenza and other respiratory virus-related emergency department visits among young children. Pediatrics. (2006) 118:e1-8. doi: 10.1542/peds.2005-2248
2. Ramaekers K, Keyaerts E, Rector A, Borremans A, Beuselinck K, Lagrou K, et al. Prevalence and seasonality of six respiratory viruses during five consecutive epidemic seasons in Belgium. J Clin Virol. (2017) 94:72-8. doi: 10.1016/j.jcv.2017.07.011
3. Zhu G, Xu D, Zhang Y, Wang T, Zhang L, Gu W, et al. Epidemiological characteristics of four common respiratory viral infections in children. Virol J. (2021) 18:10. doi: 10.1186/s12985-020-01475-y
4. Moriyama M, Hugentobler WJ, Iwasaki A. Seasonality of respiratory viral infections. Annu Rev Virol. (2020) 7:83-101. doi: 10.1146/annurev-virology-012420-022445
5. Department of Virology MUoV. Influenza - Projekt Diagnostisches Influenzanetzwerk Österreich (DINÖ). Available online at: https://www.virologie.meduniwien.ac.at/wissenschaft-forschung/virus-epidemiologie/influenza-projekt-diagnostisches-influenzanetzwerk-oesterreich-dinoe/2021 (accessed May 3, 2021).
6. Department of Virology MUoV. Österreichisches RSV Netzwerk – ÖRSN. Available online at: https://www.virologie.meduniwien.ac.at/wissenschaft-forschung/virus-epidemiologie/rsv-netzwerk-oersn/2021 (accessed May 3, 2021).
7. Bullard J, Funk D, Dust K, Garnett L, Tran K, Bello A, et al. Infectivity of severe acute respiratory syndrome coronavirus 2 in children compared with adults. CMAJ. (2021) 193:E601-E6. doi: 10.1503/cmaj.210263
8. ECDC. Guidelines for the Implementation of Non-Pharmaceutical Interventions Against COVID-19. (2020). Available online at: https://www.ecdc.europa.eu/sites/default/files/documents/covid-19-guidelines-non-pharmaceutical-interventions-september-2020.pdf (accessed May 4, 2021).
9. Federal Ministry of Social Affairs H. Care and Consumer Protection. Coronavirus. Available online at: https://www.sozialministerium.at/Informationen-zum-Coronavirus/Coronavirus---Rechtliches.html2021 (accessed May 1, 2021).
10. Redlberger-Fritz M, Kundi M, Aberle SW, Puchhammer-Stöckl E. Significant impact of nationwide SARS-CoV-2 lockdown measures on the circulation of other respiratory virus infections in Austria. J Clin Virol. (2021) 137:104795. doi: 10.1016/j.jcv.2021.104795
11. Belingheri M, Paladino ME, Piacenti S, Riva MA. Effects of COVID-19 lockdown on epidemic diseases of childhood. J Med Virol. (2021) 93:153-4. doi: 10.1002/jmv.26253
12. Schwab E, Voitl P, Loeffler-Stastka H. Primary Healthcare Center Möglichkeiten, Strukturen und Grenzen von Primärversorgungseinheiten. Psychopraxis, Neuropraxis. (2018). 21:276-81. doi: 10.1007/s00739-018-0494-y
13. Voitl P. Kinder- und Jugendheilkunde im Vergleich. Pädiatrie und Pädologie. (2016) 51:260–3. doi: 10.1007/s00608-016-0426-0
14. Rappo U, Schuetz AN, Jenkins SG, Calfee DP, Walsh TJ, Wells MT, et al. Impact of early detection of respiratory viruses by multiplex PCR assay on clinical outcomes in adult patients. J Clin Microbiol. (2016) 54:2096-103. doi: 10.1128/JCM.00549-16
15. Kotrilk J, Williams H, Khata Jabor M. Reporting and Interpreting Effect Size in Quantitative Agricultural Education Research. J Agri Educ. (2011) 52:132-42. doi: 10.5032/jae.2011.01132
16. Bewick V, Cheek L, Ball J. Statistics review 14: logistic regression. Crit Care. (2005) 9:112-8. doi: 10.1186/cc3045
17. Mallory RM, Yi T, Ambrose CS. Shedding of Ann Arbor strain live attenuated influenza vaccine virus in children 6-59 months of age. Vaccine. (2011) 29:4322-7. doi: 10.1016/j.vaccine.2011.04.022
18. European foundation for the care of newborn infants. Position paper. Respiratory Syncytial Virus (RSV) in Preterm and Ill Infants. Available online at: https://www.efcni.org/wp-content/uploads/2021/04/2021_RSV_Positionpapier.pdf2021 (accessed September 6, 2021).
19. Austria S. Day Care Centers, Childcare. (2020). Available onlne at: https://www.statistik.at/web_de/statistiken/menschen_und_gesellschaft/bildung/kindertagesheime_kinderbetreuung/index.html (accessed September 6, 2021).
20. Diseases RCoPCHESoPI. Lower respiratory tract infection. In: Mike Sharland KB, Cant A, Dagan R, Davies G, de Groot R, Elliman D, et al. editors. Manual of Childhood Infections (Oxford Specialist Handbooks in Paediatrics): The Blue Book. 4th ed. Oxford: Oxford University Press (2016).
21. Das S, Dunbar S, Tang YW. Laboratory diagnosis of respiratory tract infections in children - the state of the art. Front Microbiol. (2018) 9:2478. doi: 10.3389/fmicb.2018.02478
22. Mahony JB. Detection of respiratory viruses by molecular methods. Clin Microbiol Rev. (2008) 21:716-47. doi: 10.1128/CMR.00037-07
23. Sullivan SG, Carlson S, Cheng AC, Chilver MB, Dwyer DE, Irwin M, et al. Where has all the influenza gone? The impact of COVID-19 on the circulation of influenza and other respiratory viruses, Australia, March to September 2020. Euro Surveill. (2020) 25:2001847. doi: 10.2807/1560-7917.ES.2020.25.47.2001847
24. Tang JW, Bialasiewicz S, Dwyer DE, Dilcher M, Tellier R, Taylor J, et al. Where have all the viruses gone? Disappearance of seasonal respiratory viruses during the COVID-19 pandemic. J Med Virol. (2021) 93:4099-101. doi: 10.1002/jmv.26964
25. Takashita E, Kawakami C, Momoki T, Saikusa M, Shimizu K, Ozawa H, et al. Increased risk of rhinovirus infection in children during the coronavirus disease-19 pandemic. Influenza Other Respir Viruses. (2021) 15:488-94. doi: 10.1111/irv.12854
26. Kuitunen I, Artama M, Haapanen M, Renko M. Rhinovirus spread in children during the COVID-19 pandemic despite social restrictions-A nationwide register study in Finland. J Med Virol. (2021) 93:6063-7. doi: 10.1002/jmv.27180
27. Rucinski SL, Binnicker MJ, Thomas AS, Patel R. Seasonality of Coronavirus 229E, HKU1, NL63, and OC43 From 2014 to 2020. Mayo Clin Proc. (2020) 95:1701-3. doi: 10.1016/j.mayocp.2020.05.032
28. Haapanen M, Renko M, Artama M, Kuitunen I. The impact of the lockdown and the re-opening of schools and day cares on the epidemiology of SARS-CoV-2 and other respiratory infections in children - A nationwide register study in Finland. EClinicalMedicine. (2021) 34:100807. doi: 10.1016/j.eclinm.2021.100807
29. Vissing NH, Chawes BL, Rasmussen MA, Bisgaard H. Epidemiology and risk factors of infection in early childhood. Pediatrics. (2018) 141:e20170933. doi: 10.1542/peds.2017-0933
30. Chen Y, Williams E, Kirk M. Risk factors for acute respiratory infection in the Australian community. PLoS ONE. (2014) 9:e101440. doi: 10.1371/journal.pone.0101440
31. Nesti MM, Goldbaum M. Infectious diseases and daycare and preschool education. J Pediatr. (2007) 83:299-312. doi: 10.2223/JPED.1649
32. Friedrich F, Ongaratto R, Scotta MC, Veras TN, Stein R, Lumertz MS, et al. Early Impact of social distancing in response to COVID-19 on hospitalizations for acute bronchiolitis in infants in Brazil. Clin Infect Dis. (2020) 72:2071-5. doi: 10.1093/cid/ciaa1458
33. Nolen LD, Seeman S, Bruden D, Klejka J, Desnoyers C, Tiesinga J, et al. Impact of social distancing and travel restrictions on non-coronavirus disease 2019 (Non-COVID-19) respiratory hospital admissions in young children in Rural Alaska. Clin Infect Dis. (2021) 72:2196-8. doi: 10.1093/cid/ciaa1328
34. Shi T, McAllister DA, O'Brien KL, Simoes EAF, Madhi SA, Gessner BD, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet. (2017) 390:946-58. doi: 10.1016/S0140-6736(17)30938-8
35. Pierangeli A, Scagnolari C, Selvaggi C, Monteleone K, Verzaro S, Nenna R, et al. Virological and clinical characterization of respiratory infections in children attending an emergency department during the first autumn-winter circulation of pandemic A (H1N1) 2009 influenza virus. Clin Microbiol Infect. (2012) 18:366-73. doi: 10.1111/j.1469-0691.2011.03590.x
36. Iozzi L, Brambilla I, Foiadelli T, Marseglia GL, Ciprandi G. Paediatric emergency department visits fell by more than 70% during the COVID-19 lockdown in Northern Italy. Acta Paediatr. (2020) 109:2137-8. doi: 10.1111/apa.15458
37. Manzoni P, Militello MA, Fiorica L, Cappiello AR, Manzionna M. Impact of COVID-19 epidemics in paediatric morbidity and utilisation of Hospital Paediatric Services in Italy. Acta Paediatr. (2021) 110:1369-70. doi: 10.1111/apa.15435
38. Kuitunen I, Artama M, Mäkelä L, Backman K, Heiskanen-Kosma T, Renko M. Effect of social distancing due to the COVID-19 pandemic on the incidence of viral respiratory tract infections in children in Finland during early 2020. Pediatr Infect Dis J. (2020) 39:e423-7. doi: 10.1097/INF.0000000000002845
39. Ziebuhr J. Coronaviren. Medizinische Mikrobiologie und Infektiologie. (2016) 2016:479–82. doi: 10.1007/978-3-662-48678-8_58
40. Olsen SJ, Azziz-Baumgartner E, Budd AP, Brammer L, Sullivan S, Pineda RF, et al. Decreased influenza activity during the COVID-19 pandemic - United States, Australia, Chile, and South Africa, 2020. MMWR Morb Mortal Wkly Rep. (2020) 69:1305-9. doi: 10.15585/mmwr.mm6937a6
41. Kim D, Quinn J, Pinsky B, Shah NH, Brown I. Rates of co-infection between SARS-CoV-2 and other respiratory pathogens. JAMA. (2020) 323:2085-6. doi: 10.1001/jama.2020.6266
42. Hong H, Wang Y, Chung HT, Chen CJ. Clinical characteristics of novel coronavirus disease 2019 (COVID-19) in newborns, infants and children. Pediatr Neonatol. (2020) 61:131-2. doi: 10.1016/j.pedneo.2020.03.001
43. Byington CL, Ampofo K, Stockmann C, Adler FR, Herbener A, Miller T, et al. Community surveillance of respiratory viruses among families in the Utah better identification of germs-longitudinal viral epidemiology (BIG-LoVE) study. Clin Infect Dis. (2015) 61:1217-24. doi: 10.1093/cid/civ486
44. Blaschke AJ, Allison MA, Meyers L, Rogatcheva M, Heyrend C, Mallin B, et al. Non-invasive sample collection for respiratory virus testing by multiplex PCR. J Clin Virol. (2011) 52:210-4. doi: 10.1016/j.jcv.2011.07.015
Keywords: SARS-CoV-2, Covid-19 mitigating measures, respiratory pathogens, multiplex PCR, pediatrics
Citation: Diesner-Treiber SC, Voitl P, Voitl JJM, Langer K, Kuzio U, Riepl A, Patel P, Mühl-Riegler A and Mühl B (2021) Respiratory Infections in Children During a Covid-19 Pandemic Winter. Front. Pediatr. 9:740785. doi: 10.3389/fped.2021.740785
Received: 13 July 2021; Accepted: 21 September 2021;
Published: 18 October 2021.
Edited by:
Christoph Aebi, University Children's Hospital Bern, SwitzerlandReviewed by:
Lisa Kottanattu, Ente Ospedaliero Cantonale (EOC), SwitzerlandCopyright © 2021 Diesner-Treiber, Voitl, Voitl, Langer, Kuzio, Riepl, Patel, Mühl-Riegler and Mühl. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Susanne C. Diesner-Treiber, c3VzYW5uZS5kaWVzbmVyLXRyZWliZXJAa2luZGVyYXJ6dC5hdA==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.