- 1Department of Pediatrics, The Fourth Affiliated Hospital of China Medical University, Shenyang, China
- 2Department of Pediatrics, Shengjing Hospital of China Medical University, Shenyang, China
Background: Neuroblastoma (NB) is the most common pediatric extracranial solid neoplasm after leukemia. Intracranial metastases (IM) rarely occur in patients with NB. The present study aimed to review the clinical characteristics of NB patients from a single center presenting with IM.
Methods: Two hundred children (aged 3–91 months) with NB admitted to the Fourth Affiliated Hospital of China Medical University between January 2009 and December 2015 were enrolled, and their clinical characteristics were recorded. The patients were divided into two groups based on the presence of IM. Their clinical characteristics, including demographics, clinical features, and laboratory and imaging studies, were retrospectively analyzed.
Results: IM occurred in 22 of 200 (11%) neuroblastoma patients, with a median age of 42.5 months (range, 3–91 months), with a male-to-female ratio of 1.4:1. Seven patients had IM at the initial diagnosis. Among the 15 children who did not have IM at initial presentation, the median interval from presentation to the diagnosis of IM was 17.3 months (range, 1–55 months). Compared with the control group, NB patients with IM tended to be asymptomatic at the time of NB diagnosis, which was made incidentally during routine physical examination (5 of 22, 22.7%, p < 0.05). In addition, this group had more primary intra-abdominal sites (18 of 22, 81.8%, p < 0.001) and worse prognosis (5 of 22, 22.7%, p < 0.05).
Conclusions: NB patients with IM have insidious onset in the early stage and a lower survival rate, especially patients with primary intra-abdominal lesions. Regular neurological monitoring could improve the rate of early diagnosis and prognosis of NB children with IM. Familiarity with the characteristic findings of NB with IM is necessary to avoid misdiagnosis and initiate necessary interventions.
Introduction
Neuroblastoma (NB) is the most common extracranial solid malignancy that arises from neural crest cells in children (1, 2), accounting for 8–10% of all pediatric tumors and for ~15% of cancer-related deaths in children (3). The etiology of neuroblastoma remains obscure, and in most cases, there is no family history of the disease (4). NB is marked by its heterogeneous clinical behavior (5) and is classified into three risk groups (low, intermediate, and high) depending on age, extent of disease, histology, and cytogenetic abnormalities (6). The tumor may spontaneously regress or progress and metastasize with resistance to treatment (5). The incidence of NB is strongly age-dependent, with a median age of onset of 24 months and a peak age of 18 months (7). It is by far the most prevalent cancer diagnosed before the age of 1 year.
NB is an embryonal tumor of the peripheral sympathetic nervous system that typically originates from the adrenal glands, although it can develop along the paravertebral sympathetic chain (6), or anywhere along the sympathetic ganglia chain from the neck to the groin. It shows an array of incompletely understood and diverse clinical and biological characteristics and behaviors, related to the variety of locations of neuroblastic tumors and the differing degrees of histopathological differentiation (8).
Distant metastases, which frequently involve the bone, bone marrow, and liver, are present in up to 70% of children with NB at the time of diagnosis and confer a poor prognosis (9–11). Hematogenous spread to the head and neck is also commonly seen both at presentation and upon recurrence and manifests most often as osseous metastases involving the calvarium, orbit, or skull base (9). However, intracranial metastases (IM), including metastases to the brain parenchyma, leptomeninges, or cerebrospinal fluid, are rare and represent a serious complication indicating poor prognosis (12–15). Neuroblastoma with distant metastases is considered a stage 4 tumor according to the International Neuroblastoma Staging System (INSS) and has a poor prognosis (1, 16, 17).
IM in neuroblastoma patients are rare, and although some case series have focused on some specific topics such as treatment (10, 18), the role of neurosurgery (11), and imaging findings (19–21), scarce information exists on the neurologic manifestations of the disease. In this retrospective study, we aimed to describe the clinical findings of NB patients with IM in our institution. Our objective was to gain updated insights into the phenomenon of central nervous system (CNS) involvement by NB, including incidence rates, clinical features, risk factors, and survival rate. We also hoped to define the natural history of NB metastasis to the brain which may eventually lead to a better understanding of the pathophysiology of this rare disease.
Materials and Methods
Patient Population and Data Collection
From January 2009 to December 2015, 200 pediatric patients who were diagnosed with NB at the Fourth Affiliated Hospital of China Medical University were included and reviewed in our study. Patients with IM were defined as the IM group, while those without IM were defined as the control group. Staging was performed according to INSS. Pre-treatment risk classification was performed according to the International Neuroblastoma Risk Group system.
All NB patients were regularly followed up, and CNS imaging was deemed necessary when the patients presented with neurological symptoms, or at each half-year time point during the follow-up period. IM was confirmed by CNS imaging and was defined as all lesions located in the brain parenchyma and meninges.
The sex, age of onset, symptoms at onset, primary site of NB, time interval from symptom onset to diagnosis, metastatic sites, grade of differentiation, risk grade, N-Myc status, primary histopathology, differentiation degree, and outcome of all enrolled patients were collected and analyzed. Other clinical data, including initial neuroimaging at diagnosis, primary tumor size, histology, initial treatment, time interval from diagnosis to brain metastasis, location of IM, number of brain lesions, maximum lesion diameter, presence of edema, contrast enhancement of the lesions, type of treatment upon diagnosis of IM, neurologic outcome after treatment, concomitant extra-CNS disease at the time of IM, and survival of NB patients with IM, were reviewed. When available, autopsy reports were reviewed to determine whether the children had IM at death.
Statistics
Statistical analyses were performed using SPSS version 22.0. Continuous data with a normal distribution were expressed as the mean ± SD and further analyzed using an independent sample t-test. Continuous data with abnormal distribution were analyzed using the Wilcoxon Mann–Whitney U test. Categorical data were expressed as absolute numbers and percentages and analyzed using the chi-square test or Fisher's exact test. Data on sex, age at onset, symptoms of onset, primary site of NB, other metastatic sites, stage, risk grade, N-Myc status, primary histopathology, differentiation degree, and outcome were analyzed using multiple logistic regression analysis. Statistical significance was set at p < 0.05.
Results
Demographic and Clinical Characteristics of NB Patients
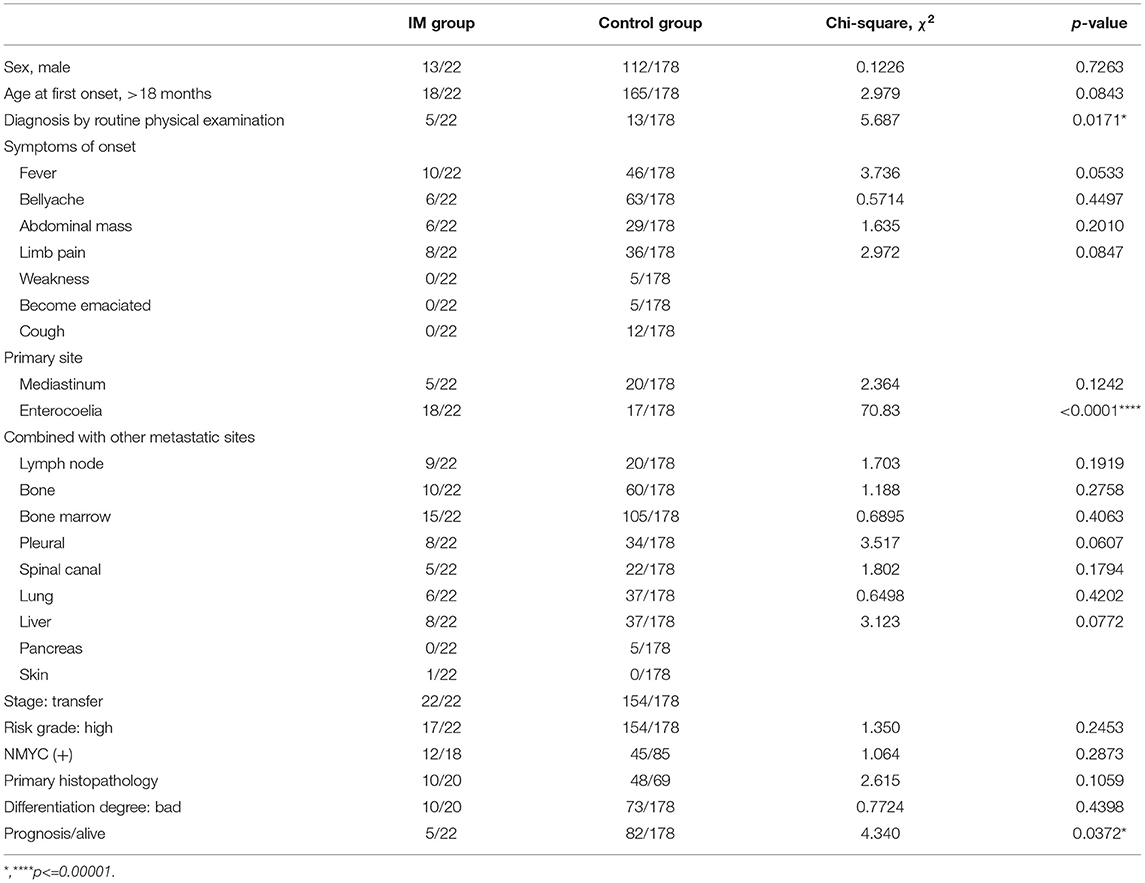
This study included 200 patients with NB. NB was more common in boys (n = 125) than in girls (n = 75), with a male-to-female sex ratio of 1.7:1. Of the 200 children almost all (91.5%, 183/200) were older than 18 months. According to the neuroimaging results, the 200 patients were divided into the IM group (n = 22) and control group (n = 178). The demographic and clinical characteristics of the two groups are shown in Table 1. There were no statistically significant differences between the two groups regarding sex, age at first onset, symptoms of onset (fever, abdominal pain, abdominal mass, limb pain, weakness, emaciation, cough), primary site of mediastinum, combined with other metastatic sites, stage, risk grade, N-Myc status, primary histopathology, and degree of differentiation. Compared with the control group, the NB patients with IM tended to be asymptomatic at the time of NB diagnosis which was made incidentally during routine physical examination (5 of 22, 22.7% p < 0.05). The primary site of enterocoelia was significantly more common in the IM group than in the control group (18 of 22, 81.8% vs. 17 of 178, 9.6%, p < 0.001). Survival (5 of 22, 22.7% p < 0.05) in the IM group was significantly worse than that in the control group (82 of 178, 46.1%).
Natural History of NB Patients With IM
Table 2 provides information on the natural history of the 22 patients with IM. The median age at diagnosis of NB in the IM group (range 3–91 months) was 42.5 months. The median time interval from symptom onset to NB diagnosis was 4.2 months (range 0.5–29 months). Among the 15 patients who did not have IM at initial presentation, the median time interval from NB diagnosis to IM was 17.3 months (range, 1–55 months). Ten patients (10 of 22, 45.5%) had solitary brain parenchymal metastasis. The N-Myc status of these tumors was positive in three patients and not available in 19 patients.
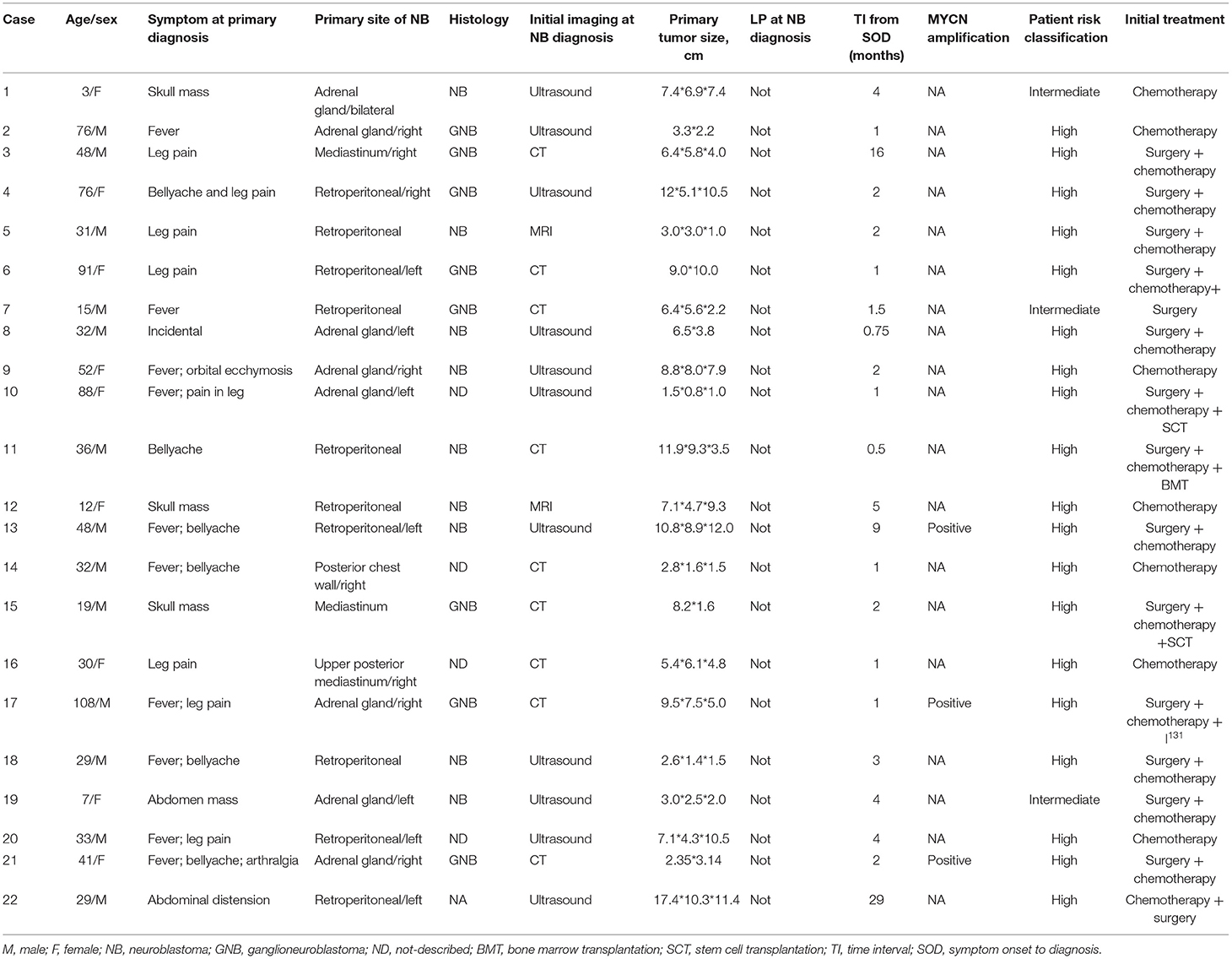
Table 2. Summary of clinical characteristics of patients at the initial diagnosis of neuroblastoma in the IM group.
Imaging at the Time of Initial NB Diagnosis
Non-contrast computed tomography scan was performed in nine patients, ultrasound scanning was the initial imaging modality of choice for 11 patients, and in two patients only contrast-enhanced magnetic resonance imaging was performed. The primary site of NB was the retroperitoneum (n = 10), adrenal glands (n = 8), and mediastinum (n = 3).
Clinical Characteristics of NB Patients With IM
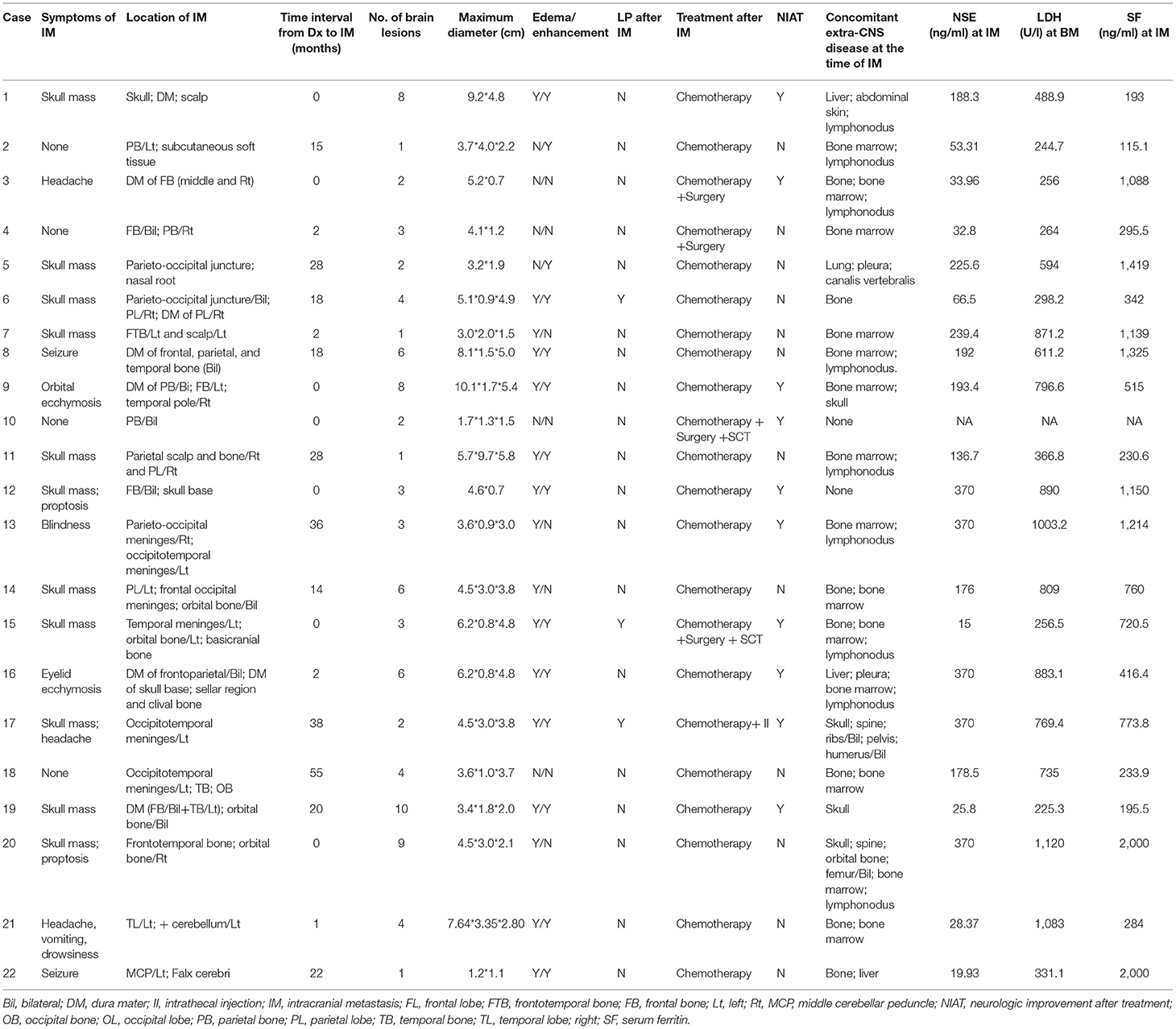
The most common initial signs and symptoms of brain metastases were skull mass in 11 patients, headaches in two patients, seizures in two patients, blindness, eyelid ecchymosis, and orbital ecchymosis in one patient each (Table 3). There were no symptoms of spinal cord involvement. Four of our patients were asymptomatic at the time of the IM diagnosis.
Brain parenchyma and cerebellum metastases were found in 8 of 200 (4%) and 2 of 200 (1%) NB patients, respectively. Meningeal metastasis was found in 12 of the 200 patients (6%).
The number of brain lesions ranged from 1 to 10. In all patients with IM, brain contrast-enhanced magnetic resonance imaging was performed (Figures 1, 2). Most patients (18 of 22, 82%) had more than one mass lesion. Four patients had only one mass lesion. Most of the intraparenchymal lesions were supratentorial (19 of 22, 86%), and three lesions were infratentorial (3 of 22, 14%). The lesions in the brain parenchyma showed varying degrees of vasogenic edema in 16 patients (16 out of 22, 73%). Contrast enhancement was observed in 14 patients (14 of 22, 64%). Leptomeningeal lesions demonstrated enhancement in eight patients.
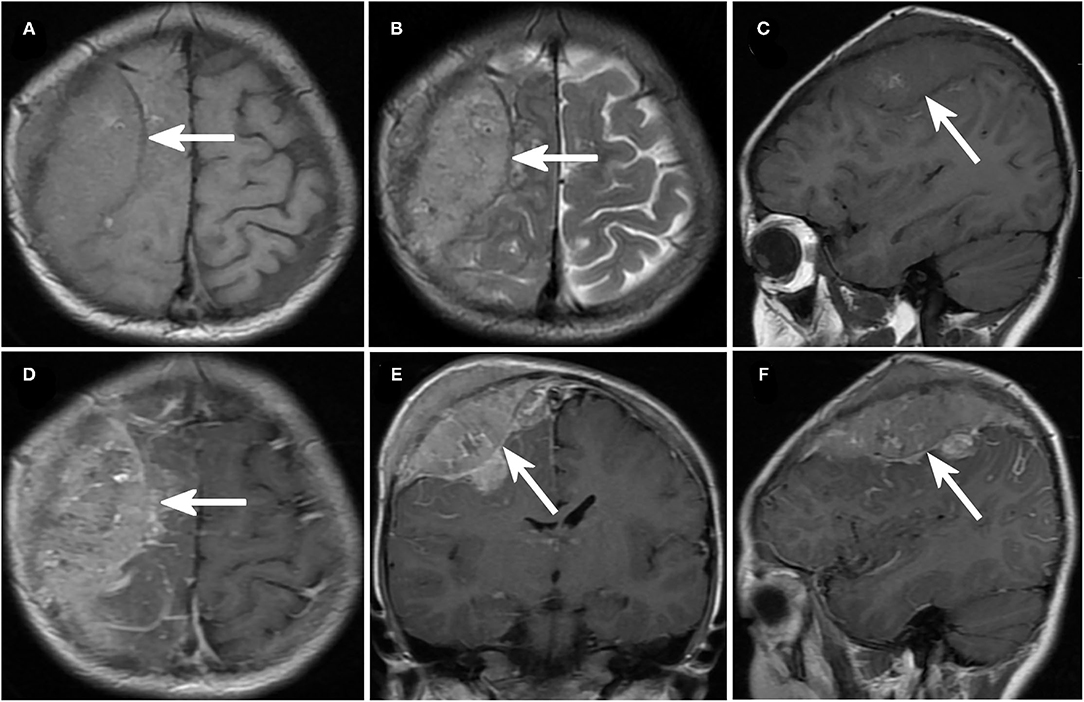
Figure 1. Imaging of neuroblastoma with intracranial metastases. (A–C) Axial (A,B) and sagittal (C) magnetic resonance imaging (MRI) in case No. 11 demonstrated a recurrent mass lesion in the right occipital lobe. (D–F) Cranial enhanced MRI of case No. 11, axial (C), coronal (D), and sagittal (F) images.
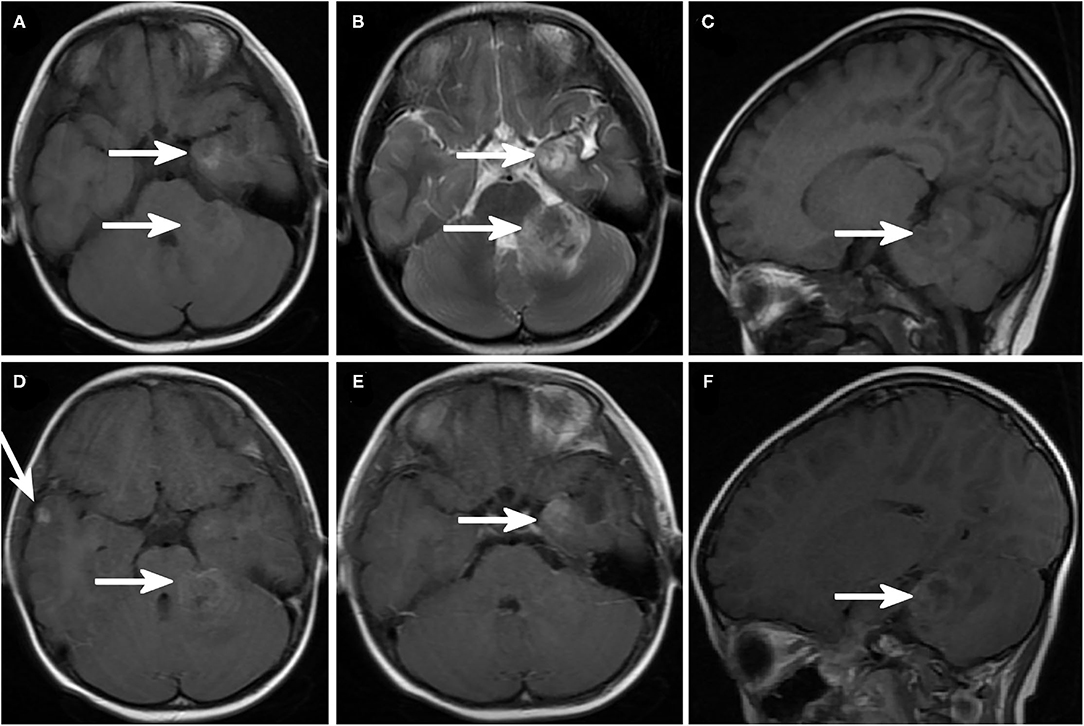
Figure 2. Brain magnetic resonance imaging (MRI) showing intracranial metastatic lesions. (A) Axial T1-weighted image and (B) axial T2-weighted image demonstrated a lesion in the left temporal lobe and left cerebellar hemisphere with prominent cerebral edema around the lesion. Sagittal T1-weighted image (C) showed a cerebellar lesion. (D–F) Enhanced MRI sagittal T1-weighted image and (D) right temporal lobe and left cerebellar hemisphere lesions. (E) Enhanced sagittal MRI image showed left cerebellar hemisphere lesions.
The treatment modalities for the 22 NB children with IM included chemotherapy in 17, chemotherapy plus surgery in 2, chemotherapy, surgery plus stem cell transplantation in 2, and chemotherapy plus intrathecal injection in 1. Twenty patients had concomitant extra-CNS disease at the time of brain metastases; the other common sites of extra-CNS involvement were bone (n = 9 patients) and bone marrow (n = 19 patients).
Seven patients had IM at initial presentation. Concurrent non-brain distant metastasis was present in five of these seven cases, including metastasis to the liver (n = 1), abdominal skin (n = 1), lymph node (n = 4), bone (n = 3), bone marrow (n = 4), and femur (n = 1).
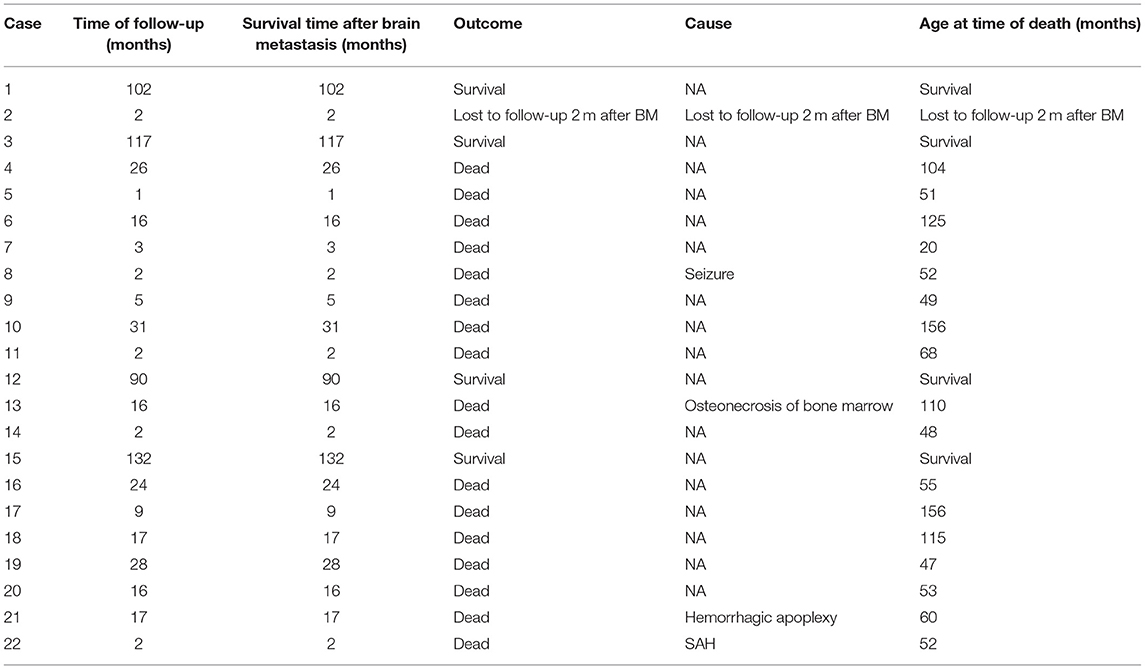
By December 31, 2019, the median follow-up period had been 12.2 months (range, 1–132 months) (Table 3). During this follow-up, 17 patients had died despite treatment, four patients were alive, and one patient was lost to follow-up (Table 4). The median survival time was 12.2 months after diagnosis of IM, with a 1-year survival rate of 59.0% and 2-year survival rate of 36.4%. Of the 17 patients who died despite treatment, patient 8 died due to untreated status convulsion. Patients 13, 21, and 22 died due to multiple-organ failure, which is caused by severe infection or multiple-organ bleeding caused by bone marrow failure, cerebral herniation caused by hemorrhage of the metastatic tumor, and subarachnoid hemorrhage, respectively.
Discussion
The objective of the present retrospective study was to describe the relevant clinical findings in NB patients with IM at our institution. We tried to gain updated insights into the phenomenon of CNS involvement by NB, including incidence rates and treatment options. To our knowledge, the present study is the largest case series to date on IM in NB patients, based on a comprehensive review of clinical features. The present study may define the natural history of IM in NB and eventually lead to a better understanding of the associated clinical characteristics with the goal of contributing to the existing knowledge of this rare disease.
NB is one of the most common extracranial solid tumors in children to metastasize to the CNS among pediatric malignant non-epithelial tumors, with an incidence of 1.7–11.7% (17, 22–24). The 22 cases reported in the present study were diagnosed with stage IV NB. Twenty-two brain metastases in a cohort of 200 neuroblastoma patients corresponds to a rate of 11%, which is close to the reported incidence in some large studies (10, 25) and higher than that reported in other studies (12–15, 17, 26). This incidence suggests that ~10% of patients will develop parenchymal brain involvement, which may justify routine CNS surveillance of this patient population. It is possible that if detected earlier, before the onset of symptoms, NB with parenchymal brain metastasis may be easier to treat.
Certain lines of evidence suggest that IM in neuroblastoma patients are rare and usually occur during disease progression (12, 13, 15, 17, 19), which was corroborated by the present study. These findings and other studies (4, 13, 27) substantiate previous indications that patients with IM have a dismal prognosis. The incidence of NB with spinal cord involvement in previous reports was 1.14–4.09% (10, 28). There was one patient (1/22, 4.5%) with involvement of the spinal cord in our study, which is consistent with previous studies.
The imaging features of IM in NB varied from single brain lesions to diffuse meningeal involvement, with a mean number of four brain lesions (range, 1–10), which is consistent with previous studies (10, 12, 27, 29, 30). Most commonly IM lesions are located in the frontal and temporal lobes (8, 31). In the 22 cases reported here, IM mainly presented as meningeal enhancement (12/22), followed by parietal lobe lesions (5/22).
It is disheartening that, by the time IM is clinically evident, it is usually too late. No consistent or effective treatment strategy exists for such patients (17, 32). The major traditional therapeutic strategies for NB are surgery, chemotherapy, radiotherapy, and autologous peripheral blood stem cell transplantation (33). Immunotherapy is an emerging potential treatment option (34, 35). In our study, chemotherapy was the mainstay treatment for NB with distant metastasis (n = 16). Two patients received more aggressive treatment with surgery plus chemotherapy, which may result in a longer median survival in a limited number of patients, although death from subsequent systemic recurrence seems inevitable.
In summary, IM remains an uncommon (10%) complication in children with NB. This study was based on a retrospective review of 22 cases with IM and aimed to describe the relevant clinical features of these patients in our institution. Despite being a single institution study, our report represents the largest series of children with IM described to date. Our findings lend support to the idea of routine CNS screening of children with NB to diagnose IM at earlier stages. Whether earlier diagnosis corresponds to improve survival should be the goal of prospective randomized studies.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Medical Ethics Committee of the Fourth Affiliated Hospital of China Medical University. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author Contributions
Material preparation, data collection, and analysis were performed by YL (1st author), LH, JZ, and YL (4th author). The first draft of the manuscript was written by YL (1st author). All authors contributed to the conception and design of the study, commented on previous versions of the manuscript, and read and approved the final manuscript.
Funding
Shenyang Science and Technology provided financial support in the form of Shenyang Science and Technology Funding (F13-221-9-43). The sponsor had no role in the design or conduct of this study.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet. (2007) 369:2106–20. doi: 10.1016/S0140-6736(07)60983-0
2. Pastor ER, Mousa SA. Current management of neuroblastoma and future direction. Crit Rev Oncol Hematol. (2019) 138:38–43. doi: 10.1016/j.critrevonc.2019.03.013
3. Pai Panandiker AS, Beltran C, Billups CA, McGregor LM, Furman WL, Davidoff AM. Intensity modulated radiation therapy provides excellent local control in high-risk abdominal neuroblastoma. Pediatr Blood Cancer. (2013) 60:761–5. doi: 10.1002/pbc.24350
4. Maris JM, Kyemba SM, Rebbeck TR, White PS, Sulman EP, Jensen SJ, et al. Familial predisposition to neuroblastoma does not map to chromosome band 1p36. Cancer Res. (1996) 56:3421–5.
5. Kholodenko IV, Kalinovsky DV, Doronin II, Deyev SM, Kholodenko RV. Neuroblastoma origin and therapeutic targets for immunotherapy. J Immunol Res. (2018) 2018:7394268. doi: 10.1155/2018/7394268
6. Herd F, Basta NO, McNally R, Tweddle DA. A systematic review of re-induction chemotherapy for children with relapsed high-risk neuroblastoma. Eur J Cancer. (2019) 111:50–8. doi: 10.1016/j.ejca.2018.12.032
7. Cotterill SJ, Parker L, Malcolm AJ, Reid M, More L, Craft AW. Incidence and survival for cancer in children and young adults in the North of England, 1968–1995: a report from the Northern Region Young Persons' Malignant Disease Registry. Br J Cancer. (2000) 83:397–403. doi: 10.1054/bjoc.2000.1313
8. Kose D, Karabagli P, Yavas G, Karabagli H, Koksal Y. Intracranial metastasis of neuroblastoma: in two different areas at the same time. Childs Nerv Syst. (2013) 29:1799–802. doi: 10.1007/s00381-013-2203-8
9. Chirathivat S, Post MJ. CT demonstration of dural metastases in neuroblastoma. J Comput Assist Tomogr. (1980) 4:316–9. doi: 10.1097/00004728-198006000-00005
10. Hu H, Zhang W, Huang D, Wang Y, Zhang Y, Yi Y, et al. Clinical characteristics, treatment and prognosis of paediatric patients with metastatic neuroblastoma to the brain. Clin Neurol Neurosurg. (2019) 184:105372. doi: 10.1016/j.clineuro.2019.105372
11. Fleck S, Marx S, Bobak C, Richter V, Nowak S, Rafaee EE, et al. Neuroblastoma with intracerebral metastases and the need for neurosurgery: a single-center experience. J Neurosurg Pediatr. (2019) 25:51–566. doi: 10.3171/2019.7.PEDS19143
12. Matthay KK, Brisse H, Couanet D, Couturier J, Bénard J, Mosseri V, et al. Central nervous system metastases in neuroblastoma: radiologic, clinical, and biologic features in 23 patients. Cancer. (2003) 98:155–65. doi: 10.1002/cncr.11448
13. DuBois SG, Kalika Y, Lukens JN, Brodeur GM, Seeger RC, Atkinson JB, et al. Metastatic sites in stage IV and IVS neuroblastoma correlate with age, tumor biology, and survival. J Pediatr Hematol Oncol. (1999) 21:181–9. doi: 10.1097/00043426-199905000-00005
14. Stefanowicz J, Izycka-Swieszewska E, Szurowska E, Bień E, Szarszewski A, Liberek A, et al. Brain metastases in paediatric patients: characteristics of a patient series and review of the literature. Folia Neuropathol. (2011) 49:271–81.
15. Wiens AL, Hattab EM. The pathological spectrum of solid CNS metastases in the pediatric population. J Neurosurg Pediatr. (2014) 14:129–35. doi: 10.3171/2014.5.PEDS13526
16. Aygun N. Biological and genetic features of neuroblastoma and their clinical importance. Curr Pediatr Rev. (2018) 14:73–90. doi: 10.2174/1573396314666180129101627
17. Kramer K, Kushner B, Heller G, Cheung NK. Neuroblastoma metastatic to the central nervous system. The memorial Sloan-Kettering cancer center experience and a literature review. Cancer. (2001) 91:1510–9. doi: 10.1002/1097-0142(20010415)91:8<1510::AID-CNCR1159>3.0.CO;2-I
18. Zhu J, Wang J, Zhen ZJ, Lu SY, Zhang F, Sun FF, et al. Brain metastasis in children with stage 4 neuroblastoma after multidisciplinary treatment. Chin J Cancer. (2015) 34:531–7. doi: 10.1186/s40880-015-0038-2
19. Nabavizadeh SA, Feygin T, Harding BN, Bilaniuk LT, Zimmerman RA, Vossough A. Imaging findings of patients with metastatic neuroblastoma to the brain. Acad Radiol. (2014) 21:329–37. doi: 10.1016/j.acra.2013.10.016
20. Zhang X, Li C, Xu C, Hao X, Yu X, Li Q. Correlation of CT signs with lymphatic metastasis and pathology of neuroblastoma in children. Oncol Lett. (2018) 16:2439–43. doi: 10.3892/ol.2018.8959
21. Piccardo A, Morana G, Massollo M, Pescetto M, Conte M, Garaventa A. Brain metastasis from neuroblastoma depicted by (18)F-DOPA PET/CT. Nucl Med Mol Imaging. (2015) 49:241–2. doi: 10.1007/s13139-015-0322-8
22. Hauser P, Jakab Z, Láng O, Kondás O, Müller J, Schuler D, et al. Incidence and survival of central nervous system involvement in childhood malignancies: Hungarian experience. J Pediatr Hematol Oncol. (2005) 27:125–8. doi: 10.1097/01.mph.0000155119.12818.7e
23. Paulino AC, Nguyen TX, Barker JL. Brain metastasis in children with sarcoma, neuroblastoma, and Wilms' tumor. Int J Radiat Oncol Biol Phys. (2003) 57:177–83. doi: 10.1016/S0360-3016(03)00502-9
24. Shaw PJ, Eden T. Neuroblastoma with intracranial involvement: an ENSG Study. Med Pediatr Oncol. (1992) 20:149–55. doi: 10.1002/mpo.2950200211
25. Wieczorek A, Balwierz W, Wyrobek Ł, Garus K, Kwiatkowski S, Kwiecińska K, et al. [Central nervous system involvement at diagnosis and at relapse in children with neuroblastoma]. Przegl Lek. (2010) 67:399–403.
26. Curless RG, Toledano SR, Ragheb J, Cleveland WW, Falcone S. Hematogenous brain metastasis in children. Pediatr Neurol. (2002) 26:219–21. doi: 10.1016/S0887-8994(01)00363-0
27. Kellie SJ, Hayes FA, Bowman L, Kovnar EH, Langston J, Jenkins JJ, et al. Primary extracranial neuroblastoma with central nervous system metastases characterization by clinicopathologic findings and neuroimaging. Cancer. (1991) 68:1999–2006. doi: 10.1002/1097-0142(19911101)68:9<1999::aid-cncr2820680926>3.0.co;2-0
28. Kraal K, Blom T, van Noesel M, Kremer L, Caron H, Tytgat G, et al. Treatment and outcome of neuroblastoma with intraspinal extension: a systematic review. Pediatr Blood Cancer. (2017) 64:1–12. doi: 10.1002/pbc.26451
29. Aronson MR, Smoker WR, Oetting GM. Hemorrhagic intracranial parenchymal metastases from primary retroperitoneal neuroblastoma. Pediatr Radiol. (1995) 25:284–5. doi: 10.1007/BF02011102
30. Quackenbush KE, Luna-Fineman S, Magee JF, Gundogan M, Golobi M, Irie T, et al. Neuroblastoma involvement of the falx cerebri. Pediatr Blood Cancer. (2009) 53:1337–9. doi: 10.1002/pbc.22192
31. D'Ambrosio N, Lyo JK, Young RJ, Haque SS, Karimi S. Imaging of metastatic CNS neuroblastoma. AJR Am J Roentgenol. (2010) 194:1223–9. doi: 10.2214/AJR.09.3203
32. Verissimo CS, Molenaar JJ, Fitzsimons CP, Vreugdenhil E. Neuroblastoma therapy: what is in the pipeline. Endocr Relat Cancer. (2011) 18:R213–31. doi: 10.1530/ERC-11-0251
33. Whittle SB, Smith V, Doherty E, Zhao S, McCarty S, Zage PE. Overview and recent advances in the treatment of neuroblastoma. Expert Rev Anticancer Ther. (2017) 17:369–86. doi: 10.1080/14737140.2017.1285230
34. Sait S, Modak S. Anti-GD2 immunotherapy for neuroblastoma. Expert Rev Anticancer Ther. (2017) 17:889–904. doi: 10.1080/14737140.2017.1364995
Keywords: neuroblastoma, intracranial metastasis (IM), neurologic manifestation, pediatric, prognosis
Citation: Liu Y, Huo L, Zhang J and Liu Y (2021) Intracranial Metastases Tend to Be Overt and Predict Poor Prognosis in Children With Neuroblastoma. Front. Pediatr. 9:716880. doi: 10.3389/fped.2021.716880
Received: 29 May 2021; Accepted: 13 September 2021;
Published: 03 November 2021.
Edited by:
Tao Liu, University of New South Wales, AustraliaReviewed by:
Joseph Louis Lasky, Cure 4 the Kids, United StatesManish Beniwal, National Institute of Mental Health and Neurosciences (NIMHANS), India
Copyright © 2021 Liu, Huo, Zhang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Liu, Y211NGgtbHlAMTI2LmNvbQ==
 Ying Liu
Ying Liu Liang Huo
Liang Huo Jinhua Zhang1
Jinhua Zhang1