
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 01 October 2021
Sec. Obstetric and Pediatric Pharmacology
Volume 9 - 2021 | https://doi.org/10.3389/fped.2021.719803
 Xiaoyan Mao1,2
Xiaoyan Mao1,2 Runxiu Yin1
Runxiu Yin1 Gaoyuan Sun3
Gaoyuan Sun3 Yan Zhou1
Yan Zhou1 Chunhui Yang1
Chunhui Yang1 Chunlian Fang1
Chunlian Fang1 Yuhong Wu1
Yuhong Wu1 Tingting Cui1
Tingting Cui1 Li Liu1
Li Liu1 Jiaxin Gan3
Jiaxin Gan3 Xin Tian1*
Xin Tian1*Background: 6-Mercaptopurine (6-MP) is the cornerstone of current antileukemia regimen and contributes greatly to improve the survival of pediatric acute lymphoblastic leukemia (ALL) patients. However, 6-MP dose-related toxicities limit its application. TPMT, NUDT15, and ITPA are pharmacogenetic markers predicting 6-MP-related toxicities, but their genetic polymorphisms differ from those of ethnic populations. In Yunnan province, a multiethnic region of China, we had no genetic data to predict 6-MP toxicities. In this study, we evaluated the most common variants involved in 6-MP metabolism—TPMT*3C (rs1142345), NUDT15 c.415C>T (rs116855232), and ITPA c.94C>A (rs1127354) variants—in our cohort of pediatric ALL patients.
Methods: A total of 149 pediatric ALL patients in the Affiliated Children's Hospital of Kunming Medical University (Yunnan Children's Medical Center) from 2017 to 2019 were enrolled in this retrospective study. We assessed the TPMT*3C (rs1142345), NUDT15 c.415C>T (rs116855232), and ITPA c.94C>A (rs1127354) frequencies and evaluated association between genotypes and 6-MP toxicities, 6-MP dose, and event-free survival (EFS) in these ALL patients.
Results: The allele frequencies of TPMT*3C (rs1142345), NUDT15 c.415C>T (rs116855232), and ITPA c.94C>A (rs1127354) were 1.34%, 14.43%, and 18.79%, respectively. Only NUDT15 c.415C>T (rs116855232) was strongly associated with 6-MP toxicity and 6-MP tolerable dose. NUDT15 c.415C>T was related to leukopenia, p = 0.008, OR = 2.743 (95% CI: 1.305–5.768). The T allele was significantly correlated with 6-MP tolerable dose, dose of NUDT15 c.415C>T wild genotype CC 39.80 ± 1.32 mg/m2, heterozygotes CT 35.20 ± 2.29 mg/m2, and homozygotes TT 18.95 ± 3.95 mg/m2. 6-MP tolerable dose between CC and TT had a significant difference, p = 0.009. Between CC and CT, and CT and TT, they had no significant difference. EFS showed no significant difference among NUDT15 c.415C>T genotypes.
Conclusion: NUDT15 c.415C>T (rs116855232) was an optimal predictor for 6-MP toxicity and tolerable dose in pediatric ALL patients from Yunnan province, a multiethnic region in China, and would play an important role in precise therapy for ALL.
Acute lymphoblastic leukemia (ALL) is the most common pediatric malignant cancer. Chemotherapy remains the major treatment, including induction, consolidation, and maintenance, with overall survival rate of 80–90% (1–3). 6-Mercaptopurine (6-MP), one of purine antimetabolites, is the cornerstone of current antileukemia regimen. Especially in maintenance therapy, 6-MP and methotrexate (MTX) are primary components and play important roles in long-term remission (4). In a clinic setting, 6-MP has severe toxicities including myelosuppression, hepatotoxicity, gastrointestinal distress, and alopecia. In particular, myelosuppression is complicated with severe infection, leading to drug reduction or withdrawal, even treatment interruption, and patient death (5).
Thiopurine methyltransferase (TPMT) is the first described enzyme linked to 6-MP intolerance, and its decreased enzyme activity is related to increased drug toxicities (6). Three TPMT genetic polymorphisms (*2, *3A, and *3C) account for 95% low enzyme activity (7), but TPMT genetic polymorphisms cannot explain all 6-MP intolerance. In fact, the frequency of TPMT genetic polymorphism differs among ethnic groups: high in European population but low in Asian population (8). In China, total frequency of variant TPMT alleles is 2.91%, and the most common genetic polymorphism is *3C, differing between ethnic groups (9).
Nucleoside diphosphate-linked moiety X-type motif 15 (NUDT15) is a newly found important enzyme associated with 6-MP metabolism. In 2014, Yang et al. first identified the relationship between NUDT15 genetic polymorphism and myelosuppression caused by thioprine drugs (10). They reported NUDT15 c.415 C>T (rs116855232) linked to thiopurine-induced leukopenia in patients with inflammatory bowel disease (IBD), and the variant frequency of NUDT15 is much higher than that of TPMT in Korean population. The updated guideline from Clinical Pharmacogenetics Implementation Consortium Guideline for Thiopurine Dosing Based on TPMT and NUDT15 Genotypes included nine NUDT15 single-nucleotide polymorphisms (SNPs) (8), and the most frequent variant is NUDT15 *3, rs116855232 (c.415C>T; p.R139C). NUDT15 genetic polymorphisms are variable across different ethnic populations. In contrast to TPMT gene, NUDT15 variation is rarely found in European and African populations as compared with Asian population (11). In China, the frequency of variant NUDT15 is higher than that of TPMT, and NUDT15 polymorphisms are responsible for 6-MP intolerance (12, 13).
Inosine triphosphate (ITPA) is also related to thioprine metabolism. In 2004, Mariaki et al. reported the variant of ITPA c.94C>A inducing decreased enzyme activity, thus causing azathioprine intolerance. It first confirmed the association between ITPA genetic variant and thioprine drug intolerance (14). ITPA c.94C>A is one of the most frequent variants decreasing enzyme activity, and variant frequency also differs between ethnic groups. Data from the National Institutes of Health (NIH) show the frequency of ITPA c.94C>A in Asia population of 18.5%, European population of 7.2%, and African population of 5.3%. In a clinic setting, the relationship between ITPA genetic polymorphisms and thioprine toxicity has not been unified, especially in different ethnic populations (15–17).
TPMT, NUDT15, and ITPA genetic polymorphisms associate with thioprine toxicity; and their frequencies of variants differ among ethnic populations. In Asia, China has 56 ethnic groups, and Yunnan province is a multiethnic region with 25 ethnic groups. The frequencies of TPMT, NUDT15, and ITPA genetic polymorphisms for pediatric ALL patients in this area have not been reported. The aim of this study is to measure the frequencies of the most common variants involved in 6-MP metabolism—TPMT*3C (rs1142345), NUDT15 c.415C>T (rs116855232), and ITPA c.94C>A (rs1127354)—in children with ALL from Yunnan province and to indicate whether these genetic variants could predict 6-MP toxicity and tolerable dose during ALL maintenance therapy.
This study was conducted in accordance with the Declaration of Helsinki guidelines and was approved by ethics committee of a children's hospital affiliated to Kunming Medical University (No. 2020-03-200-k01). Written informed consent was obtained from parents of ALL patients before this study.
Children with ALL hospitalized in the Affiliated Children's Hospital of Kunming Medical University (Yunnan Children's Medical Center) from 2017 to 2019 were enrolled in this retrospective study. They received CCLG-ALL-2015 therapy, had finished at least 6 months' maintenance therapy, and were followed up until 2021 with complete material.
The maintenance phase consisted of daily oral 6-MP (50 mg/m2/day), weekly oral MTX (20 mg/m2/time), monthly intravenous vincristine (VCR; 1.5 mg/m2/time, <2 mg/time), and monthly oral dexamethasone (DEX; 6 mg/m2/day, 1–5 days). Blood count was performed once or twice a week at the beginning of maintenance treatment and then at a 2-week interval to maintain white blood cell (WBC) count of 2.0–3.0 × 109/L. Leukopenia was defined as WBC count <2.0 × 109/L. Liver function was performed every month, and hepatotoxicity was defined as alanine aminotransferase (ALT) or aspartate aminotransferase (AST) level >5-fold of normal.
DNA was extracted from 200 μl of EDTA-treated peripheral blood by paramagnetic particle method according to the instruction of nucleic acid extraction and purification kit (Huaxia, Beijing, China). TPMT*3C (rs1142345), NUDT15 c.415C>T (rs116855232), and ITPA c.94C>A (rs1127354) were genotyped by fluorescence in situ hybridization using thioprine SNP locus genotype kit (Huaxia, Beijing, China). All analyses were performed in the laboratory of the Affiliated Children's Hospital of Kunming Medical University (Yunnan Children's Medical Center).
According to type of data, patient's characteristics were analyzed by Pearson's chi-square test, Fisher's exact test, or the Mann–Whitney U-test. Odds ratios and 95% confidence intervals were determined using logistic regression analysis. Receiver operating characteristic (ROC) curves were obtained to plot the sensitivity and specificity for genotypes to predict the development of leucopenia. Event-free survival (EFS) was calculated by the Kaplan–Meier method, and differences were compared using the log-rank test. All genotype frequencies were computed and tested for the Hardy–Weinberg equilibrium. All the tests were two-tailed, and probability values <0.05 were considered statistically significant. The analyses were performed using the SPSS (version 24.0), and the figures were performed by GraphPad Prism software (version 7.0).
A total of 149 pediatric ALL patients were enrolled in this study, including 115 ethnic Han and 34 ethnic minorities. Baseline characteristics of these patients, including the specific ethnic minorities and variant frequencies of TPMT, NUDT15, and ITPA, are shown in Table 1.
For each minority, the number of patients was too little, with most minority with only one patient, so we only compare the frequency of gene variant in this region and not between ethnicities. The frequency of TPMT*3C in this region is 2.68% (4/149), and the allele frequency is 1.34%. The frequency of NUDT15 c.415C>T in this region is 26.84% (40/149), and the allele frequency is 14.43%. The frequency of ITPA c.94C>A in this region is 32.89% (49/149), and the allele frequency is 18.79%. The frequencies of these genotypes and alleles did not deviate from the Hardy–Weinberg equilibrium (p > 0.05).
Allele frequency = (frequency of heterozygotes + frequency of homozygotes × 2)/(Total sample numbers × 2) × 100%.
During maintenance therapy, leukopenia happened at 1 week after 6-MP treatment (1, 1–12 weeks), while hepatotoxicity usually at 1.5 months later (1.5 and 1–18 months). To our surprise, only the variant of NUDT15 c.415C>T was related to leukopenia, p = 0.008, OR = 2.743 (95% CI: 1.305–5.768) (Table 2). The area under the curve (AUC) of NUDT15 c.415C>T predicting leukopenia is 0.600 (Figure 1). The variants of TPMT*3C and ITPA c.94C>A were not related to leukopenia. All of the three genetic variants had no relationship with hepatotoxicity (Table 2).
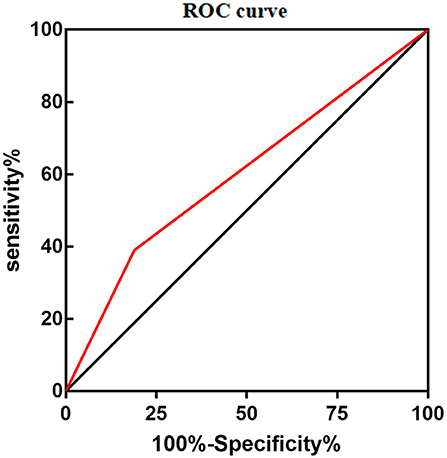
Figure 1. Area under the curve (AUC) of NUDT15 c.415C > T predicting 6-MP related leucopenia was 0.600.
Considering NUDT15 c.415C>T associated with leucopenia caused by 6-MP, we further found evidence that it also caused 6-MP tolerable dose decrease, p = 0.021, OR = 2.514 (95% CI: 1.132–5.583), and the AUC of NUDT15 c.415C>T predicting 6-MP tolerable dose decrease was 0.598 (Figure 2). In 149 patients with ALL, 109 patients had wild NUDT15 c.415C>T (CC), 37 patients had heterozygotes NUDT15 c.415C>T (CT), and three patients had homozygotes NUDT15 c.415C>T (TT). In order to evaluate effect of NUDT15 c.415C>T on 6-MP tolerable dose, we further compared 6-MP tolerable doses for each genotype separately. To maintain WBC count of 2.0–3.0 × 109/L, 6-MP tolerable dose differed among different genotypes of NUDT15 c.415C>T, CC with 39.80 ± 1.32 mg/m2, CT with 35.20 ± 2.29 mg/m2, and TT with 18.95 ± 3.95 mg/m2. 6-MP tolerable dose between CC and TT had a significant difference, p = 0.009. While in the comparison between CC and CT, and CT and TT, they had no significant difference (Figure 3). Although variants of NUDT15 c.415C>T may decrease 6-MP tolerable dose in maintenance therapy, EFS shows no difference between them (Figure 4).
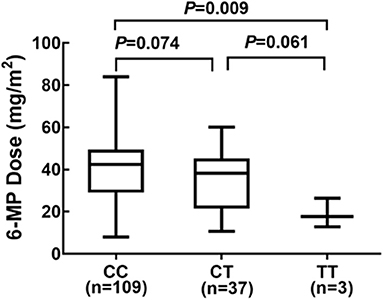
Figure 3. Genotype of NUDT15 c.415C > T and 6-MP dose at maintenance period 109 patients with CC, and 6-MP dose 39.80 ± 1.32 mg/m2; 37 patients with CT and 6-MP dose 35.20 ± 2.29 mg/m2; 3 patients with TT and 6-MP dose 18.95 ± 3.95 mg/m2.
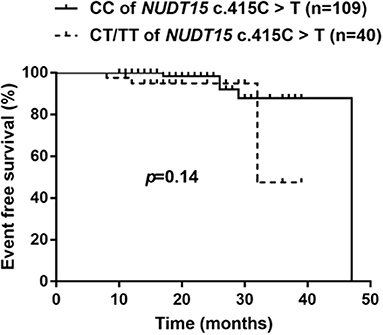
Figure 4. Kaplan–Meier's plots of EFS for NUDT15 c.415C > T variants (CC and CT/TT), P = 0.14. In total 149 patients, followed 18 (8–47) months, NUDT15 c.415C > T variants had no effect on long-time survival for these ALL patients in this region.
In this study, there were 10 patients with combined NUDT15 c.415C>T (CT) and ITPA c.94C>A (CA) variants. We further evaluated the mixed effects of both NUDT15 and ITPA variants on 6-MP tolerable dose. Twenty-seven patients had single heterozygotes NUDT15 c.415C>T (CT), with 6-MP tolerable dose 33.56 ± 2.70 mg/m2. Thirty-two patients had single heterozygotes ITPA c.94C>A (CA), with 6-MP tolerable dose 37.25 ± 2.80 mg/m2. Ten patients had combined heterozygotes NUDT15 c.415C>T (CT) and ITPA c.94C>A (CA), with 6-MP tolerable dose 39.62 ± 4.24 mg/m2. The 6-MP tolerable dose among these three groups had no significant difference (Figure 5).
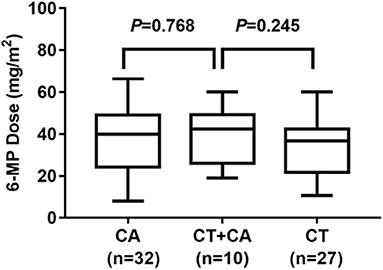
Figure 5. 6-MP dose for patients with ITPA c.94C>A (CA) and NUDT15 c.415C>T (CT) 27 patients with CT, and 6-MP dose 33.56 ± 2.70 mg/m2; 32 patients with CA, and 6-MP dose 37.25 ± 2.80 mg/m2; 10 patients with combined CT and CA, with 6-MP dose 39.62 ± 4.24 mg/m2. 6-MP dose had no significant difference between these three groups.
With the development of next-generation sequencing techniques, genomic data have been incorporated into ALL risk classification, treatment, and prognostic system and greatly improved survival of pediatric ALL patients (18). Clinicians could reduce drug resistance, avoid adverse events, and improve overall survival based on pharmacogenomics. TPMT is the best example of application of pharmacogenomics to clinical practice. In 2019, the Clinical Pharmacogenetics Implementation Consortium updated 6-MP dose in a clinic setting based on TPMT genotype. However, differences in genotype distribution and frequency of TPMT alleles among different ethnic populations limit its predictive value. In this study, we focused on TPMT*3C, the most frequent TPMT genotype in China (19–21). In 149 pediatric ALL, only four TPMT*3C heterozygotes were found with allele frequency of 1.34%, a little lower than that in Chinese children with ALL (2.9%) reported by Zhou et al. (12), but higher than in Korean (0.6%) (22) and Indonesian pediatric ALL (0.95%) patients (23). Meanwhile, no significant association between TPMT*3C and 6-MP toxicities was found in our study, which was consistent with previous reports (23–25) but not compatible with another report from Chinese pediatric ALL by Zhou et al. (12). This discrepancy may be attributed to various factors, such as patient characteristics. In Zhou et al. study, the frequency of TPMT*3C is relatively high, so additional studies on a larger scale are warranted to make it clear in the future. In this study, the four TPMT*3C heterozygotes included three ethnic Han and one ethnic minority (ethnic Hani); the allele frequency of ethnic Han (1.3%) was compatible with that of other reports at 1.0% (19).
Considering the low frequency of TPMT polymorphism but comparable rates of 6-MP myelotoxicity in Asians, researchers have noted NUDT15 polymorphisms as an important determinant factor for 6-MP myelotoxicity, especially NUDT15 c.415C>T (rs116855232) (10, 26). Our study first described the frequency of NUDT15 c.415C>T variant and association with 6-MP in pediatric ALL patients in this Yunnan province, a multiethnic region. In 149 pediatric ALL patients, 37 heterozygotes (CT) and three homozygotes (TT) were found with an allele frequency of 14.43%, consistent with that of another Chinese ALL group (15.7%) and other East Asia populations (9.8–16.8%) (Table 3), much higher than in European population (0.2–0.4%) (Table 3). In terms of 6-MP toxicity, we found a strong association between NUDT15 c.415C>T with elevated risk of 6-MP-associated leucopenia (p = 0.008), but no association with hepatotoxicity (p = 0.37). In a clinic setting, 6-MP dose would be decreased for leucopenia or hepatotoxicity. In a further study, we found statistical significance NUDT15 c.415C>T caused by 6-MP tolerable dose decrease (p = 0.021). In particular, the T allele was significantly correlated with 6-MP tolerable dose decreased. Compared with NUDT15 c.415C>T wild (CC) and heterozygotes (CT), homozygote genotype (TT) was more intolerant to 6-MP, and 6-MP tolerable dose was decreased to 18.95 ± 3.95 mg/m2 (CC 39.80 ± 1.32 mg/m2, CT 35.20 ± 2.29 mg/m2). Considering different treatment protocols with different 6-MP doses in a maintenance period, most studies used 6-MP dose intensity. In this study, we used 6-MP tolerable dose to illustrate the treatment strength directly and found it different from other reports, such as Taiwan Chinese (TT 9.4 mg/m2, CT 30.7 mg/m2, and CC 44.1 mg/m2) (27) and other Chinese groups (TT 30.14 mg/m2, CT 41.92 mg/m2, and CC 47.12 mg/m2) (12). There may be various factors attributed to it, such as patient characteristics, clinician decision, complexity of pharmacogenomics, and medical level in different regions. These suggested that the precision therapy relied on not one factor but multiple factors; thus, more studies on a larger scale with different 6-MP regimens are needed to fully elucidate 6-MP individualized dose adjustment.
A retrospective study showed reducing 6-MP starting dose based on TPMT polymorphisms reduced second malignant neoplasm (SMN) risk but increased relapse risk (30). In this study, we evaluated the risk of NUDT15 c.415C>T on long-term survival of patients with ALL. Our results indicated that there was no significant difference of EFS between CC and CT/TT patients, which was consistent with report by Tanaka (26). But as Tanaka suggested, EFS probabilities appeared lower in patients with CT and TT genotypes. A larger-scale study with longer time followed up is needed.
ITPA is related to thioprine metabolism and also a potential predictor of 6-MP toxicity, but the clinical relevance of ITPA polymorphisms in 6-MP intolerance is still controversial (12, 31). In our study, frequency of ITPA c.94C>A was higher than that of NUDT15 c.415C>T; 42 heterozygotes (CA) and seven homozygotes (AA) were found with allele frequency of 18.79%. We found no significant association with 6-MP toxicities, which was consistent with previous report in other Chinese pediatric ALL patients (12). Meanwhile, there were 10 patients with combined NUDT15 c.415C>T (CT) and ITPA c.94C>A (CA) variants, but we did not discover any statistically significant differences of 6-MP dose between the NUDT15 carriers, or ITPA carriers, or both carriers (Figure 5). In the future, a study of larger populations and additional other variants is needed to evaluate its effects on 6-MP.
Our study also had some limitations. First, we only focused on one variant of each gene and missed some other potential genetic polymorphisms. Second, our study had a small sample size, especially the small number of ethnic minorities, resulting in a low power to detect differences. Third, we only discovered a significant relationship of NUDT15 c.415C>T with neutropenia and did not elaborate the underlying mechanism on how this variant influenced the toxicity of 6-MP. Valerie et al. found that NUDT15 c.415C>T did not affect enzymatic activity but negatively influenced protein stability, which thus lost supportive intramolecular bonds and caused rapid NUDT15 proteasomal degradation, which finally induced DNA damage checkpoint and cancer cell death by 6-thioguanine (32). Moreover, 6-MP tolerable dose was adjusted by many factors, such as 6-MP toxicity, the combined use of MTX, and patient compliance, but we only focused on 6-MP toxicity. Further studies will focus on these questions to achieve 6-MP precise treatment in a clinic setting.
We first elucidated the variant frequencies of TPMT*3C (rs1142345), NUDT15 c.415C>T (rs116855232), and ITPA c.94C>A (rs1127354) in pediatric ALL from Yunnan province, a multiethnic region in China. Among these genetic variants, we found that NUDT15 c.415C>T (rs116855232) could predict 6-MP toxicity and intolerance during ALL maintenance therapy, but the 6-MP tolerable dose in our data was not so consistent with that of other reports of Asian population. We also found no statistical difference of EFS in ALL patients with NUDT15 variants. Further clinical studies in larger scale with more genetic polymorphisms are required to develop better and precise treatment strategies in ALL patients.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by Ethics Committee of children's hospital affiliated to Kunming Medical University. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
XT designed the study, analyzed the patient data, and concluded the value of the research. XM performed the research and was a major contributor in writing the manuscript. RY, GS, YZ, and CY collected the patient data. CF, YW, TC, LL, and JG did the follow-up work. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (No. 81760032) to XT.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Thanks all the patients, families, and physicians for their participation in our study.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2021.719803/full#supplementary-material
1. Shen S, Cai J, Chen J, Xue H, Pan C, Gao Y, et al. Long-term results of the risk-stratified treatment of childhood acute lymphoblastic leukemia in China. Hematol Oncol. (2018) 36:679–88. doi: 10.1002/hon.2541
2. Inaba H, Greaves M, Mullighan CG. Acute lymphoblastic leukaemia. Lancet. (2013) 381:1943–55. doi: 10.1016/S0140-6736(12)62187-4
3. Pui CH, Mullighan CG, Evans WE, Relling MV. Pediatric acute lymphoblastic leukemia: where are we going and how do we get there? Blood. (2012) 120:1165–74. doi: 10.1182/blood-2012-05-378943
4. Schmiegelow K, Heyman M, Kristinsson J, Mogensen U, Rosthøj S, Vettenranta K, et al. Oral methotrexate/6-mercaptopurine may be superior to a multidrug LSA2L2 Maintenance therapy for higher risk childhood acute lymphoblastic leukemia: results from the NOPHO ALL-92 study. J Pediatric Hematol Oncol. (2009) 31:385–92. doi: 10.1097/MPH.0b013e3181a6e171
5. Schmiegelow K, Nielsen SN, Frandsen TL, Nersting J. Mercaptopurine/methotrexate maintenance therapy of childhood acute lymphoblastic leukemia: clinical facts and fiction. J Pediatr Hematol Oncol. (2014) 36:503–17. doi: 10.1097/MPH.0000000000000206
6. Lennard L, Lilleyman J, Van Loon J, Weinshilboum R. Genetic variation in response to 6-mercaptopurine for childhood acute lymphoblastic leukaemia. Lancet. (1990) 336:225–9. doi: 10.1016/0140-6736(90)91745-V
7. Evans W. Pharmacogenetics of thiopurine S-methyltransferase and thiopurine therapy. Therap Drug Monitor. (2004) 26:186–91. doi: 10.1097/00007691-200404000-00018
8. Relling M, Schwab M, Whirl-Carrillo M, Suarez-Kurtz G, Pui C, Stein C, et al. Clinical pharmacogenetics implementation consortium guideline for thiopurine dosing based on TPMT and NUDT15 Genotypes: 2018 update. Clin Pharmacol Therap. (2019) 105:1095–105. doi: 10.1002/cpt.1304
9. Yueping L, Hanqing X, Xiang Y, Qing H, Weiling F. Distribution of the TPMT genotype in the Chinese population. J Clin Lab. (2015) 33:711–4. doi: 10.13602/j.cnki.jcls.2015.09.20
10. Yang S, Hong M, Baek J, Choi H, Zhao W, Jung Y, et al. A common missense variant in NUDT15 confers susceptibility to thiopurine-induced leukopenia. Nat Genet. (2014) 46:1017–20. doi: 10.1038/ng.3060
11. Yang JJ, Landier W, Yang W, Liu C, Hageman L, Cheng C, et al. Inherited NUDT15 variant is a genetic determinant of mercaptopurine intolerance in children with acute lymphoblastic leukemia. J Clin Oncol. (2015) 33:1235–42. doi: 10.1200/JCO.2014.59.4671
12. Zhou H, Li L, Yang P, Yang L, Zheng JE, Zhou Y, et al. Optimal predictor for 6-mercaptopurine intolerance in Chinese children with acute lymphoblastic leukemia: NUDT15, TPMT, or ITPA genetic variants? BMC Cancer. (2018) 18:516. doi: 10.1186/s12885-018-4398-2
13. Huang PW, Tseng YH, Tsai TF. Predictive value of NUDT15 variants on neutropenia among Han Chinese patients with dermatologic diseases: a single-center observational study. Dermatol Ther (Heidelb). (2020) 10:263–71. doi: 10.1007/s13555-020-00360-4
14. Marinaki A, Ansari A, Duley J, Arenas M, Sumi S, Lewis C, et al. Adverse drug reactions to azathioprine therapy are associated with polymorphism in the gene encoding inosine triphosphate pyrophosphatase (ITPase). Pharmacogenetics. (2004) 14:181–7. doi: 10.1097/00008571-200403000-00006
15. Azimi F, Mortazavi Y, Alavi S, Khalili M, Ramazani A. Frequency of ITPA gene polymorphisms in Iranian patients with acute lymphoblastic leukemia and prediction of its myelosuppressive effects. Leuk Res. (2015) 39:1048–54. doi: 10.1016/j.leukres.2015.06.016
16. Citterio-Quentin A, Moulsma M, Gustin MP, Lachaux A, Boulieu R. ITPA activity in children treated by azathioprine: relationship to the occurrence of adverse drug reactions and inflammatory response. Basic Clin Pharmacol Toxicol. (2018) 122:588–95. doi: 10.1111/bcpt.12958
17. Yushan T, Yu L. Relationship between ITPA activity and 6-mercaptopurine toxicity in children with acute lymphoblastic leukemia during maintenance treatment. J Clin Pediatr. (2013) 31:412–6. doi: 10.3969/j.issn.1000-3606.2013.05.004
18. Kato M, Manabe A. Treatment and biology of pediatric acute lymphoblastic leukemia. Pediatr Int. (2018) 60:4–12. doi: 10.1111/ped.13457
19. Zhang JP, Zhou SF, Chen X, Huang M. Determination of intra-ethnic differences in the polymorphisms of thiopurine S-methyltransferase in Chinese. Clin Chim Acta. (2006) 365:337–41. doi: 10.1016/j.cca.2005.09.005
20. Zhang J, Guan Y, Wu J, Jiang W, Huang M. Genetic polymorphism of the thiopurine S-methyltransferase of healthy Han Chinese. Chin J Cancer. (2003) 22:385–8. doi: 10.3321/j.issn:1000-467X.2003.04.011
21. Zhang J, Huang M, Guan Y, Xu A, Wu J. Mutant thiopurine S-methyltransferase alleles among Jing Chinese in Guangxi province. Chin J Med Genet. (2003) 20:303–6. doi: 10.3760/j.issn:1003-9406.2003.04.007
22. Lee JM, Shim YJ, Kim DH, Jung N, Ha JS. The effect of NUDT15, TPMT, APEX1, and ITPA genetic variations on mercaptopurine treatment of pediatric acute lymphoblastic leukemia. Children (Basel). (2021) 8:2224. doi: 10.3390/children8030224
23. Rosdiana DS, Setiabudy R, Andalusia R, Gatot D, Louisa M, Bardosono S, et al. TPMT genetic variability and its association with hematotoxicity in Indonesian children with acute lymphoblastic leukemia in maintenance therapy. Pharmgenomics Pers Med. (2021) 14:199–210. doi: 10.2147/PGPM.S288988
24. Cao Q, Zhu Q, Shang Y, Gao M, Si J. Thiopurine methyltransferase gene polymorphisms in Chinese patients with inflammatory bowel disease. Digestion. (2009) 79:58–63. doi: 10.1159/000205268
25. Takatsu N, Matsui T, Murakami Y, Ishihara H, Hisabe T, Nagahama T, et al. Adverse reactions to azathioprine cannot be predicted by thiopurine S-methyltransferase genotype in Japanese patients with inflammatory bowel disease. J Gastroenterol Hepatol. (2009) 24:1258–64. doi: 10.1111/j.1440-1746.2009.05917.x
26. Tanaka Y, Kato M, Hasegawa D, Urayama K, Nakadate H, Kondoh K, et al. Susceptibility to 6-MP toxicity conferred by a NUDT15 variant in Japanese children with acute lymphoblastic leukaemia. Br J Haematol. (2015) 171:109–15. doi: 10.1111/bjh.13518
27. Liang DC, Yang CP, Liu HC, Jaing TH, Chen SH, Hung IJ, et al. NUDT15 gene polymorphism related to mercaptopurine intolerance in Taiwan Chinese children with acute lymphoblastic leukemia. Pharmacogenomics J. (2016) 16:536–9. doi: 10.1038/tpj.2015.75
28. Buaboonnam J, Sripatanatadasakul P, Treesucon A, Glomglao W, Siraprapapat P, Narkbunnam N, et al. Effect of NUDT15 on incidence of neutropenia in children with acute lymphoblastic leukemia. Pediatr Int. (2019) 61(8):754–758. doi: 10.1111/ped.13905
29. Moradveisi B, Muwakkit S, Zamani F, Ghaderi E, Mohammadi E, Zgheib NK, et al. ITPA, TPMT, and NUDT15 Genetic Polymorphisms Predict 6-Mercaptopurine Toxicity in Middle Eastern Children With Acute Lymphoblastic Leukemia. Front Pharmacol. (2019) 10:916. doi: 10.3389/fphar.2019.00916.eCollection2019
30. Levinsen M, Rotevatn E, Rosthøj S, Nersting J, Abrahamsson J, Appell M, et al. Pharmacogenetically based dosing of thiopurines in childhood acute lymphoblastic leukemia: influence on cure rates and risk of second cancer. Pediatric Blood Cancer. (2014) 61:797–802. doi: 10.1002/pbc.24921
31. Milosevic G, Kotur N, Krstovski N, Lazic J, Zukic B, Stankovic B, et al. Variants in TPMT, ITPA, ABCC4 and ABCB1 genes as predictors of 6-mercaptopurine induced toxicity in children with acute lymphoblastic leukemia. J Med Biochem. (2018) 37:320–7. doi: 10.1515/jomb-2017-0060
Keywords: TPMT, NUDT15, ITPA, 6-mercaptopurine, pediatric patients, acute lymphoblastic leukemia
Citation: Mao X, Yin R, Sun G, Zhou Y, Yang C, Fang C, Wu Y, Cui T, Liu L, Gan J and Tian X (2021) Effects of TPMT, NUDT15, and ITPA Genetic Variants on 6-Mercaptopurine Toxicity for Pediatric Patients With Acute Lymphoblastic Leukemia in Yunnan of China. Front. Pediatr. 9:719803. doi: 10.3389/fped.2021.719803
Received: 03 June 2021; Accepted: 20 August 2021;
Published: 01 October 2021.
Edited by:
Venkata Kashyap Yellepeddi, The University of Utah, United StatesReviewed by:
Evelyne Jacqz-Aigrain, Institut National de la Santé et de la Recherche Médicale (INSERM), FranceCopyright © 2021 Mao, Yin, Sun, Zhou, Yang, Fang, Wu, Cui, Liu, Gan and Tian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin Tian, ODc2MzQ2ODY1QHFxLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.