
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Pediatr., 10 August 2021
Sec. Pediatric Critical Care
Volume 9 - 2021 | https://doi.org/10.3389/fped.2021.719679
This article is part of the Research TopicCritical Complications In Pediatric Oncology and Hematopoietic Cell TransplantView all 31 articles
 Robert B. Lindell1*
Robert B. Lindell1* Michael S. Wolf2
Michael S. Wolf2 Alicia M. Alcamo1
Alicia M. Alcamo1 Michael A. Silverman3
Michael A. Silverman3 Daniel E. Dulek4
Daniel E. Dulek4 William R. Otto3
William R. Otto3 Timothy S. Olson5
Timothy S. Olson5 Carrie L. Kitko6
Carrie L. Kitko6 Paisit Paueksakon7
Paisit Paueksakon7 Kathleen Chiotos1,3
Kathleen Chiotos1,3Disseminated toxoplasmosis is an uncommon but highly lethal cause of hyperferritinemic sepsis after hematopoietic cell transplantation (HCT). We report two cases of disseminated toxoplasmosis from two centers in critically ill adolescents after HCT: a 19-year-old who developed fever and altered mental status on day +19 after HCT and a 20-year-old who developed fever and diarrhea on day +52 after HCT. Both patients developed hyperferritinemia with multiple organ dysfunction syndrome and profound immune dysregulation, which progressed to death despite maximal medical therapies. Because disseminated toxoplasmosis is both treatable and challenging to diagnose, it is imperative that intensivists maintain a high index of suspicion for Toxoplasma gondii infection when managing immunocompromised children, particularly in those with known positive T. gondii serologies.
Toxoplasma gondii is an intracellular protozoan parasite transmitted by meat containing tissue cysts and through fecal–oral transmission that typically causes an asymptomatic or mild self-limited infection (1). While the population prevalence of toxoplasmosis is decreasing, substantial regional heterogeneity in seroprevalence remains (2, 3). Among immunocompromised patients, toxoplasmosis can cause a disseminated primary infection, but most often represents reactivation of a prior infection. Neurologic findings are the most common clinical manifestations (4).
Toxoplasmosis after hematopoietic cell transplantation (HCT) generally occurs within 6 months of transplant (5). Features of toxoplasmosis typically include fever and non-specific signs, including respiratory and neurologic symptoms that overlap with other opportunistic infections (6). Serologic testing for T. gondii can be unreliable in immunocompromised patients; thus, diagnosis is often made by polymerase chain reaction (PCR) testing or microscopic identification of parasites in blood or tissue (7, 8). Pre-transplant serologic testing can identify patients at risk of reactivation, which may prompt earlier diagnostic evaluation. Exposed patients may benefit from PCR-based surveillance for T. gondii (9).
Here, we report two cases of disseminated toxoplasmosis in critically ill adolescents after HCT presenting with hyperferritinemic sepsis and multiple organ dysfunction syndrome (MODS). We review the heterogeneity of their presentation, treatment, and outcome and highlight the importance of prompt diagnosis and treatment of this highly lethal condition. This report is considered not a research on human subjects by both Institutional Review Boards (IRBs); consent was not required by the IRB and not obtained from the decedents.
A 19-year-old male with relapsed Philadelphia chromosome-like B cell acute lymphoblastic leukemia who received a haploidentical stem cell transplant developed fever on post-transplant day +16. Blood culture was positive for Bacillus cereus, which was treated with vancomycin and cefepime.
On day +17, he developed a headache and became somnolent. Computed tomography (CT) scan of the head showed a small left frontal intraparenchymal hemorrhage. Magnetic resonance imaging (MRI) of the brain revealed multiple ring-enhancing lesions consistent with possible septic emboli, shown in Figure 1A. Magnetic resonance angiography did not show evidence of intracranial aneurysm. Transthoracic echocardiogram showed no evidence of intracardiac shunt, valve disease, or vegetation. The patient was broadened to vancomycin and meropenem for treatment of presumed disseminated B. cereus. Due to persistent fever despite bloodstream clearance of B. cereus, MRI with ring-enhancing lesions, and the patient's known immunoglobulin G (IgG) seropositivity for T. gondii, trimethoprim/sulfamethoxazole (TMP/SMX) was started empirically from days +22 to +25. This was discontinued given the negative T. gondii PCR testing from blood, cerebrospinal fluid, and bronchoalveolar lavage (BAL) specimens. Diagnostic workup was negative for Aspergillus, Pneumocystis jirovecii, Cryptococcus, and viral infection.
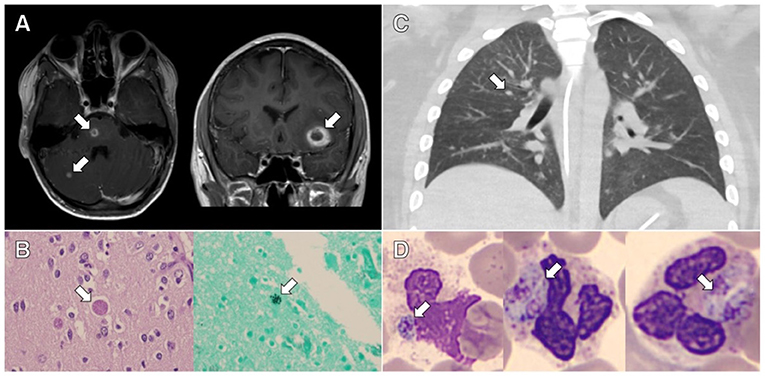
Figure 1. Clinical and pathological evidence of disseminated toxoplasmosis from the cases presented. (A) Axial and coronal contrast-enhanced magnetic resonance imaging of the brain from case 1 showing rim-enhancing lesions. (B) Representative slides stained with hematoxylin and eosin (HE; left image) and Grocott's methenamine silver stain (right image) showing cysts containing Toxoplasma bradyzoites in the brain tissue from case 1. (C) Axial contrast-enhanced chest computed tomography from case 2 showing bilateral centrilobular nodules and ground glass opacities. (D) Representative slides stained with HE showing neutrophils containing Toxoplasma tachyzoites in peripheral blood from case 2.
Given the ongoing fever, development of vomiting, and diarrhea, an endoscopy was performed on day +49. Methylprednisolone was given from day +49 to +52 for empiric management of gastrointestinal graft-vs.-host disease (GVHD), but pathology showed rare apoptotic bodies in the duodenum and colon and did not support a diagnosis of GVHD.
On day +51, the patient developed rapid-onset respiratory failure and progressive somnolence. CT scan of the chest showed diffuse ground glass opacities. He was transferred to the pediatric intensive care unit (PICU), where he developed MODS with severe hypoxemic respiratory failure and septic shock requiring mechanical ventilation and significant vasoactive support. He was treated empirically with gentamicin, meropenem, TMP/SMX, amphotericin, cidofovir, albendazole, and ivermectin, with plan to start pyrimethamine/sulfadiazine when available. PCR testing of repeat BAL fluid identified T. gondii. A splenic lesion was also identified on ultrasound and biopsied, with PCR testing positive for T. gondii. All other bacterial, viral, and fungal studies were negative. TMP/SMX was dose-adjusted for the treatment of disseminated toxoplasmosis.
In addition to antimicrobials, the patient was concurrently treated with plasma exchange and intravenous immunoglobulin for thrombocytopenia-associated multiple organ failure from day +55 to +57. Despite therapy, he developed hyperferritinemia (peak level, 49,000 ng/mL) with concurrent hepatobiliary dysfunction and coagulopathy. The results of his clinical cytokine panel from day +54 are shown in Table 1. He was also treated with anakinra based on a reanalysis of a phase III randomized trial showing a mortality benefit of interleukin-1 receptor antagonist treatment in adult severe sepsis (10). A timeline of his immune dysregulation and associated therapeutics is shown in Figure 2A.
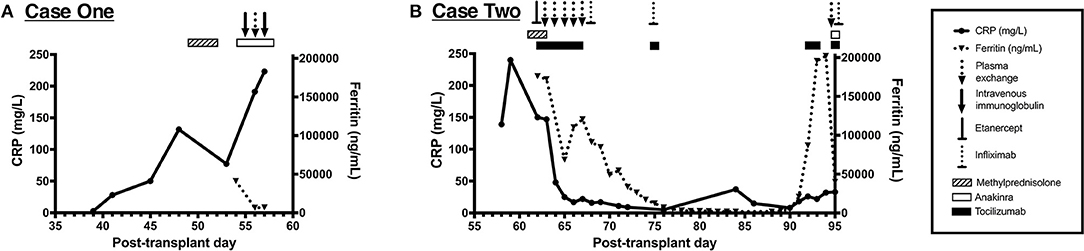
Figure 2. Immune dysregulation and exposure to associated immunomodulatory therapies from the cases presented. (A) Timeline of C-reactive protein (CRP) and ferritin values from case 1 showing progressive immune dysregulation. This patient was exposed to methylprednisolone, anakinra, intravenous immunoglobulin (IVIG), and plasma exchange, as shown along the top of the panel. (B) Timeline of CRP and ferritin values from case 2 showing two distinct periods of immune dysregulation. This patient was exposed to methylprednisolone, tocilizumab, anakinra, etanercept, infliximab, and plasma exchange, as shown along the top of the panel.
Given the patient's declining respiratory status and persistent MODS despite maximal support, his family elected to discontinue life-sustaining therapy on day +58. Autopsy findings were notable for evidence of disseminated toxoplasmosis, as shown in Figure 1B. Numerous inflammatory foci and T. gondii bradyzoites were seen in the myocardium and diaphragm. Neuropathologic evaluation revealed numerous hemorrhagic subcortical infarcts containing granulomatous inflammation and T. gondii bradyzoite cysts.
A 20-year-old male with immune-mediated aplastic anemia refractory to prior immune suppression therapy received an unrelated donor peripheral stem cell transplant. Toxoplasma serologies were not obtained during his pre-transplant evaluation as per institutional practice. His initial transplant course was uncomplicated, and he was discharged home on post-transplant day +21. On day +52, he was readmitted for fever and diarrhea. Due to fever and hypoxemia, a CT scan of the chest was obtained on day +55 and showed bilateral centrilobular nodules with right-sided predominance, as shown in Figure 1C. His infectious workup included multiple BALs, blood, and urine cultures and evaluation for Pneumocystis jirovecii and viral etiologies, which were all negative. He was maintained on broad-spectrum antibiotics and antifungal medications given the persistent fever despite negative cultures. Due to progressive hypoxemic respiratory failure, he was transferred to the PICU on day +61, where he required intubation and mechanical ventilation. Given the refractory hypoxemic respiratory failure, he was cannulated to venous–venous extracorporeal membrane oxygenation (ECMO) within 24 h of PICU transfer. His ECMO course was complicated by shock, hyperferritinemia, and acute kidney injury requiring renal replacement therapy.
Multimodal therapy for his immune dysregulation included pulse dose methylprednisolone (2 g/day) followed by a prolonged methylprednisolone course, plasma exchange, tocilizumab for IL-6 blockade, and etanercept for tumor necrosis factor alpha blockade, which was later transitioned to infliximab. A timeline of the immunomodulatory therapies is shown in Figure 2B. With improvement in his hyperinflammatory state, he was decannulated from ECMO on day +73.
On day +90, he had recurrence of fever without other changes in exam in the setting of a methylprednisolone taper; after a multidisciplinary discussion, tocilizumab, infliximab, and pulse dose methylprednisolone were administered for the management of immune dysregulation. The results of his clinical cytokine panel from day +64 to +92 are shown in Table 1. Despite these interventions, he had worsening hyperferritinemia by day +94 (peak level, 202,000 ng/mL), so anakinra was administered and plasma exchange was repeated. Despite immunomodulation, he continued to have refractory MODS with severe hypoxemic respiratory failure and catecholamine-refractory shock. At the request of his family, resuscitative efforts were discontinued on day +95. On the day of his death, T. gondii tachyzoites were noted on his peripheral blood smear (shown in Figure 1D), which were subsequently confirmed to be T. gondii by PCR and immunostaining. Postmortem analyses of the BAL specimens from day +60 and +65 were also positive for T. gondii by PCR. Postmortem T. gondii serologic testing was performed on a pre-transplant blood specimen and was positive for IgG.
Toxoplasmosis is a rare but severe complication after HCT that generally occurs within the first 6 months of transplant and is associated with substantial morbidity and mortality (3–5). These two cases highlight the potential for T. gondii reactivation to result in hyperferritinemic sepsis and MODS after HCT (11) and the lack of “classic” signs and symptoms of T. gondii in critically ill patients after HCT. Management of the immunocompromised patient with hyperferritinemic sepsis requires a highly collaborative approach, incorporating evaluation from critical care medicine, infectious disease, and stem cell transplant. Reaching a diagnosis is challenging but critical in these patients as some potential etiologies, including T. gondii, may be successfully treated if the diagnosis is made early in the clinical presentation. Several previous reports focused on pediatric HCT patients have characterized the association between toxoplasmosis and poor clinical outcomes, as summarized in Table 2.
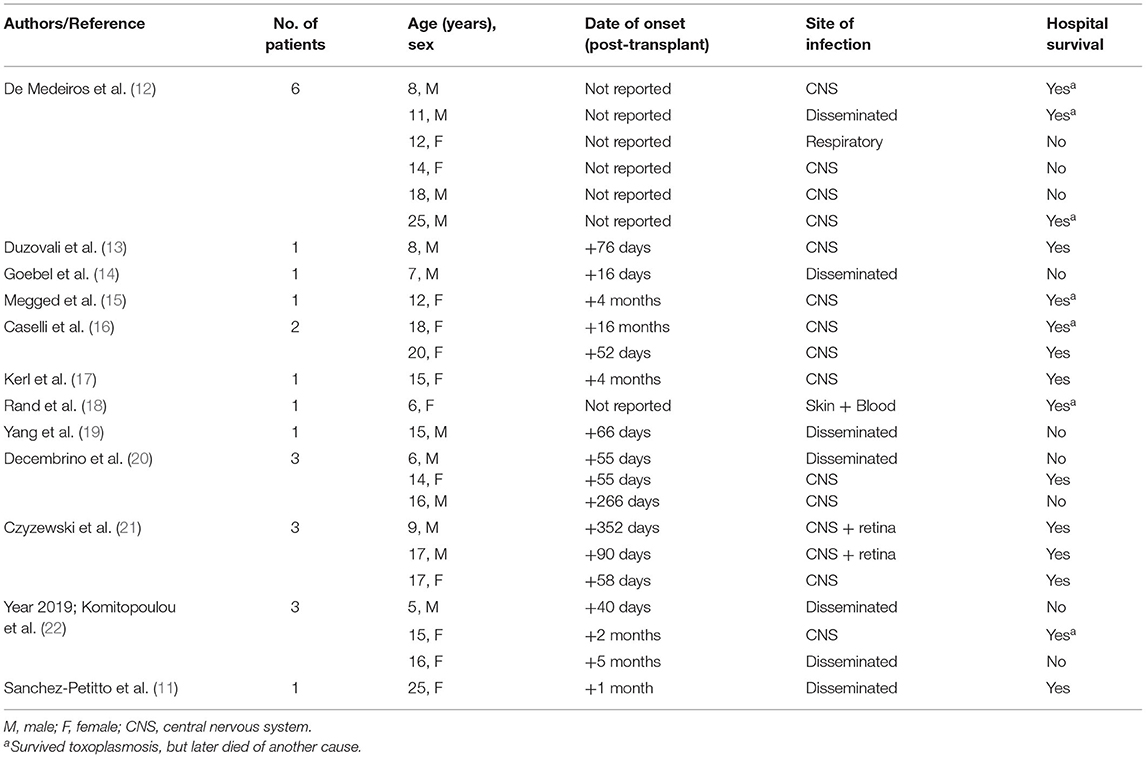
Table 2. Previously reported cases of toxoplasmosis in adolescent and young adult patients (age ≤ 25 years) after HCT (since 2000).
In addition to progressive MODS, both patients developed significant immune dysregulation during their clinical course, as summarized in Figure 2. Immune dysregulation is a feature of certain forms of critical illness and is highly associated with mortality after sepsis (23), trauma (24), and cardiopulmonary bypass (25). Immunocompromised patients, including allogeneic HCT recipients, have an increased risk of immune dysregulation and mortality in the setting of critical illness (26, 27). In the case of toxoplasmosis, hyperferritinemia is thought to be precipitated by several T. gondii virulence factors that influence macrophage activation (28, 29). This activation also occurs in the microglia (30) and may explain the predominance of the neurologic signs and symptoms commonly seen in T. gondii reactivation.
Successful treatment of severe toxoplasmosis due to reactivation in immunocompromised patients is challenging, with limited data to support the recommended antibiotic regimens. The combination of pyrimethamine, sulfadiazine, and leucovorin is the recommended regimen for the treatment of toxoplasmosis reactivation in an immunocompromised host (31, 32). TMP/SMX is an alternative therapy if pyrimethamine is unavailable or not tolerated. Both regimens combine a sulfonamide antibiotic with a dihydrofolate reductase inhibitor. Two small, prospective randomized controlled trials comparing these combination agents have shown similar efficacy and a favorable side effect profile associated with the use of TMP/SMX (33, 34). Both pyrimethamine/sulfadiazine and TMP/SMX achieve adequate tissue exposure, including within the central nervous system (CNS) (35–37), and are treatments of choice for disease involving the CNS (32).
The diagnostic and management challenges inherent to critical illness after HCT also highlight the growing role for physicians with clinical expertise in pediatric onco-critical care. Immunocompromised children represent a small subset of patients with critical illness, but account for a substantial fraction of ICU mortality due to sepsis (27). As our mechanistic understanding of immune dysregulation in these high-risk patients continues to grow, we will have increasing opportunities to study the role of novel targeted therapeutics in modifying complex host–pathogen interactions. In the meantime, because toxoplasmosis is an uncommon but treatable cause of MODS, it is imperative that clinicians maintain a high index of suspicion for T. gondii infection when managing immunocompromised children with progressive organ failure despite maximal medical therapies, particularly those with serologic evidence of prior T. gondii exposure.
The original contributions generated for this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
RL, MW, AA, MS, DD, WO, TO, CK, and KC provided patient care and contributed to the conception of this case report. PP prepared the neuropathology slides for this report. RL wrote the first draft. All authors contributed to manuscript revision, read, and approved the submitted version.
Financial support was provided by the Endowed Chair, Department of Anesthesiology and Critical Care, Children's Hospital of Philadelphia and The University of Pennsylvania Perelman School of Medicine. RL is also supported by the Thrasher Research Fund. KC is supported by the Agency for Healthcare Research and Quality K12-HS026393. No other authors report conflicts of interest or relevant funding.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: from animals to humans. Int J Parasitol. (2000) 30:1217–58. doi: 10.1016/S0020-7519(00)00124-7
2. Jones JL, Kruszon-Moran D, Sanders-Lewis K, Wilson M. Toxoplasma gondii infection in the United States, 1999 2004, decline from the prior decade. Am J Trop Med Hyg. (2007) 77:405–10. doi: 10.4269/ajtmh.2007.77.405
3. Wang ZD, Liu HH, Ma ZX, Ma HY, Li ZY, Yang ZB, et al. Toxoplasma gondii infection in immunocompromised patients: a systematic review and meta-analysis. Front Microbiol. (2017) 8:389. doi: 10.3389/fmicb.2017.00389
4. Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet. (2004) 363:1965–76. doi: 10.1016/S0140-6736(04)16412-X
5. Gajurel K, Dhakal R, Montoya JG. Toxoplasma prophylaxis in haematopoietic cell transplant recipients: a review of the literature and recommendations. Curr Opin Infect Dis. (2015) 28:283–92. doi: 10.1097/QCO.0000000000000169
6. Schmidt M, Sonneville R, Schnell D, Bige N, Hamidfar R, Mongardon N, et al. Clinical features and outcomes in patients with disseminated toxoplasmosis admitted to intensive care: a multicenter study. Clin Infect Dis. (2013) 57:1535–41. doi: 10.1093/cid/cit557
7. Montoya JG. Laboratory diagnosis of Toxoplasma gondii infection and toxoplasmosis. J Infect Dis. (2002) 185(Suppl. 1):S73–82. doi: 10.1086/338827
8. Benson CA Kaplan JE Masur H Pau A Holmes KK Cdc National Institutes of H . Treating opportunistic infections among HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association/Infectious Diseases Society of America. MMWR Recomm Rep. (2004) 53:1–112.
9. Robert-Gangneux F, Sterkers Y, Yera H, Accoceberry I, Menotti J, Cassaing S, et al. Molecular diagnosis of toxoplasmosis in immunocompromised patients: a 3-year multicenter retrospective study. J Clin Microbiol. (2015) 53:1677–84. doi: 10.1128/JCM.03282-14
10. Shakoory B, Carcillo JA, Chatham WW, Amdur RL, Zhao H, Dinarello CA, et al. Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome: reanalysis of a prior phase III trial. Crit Care Med. (2016) 44:275–81. doi: 10.1097/CCM.0000000000001402
11. Sanchez-Petitto G, Holtzman NG, Bukhari A, Brown M, Morales MK, Koka M, et al. Toxoplasma-induced hemophagocytic lymphohistiocytosis after haploidentical allogeneic stem cell transplantation. Transpl Infect Dis. (2020) 22:e13242. doi: 10.1111/tid.13242
12. De Medeiros BC, De Medeiros CR, Werner B, Loddo G, Pasquini R, Bleggi-Torres LF. Disseminated toxoplasmosis after bone marrow transplantation: report of 9 cases. Transpl Infect Dis. (2001) 3:24–8. doi: 10.1034/j.1399-3062.2001.003001024.x
13. Duzovali O, Choroszy MS, Chan KW. Hyponatremia as the presenting feature of cerebral toxoplasmosis. Bone Marrow Transplant. (2005) 35:1221–2. doi: 10.1038/sj.bmt.1704989
14. Goebel WS, Conway JH, Faught P, Vakili ST, Haut PR. Disseminated toxoplasmosis resulting in graft failure in a cord blood stem cell transplant recipient. Pediatr Blood Cancer. (2007) 48:222–6. doi: 10.1002/pbc.20537
15. Megged O, Shalit I, Yaniv I, Stein J, Fisher S, Levy I. Breakthrough cerebral toxoplasmosis in a patient receiving atovaquone prophylaxis after a hematopoietic stem cell transplantation. Pediatr Transplant. (2008) 12:902–5. doi: 10.1111/j.1399-3046.2007.00891.x
16. Caselli D, Andreoli E, Paolicchi O, Savelli S, Guidi S, Pecile P, et al. Acute encephalopathy in the immune-compromised child: never forget toxoplasmosis. J Pediatr Hematol Oncol. (2012) 34:383–6. doi: 10.1097/MPH.0b013e318257a15c
17. Kerl K, Ehlert K, Brentrup A, Schiborr M, Keyvani K, Becker K, et al. Cerebral toxoplasmosis in an adolescent post allogeneic hematopoietic stem cell transplantation: successful outcome by antiprotozoal chemotherapy and CD4+ T-lymphocyte recovery. Transpl Infect Dis. (2015) 17:119–24. doi: 10.1111/tid.12344
18. Rand AJ, Buck AB, Love PB, Prose NS, Selim MA. Cutaneous acquired toxoplasmosis in a child: a case report and review of the literature. Am J Dermatopathol. (2015) 37:305–10. doi: 10.1097/DAD.0000000000000072
19. Yang WL, Chen JS, Lee CN, Lee JY. Toxoplasmosis presenting with a subtle maculopapular eruption in a child post hematopoietic stem cell transplantation. J Formos Med Assoc. (2016) 115:887–8. doi: 10.1016/j.jfma.2016.06.001
20. Decembrino N, Comelli A, Genco F, Vitullo A, Recupero S, Zecca M, et al. Toxoplasmosis disease in paediatric hematopoietic stem cell transplantation: do not forget it still exists. Bone Marrow Transplant. (2017) 52:1326–9. doi: 10.1038/bmt.2017.117
21. Czyzewski K, Fraczkiewicz J, Salamonowicz M, Pieczonka A, Zajac-Spychala O, Zaucha-Prazmo A, et al. Low seroprevalence and low incidence of infection with Toxoplasma gondii (Nicolle et Manceaux, 1908) in pediatric hematopoietic cell transplantation donors and recipients: polish nationwide study. Folia Parasitol (Praha). (2019) 66:2019.019. doi: 10.14411/fp.2019.019
22. Komitopoulou A, Goussetis E, Oikonomopoulou C, Paisiou A, Kaisari K, Ioannidou E, et al. Toxoplasma gondii: how fatal is it in pediatric allogeneic bone marrow transplantation setting? Transpl Infect Dis. (2020) 22:e13226. doi: 10.1111/tid.13226
23. Hotchkiss RS, Coopersmith CM, Mcdunn JE, Ferguson TA. The sepsis seesaw: tilting toward immunosuppression. Nat Med. (2009) 15:496–7. doi: 10.1038/nm0509-496
24. Lord JM, Midwinter MJ, Chen YF, Belli A, Brohi K, Kovacs EJ, et al. The systemic immune response to trauma: an overview of pathophysiology and treatment. Lancet. (2014) 384:1455–65. doi: 10.1016/S0140-6736(14)60687-5
25. Hadley JS, Wang JE, Michaels LC, Dempsey CM, Foster SJ, Thiemermann C, et al. Alterations in inflammatory capacity and TLR expression on monocytes and neutrophils after cardiopulmonary bypass. Shock. (2007) 27:466–73. doi: 10.1097/01.shk.0000245033.69977.c5
26. Lindell RB, Gertz SJ, Rowan CM, Mcarthur J, Beske F, Plunkett A, et al. High levels of morbidity and mortality among pediatric hematopoietic cell transplant recipients with severe sepsis: insights from the sepsis prevalence, outcomes, and therapies international point prevalence study. Pediatr Crit Care Med. (2017) 18:1114–25. doi: 10.1097/PCC.0000000000001338
27. Lindell RB, Nishisaki A, Weiss SL, Traynor DM, Fitzgerald JC. Risk of mortality in immunocompromised children with severe sepsis and septic shock. Crit Care Med. (2020) 48:1026–33. doi: 10.1097/CCM.0000000000004329
28. Jensen KD, Wang Y, Wojno ED, Shastri AJ, Hu K, Cornel L, et al. Toxoplasma polymorphic effectors determine macrophage polarization and intestinal inflammation. Cell Host Microbe. (2011) 9:472–83. doi: 10.1016/j.chom.2011.04.015
29. Almeida F, Sardinha-Silva A, Da Silva TA, Pessoni AM, Pinzan CF, Alegre-Maller AC, et al. Toxoplasma gondii chitinase induces macrophage activation. PLoS ONE. (2015) 10:e0144507. doi: 10.1371/journal.pone.0144507
30. Li Y, Severance EG, Viscidi RP, Yolken RH, Xiao J. Persistent Toxoplasma infection of the brain induced neurodegeneration associated with activation of complement and microglia. Infect Immun. (2019) 87:e00139–19. doi: 10.1128/IAI.00139-19
31. AAP Committee on Infectious Diseases. Red Book: 2021 Report of the Committee on Infectious Diseases. 32nd Ed. Elk Grove Village, IL: American Academy of Pediatrics (2021).
32. Panel on Opportunistic Infections in HIV-Exposed HIV-Infected Children Guidelines for the Prevention Treatment of Opportunistic Infections in HIV-Exposed and HIV-Infected Children. Department of Health and Human Services. Available online at: https://clinicalinfo.hiv.gov/sites/default/files/inline-files/oi_guidelines_pediatrics.pdf (accessed June 23, 2021).
33. Torre D, Casari S, Speranza F, Donisi A, Gregis G, Poggio A, et al. Randomized trial of trimethoprim-sulfamethoxazole versus pyrimethamine-sulfadiazine for therapy of toxoplasmic encephalitis in patients with AIDS. Italian Collaborative Study Group. Antimicrob Agents Chemother. (1998) 42:1346–9. doi: 10.1128/AAC.42.6.1346
34. Soheilian M, Sadoughi MM, Ghajarnia M, Dehghan MH, Yazdani S, Behboudi H, et al. Prospective randomized trial of trimethoprim/sulfamethoxazole versus pyrimethamine and sulfadiazine in the treatment of ocular toxoplasmosis. Ophthalmology. (2005) 112:1876–82. doi: 10.1016/j.ophtha.2005.05.025
35. Weiss LM, Harris C, Berger M, Tanowitz HB, Wittner M. Pyrimethamine concentrations in serum and cerebrospinal fluid during treatment of acute Toxoplasma encephalitis in patients with AIDS. J Infect Dis. (1988) 157:580–3. doi: 10.1093/infdis/157.3.580
36. Mcleod R, Mack D, Foss R, Boyer K, Withers S, Levin S, et al. Levels of pyrimethamine in sera and cerebrospinal and ventricular fluids from infants treated for congenital toxoplasmosis. Toxoplasmosis Study Group. Antimicrob Agents Chemother. (1992) 36:1040–8. doi: 10.1128/AAC.36.5.1040
Keywords: hematopoietic cell transplant, toxoplasmosis, immune dysregulation, multiple organ dysfunction syndrome, hyperferritinemia
Citation: Lindell RB, Wolf MS, Alcamo AM, Silverman MA, Dulek DE, Otto WR, Olson TS, Kitko CL, Paueksakon P and Chiotos K (2021) Case Report: Immune Dysregulation Due to Toxoplasma gondii Reactivation After Allogeneic Hematopoietic Cell Transplant. Front. Pediatr. 9:719679. doi: 10.3389/fped.2021.719679
Received: 02 June 2021; Accepted: 08 July 2021;
Published: 10 August 2021.
Edited by:
Kris Michael Mahadeo, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Matteo Di Nardo, Bambino Gesù Children Hospital (IRCCS), ItalyCopyright © 2021 Lindell, Wolf, Alcamo, Silverman, Dulek, Otto, Olson, Kitko, Paueksakon and Chiotos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Robert B. Lindell, bGluZGVsbHJAY2hvcC5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.