- 1Department of Pediatric Emergency, Guangzhou Women and Children's Medical Center, Guangzhou Medical University, Guangzhou, China
- 2Pediatric Intensive Care Unit, Affiliated Hospital of Guangdong Medical University, Zhanjiang, China
- 3Department of Pediatric Neurology, Guangzhou Women and Children's Medical Center, Guangzhou Medical University, Guangzhou, China
- 4Children's Health Section, Guangzhou Women and Children's Medical Center, Guangzhou Medical University, Guangzhou, China
Background: This study aimed to identify potential risk factors for severe hand-foot-mouth disease (HFMD).
Methods: The PubMed, Embase, the Cochrane Library, Sinomed, WanFang, CNKI, and VIP databases were searched (up to August 2021).
Results: Twenty-nine studies (9,241 and 927,355 patients with severe HFMD and controls, respectively; all from China) were included. EV71 was associated with higher odds of severe HFMD compared with other agents (OR = 4.44, 95%CI: 3.12–6.33, p < 0.001). Being home-raised (OR = 1.99, 95%CI: 1.59–2.50, p < 0.001), higher number of children in the family (OR = 2.09, 95%CI: 1.93–2.27, p < 0.001), poor hand hygiene (OR = 2.74, 95%CI: 1.78–4.23, p < 0.001), and no breastfeeding (OR = 2.01, 95%CI: 1.45–2.79, p < 0.001) were risk factors for severe HFMD. First consulting to a district-level or above hospital (OR = 0.34, 95%CI: 0.25–0.45, p < 0.001) and diagnosis of HFMD at baseline (OR = 0.17, 95%CI: 0.13–0.24, p < 0.001) were protective factors against severe HFMD. Fever, long fever duration, vomiting, lethargy, leukocytosis, tic, and convulsions were each associated with severe HFMD (all p < 0.05), while rash was not.
Conclusions: EV71, lifestyle habits, frequent hospital visits, and symptoms are risk factors for severe HFMD in children in China, while early diagnosis and admission to higher-level hospitals are protective factors.
Introduction
Hand-foot-mouth disease (HFMD) is widespread among children and is generally characterized by fever, oral ulcers, and rash on the hands, feet, and buttocks (1–3). The main causative pathogens include human enterovirus 71 (EV 71), Coxsackievirus A16 (CA 16), and other enteroviruses (1–3). The common route of transmission of HFMD is through the fecal-oral route, respiratory droplets, contact with blister fluid, or close contact with infected children (1, 2). Although most cases are mild and self-limited, HFMD is a serious public health concern in children throughout Asia (4). Indeed, the majority of outbreaks are reported in Malaysia, Vietnam, China, Cambodia, and India (2), with an incidence of 115–197 cases per 100,000 person-years (5, 6). Severe or fatal HFMD cases have been reported in epidemiological studies as being frequent in Malaysia, Japan, Vietnam, and China and have received extensive attention from the global public health sector (4, 7–10). Studies from China have reported 27,908 children with HFMD and 905 children with fatal HFMD in 2010 (11, 12).
The most common manifestations of fatal HFMD are encephalitis and subsequent pulmonary edema, but myocarditis, neurologic complications, and paroxysmal tachycardia have been reported (1–3, 8, 13–15). Moreover, brainstem and thalamus symptoms occur at an early stage and are associated with a poor prognosis of HFMD (16).
Therefore, it is important to examine the biological and behavioral characteristics of children to identify the risk factors associated with progression to severe HFMD due to the additional fatality risk in severe cases. Several factors were shown to be associated with an increased risk of severe HFMD in China (17). Various risk factors for severe HFMD were reported in the literature (18–20), but there is a lack of consensus because of different factors being analyzed in the first place, different outcomes, and different study populations.
Hence, these results were not consistent, so continued investigations are needed to confirm known and identify additional potential risk factors. The current meta-analysis was conducted to explore the potential factors associated with the progression to severe HFMD.
Methods
Data Sources, Search Strategy, and Selection Criteria
This study was conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) Statement (21, 22). Any study (irrespective of the language) that investigated the risk factors for severe HFMD was eligible for inclusion in this study. The electronic databases of PubMed, Embase, the Cochrane Library, Sinomed, WanFang, CNKI, and VIP were systematically searched from their inception up to August 2021. The core search terms included “hand foot mouth disease” OR “coxsackievirus a16” OR “enterovirus 71” AND “risk factors.” The reference lists from the retrieved studies were also reviewed to identify any potentially eligible studies.
Two authors independently conducted the literature search and study selection following a standardized form, and any disagreement was settled by a third author. The inclusion criteria were (1) study design: prospective or retrospective observational study, (2) participants: individuals with HFMD, (3) exposure: the study reported the effect estimates of potential risk factors for severe HFMD, (4) outcomes: severe HFMD [i.e., the development of nervous system involvement, physical signs of meningeal irritation, or weakened or missing tendon reflexes (23)]; and (5) sample size >30. Severe HFMD was diagnosed according to the Chinese guidelines that were current when the included studies were conducted; the most recent version is 2018 (24).
Data Collection and Quality Assessment
The data abstraction and quality assessment were conducted by two authors, and any inconsistency was resolved by a third author after referring to the original article. The following variables were collected: first author's surname, publication year, study design, country, sample size, number of boys and girls, mean age, the definition of severe cases, and reported factors. The study quality was assessed using the Newcastle-Ottawa score (NOS) scale, which is based on selection (four items), comparability (one item), and outcome (three items), and the star system ranges from 0 to 9 (25).
Statistical Analysis
The odds ratios (ORs) and their 95% confidence intervals (CIs) were extracted from the multivariable logistic regressions presented in the original papers. The summary ORs were calculated using the random-effects model to account for heterogeneity in study design and populations among studies (26, 27). Heterogeneity across included studies was assessed by I2- and Q-statistics, and p-value < 0.10 was regarded as significant heterogeneity (28, 29). Sensitivity analyses were conducted for risk factors reported in more than four studies to assess the impact of a single study on the overall analysis (30), as well as for EV 71. Publication biases were evaluated using the funnel plot, Egger test (31), Begg test (32), and trim and fill analysis (33). Two-sided p < 0.05 was considered statistically significant. All statistical analyses were conducted using STATA 10.0 (Stata Corporation, College Station, TX, USA).
Results
Literature Search
Figure 1 presents the study selection process. The initial search yielded 993 entries, and 664 were screened after removing the duplicates. From these, 608 were excluded, and 56 full-text papers were screened. Then, 27 studies were excluded (sample size, n = 2; unclear diagnostic criteria, n = 12; data could be extracted, n = 8; outcome, n = 5). Finally, 29 studies were included.
Characteristics of the Studies
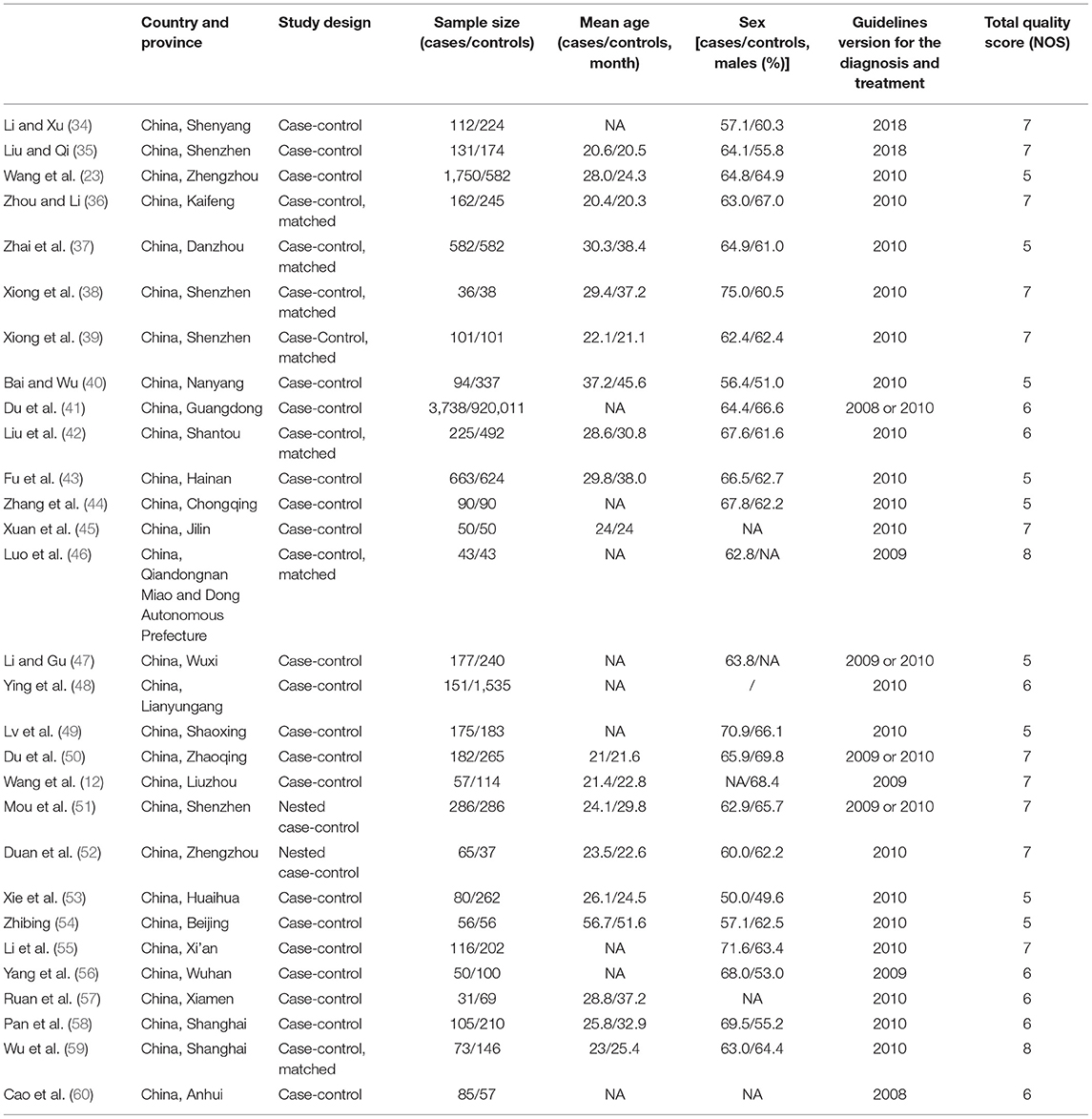
Table 1 presents the characteristics of the studies. There were 19 case-control studies, seven matched case-control studies, and two nested case-control studies. There were 9,241 patients with severe HFMD (31–3,738/study) and 927,355 controls (37–920,011/study). Regarding the NOS, nine studies scored five stars, seven scored six stars, eleven scored seven stars, and two scored eight stars out of a possible total of nine stars.
Association of EV 71 With Severe HFMD
Nineteen studies presented data regarding the association of EV 71 with severe HFMD. The overall meta-analysis showed that EV 71 is associated with higher odds of severe HFMD compared with other etiologic agents (OR = 4.44, 95%CI: 3.12–6.33, p < 0.001, I2 = 92.9%, pheterogeneity < 0.001) (Figure 2). This association was observed irrespective of the type of study: case-control (OR = 4.41, 95%CI: 2.86–6.79, p < 0.001, I2 = 94.8%, pheterogeneity < 0.001), matched case-control (OR = 5.07, 95%CI: 2.29–11.24, p < 0.001, I2 = 74.1%, pheterogeneity = 0.004), and nested case-control (OR = 3.91, 95%CI: 1.32–11.55, p = 0.014).
The sensitivity analysis (Supplementary Figure 1A) showed that Du 2018 (41) influenced the results of the association of EV 71 with severe HFMD. It can be seen that the I2 decreased to 78.1% after Du 2018 (41) was removed (Supplementary Figure 1B).
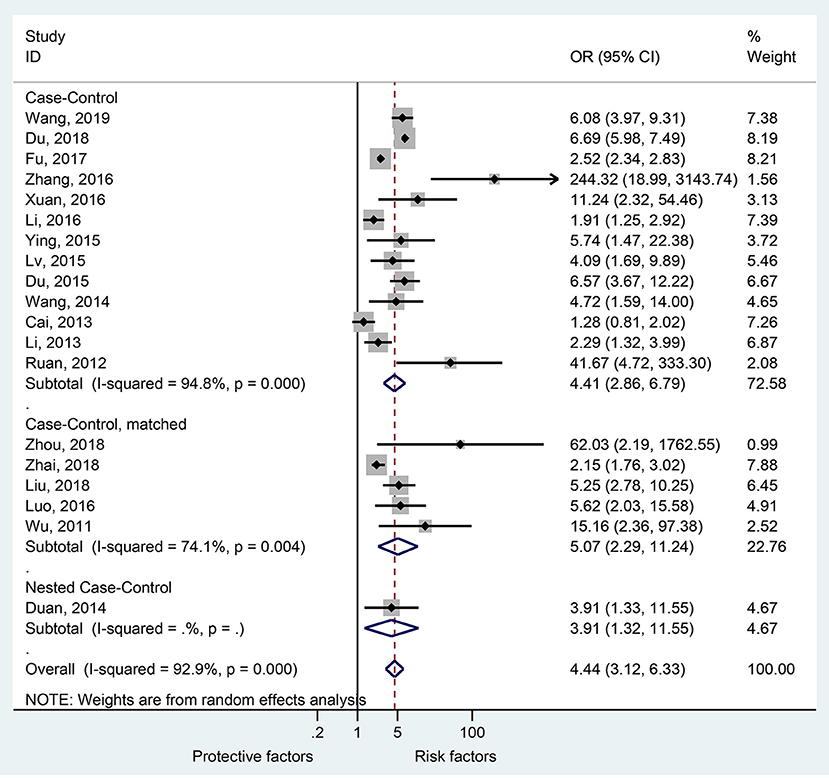
Demographic Factors
Figure 3 and Supplementary Figure 2 shows that age (seven studies, OR = 0.98, 95%CI: 0.95–1.02, p = 0.35, I2 = 77.6%, pheterogeneity < 0.001) and male sex (five studies, OR = 1.20, 95%CI: 0.90–1.61, p = 0.22, I2 = 51.7%, pheterogeneity = 0.082) were not associated with severe HFMD.
Socio-Economic Status and Lifestyle
Figure 4 and Supplementary Figure 2 shows that rural residency (OR = 1.99, 95%CI: 0.97–4.08, p < 0.059, I2 = 84.2%, pheterogeneity < 0.001) was not a risk factor for severe HFMD, while being home-raised (i.e., not frequenting kindergarten) (OR = 1.99, 95%CI: 1.59–2.50, p < 0.001, I2 = 81.8%, pheterogeneity < 0.001), higher number of children in the family (OR = 2.09, 95%CI: 1.93–2.27, p < 0.001, I2 = 0.0%, pheterogeneity = 0.790), poor hand hygiene (OR = 2.74, 95%CI: 1.78–4.23, p < 0.001, I2 = 57.8%, pheterogeneity = 0.093), and no breastfeeding (OR = 2.01, 95%CI: 1.45–2.79, p < 0.001, I2 = 0.0%, pheterogeneity = 0.558) were risk factors for severe HFMD.
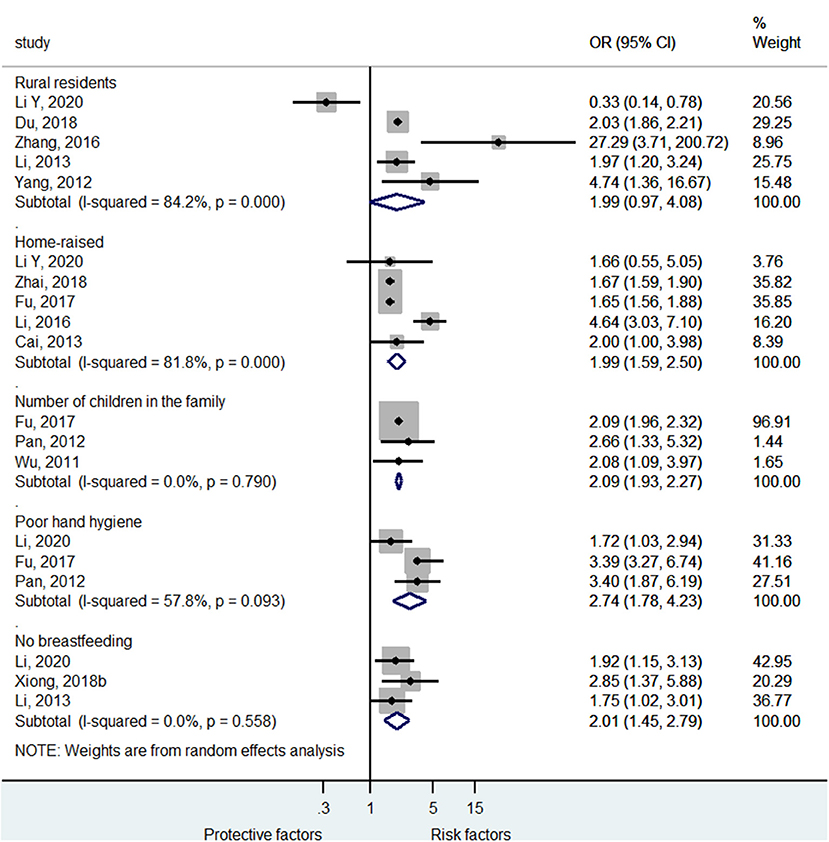
Figure 4. Forest plot for the association of lifestyle of life habits with severe hand foot mouth disease.
Clinical Pathway
Among the clinical pathway factors that could influence the outcomes of HFMD, first consulting to a district-level or above hospital (OR = 0.34, 95%CI: 0.25–0.45, p < 0.001, I2 = 0.0%, pheterogeneity = 0.649) and diagnosis of HFMD at baseline (OR = 0.17, 95%CI: 0.13–0.24, p < 0.001, I2 = 42.9%, pheterogeneity = 0.081) were protective factor against severe HFMD (Figure 5; Supplementary Figure 2), while >3 visits at the clinic in the past 6 months was a risk factor for severe HFMD (OR = 1.80, 95%CI: 1.03–3.14, p = 0.037, I2 = 97.8%, pheterogeneity < 0.001). The time from onset to the first visit at the hospital was not associated with severe HFMD (OR = 1.60, 95%CI: 0.45–5.70, p = 0.467, I2 = 87.0%, pheterogeneity < 0.001).
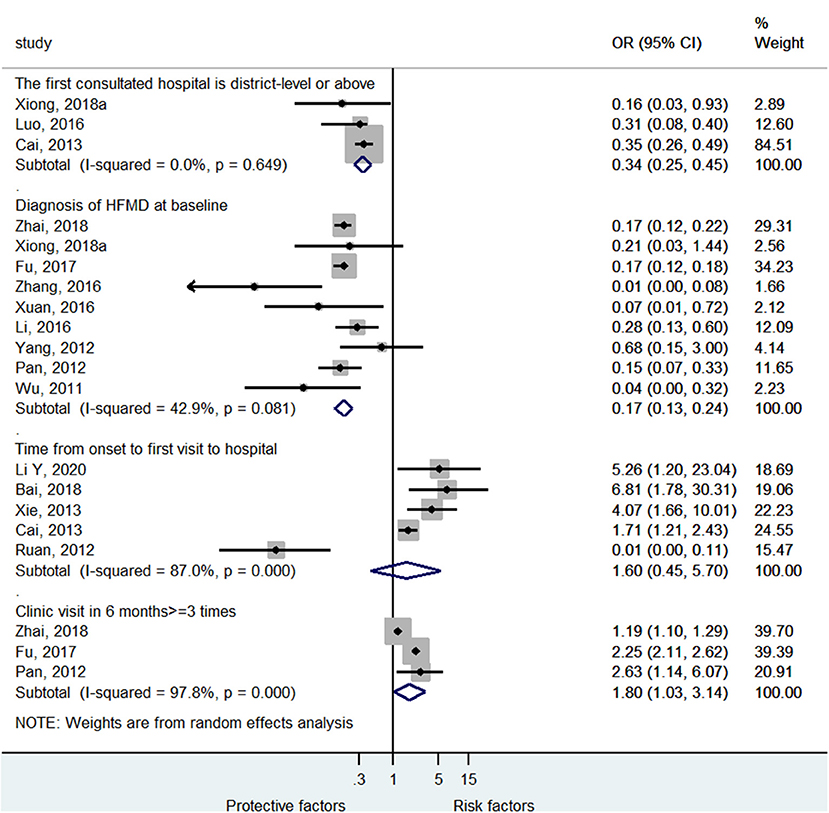
Figure 5. Forest plot for the association of the clinical pathway characteristics with severe hand foot mouth disease.
Symptoms
Supplementary Table 1 and Supplementary Figure 2 show that fever (OR = 3.23, 95%CI: 2.31–4.70, p < 0.001, I2 = 66.2%, pheterogeneity < 0.001), long fever duration (OR = 5.36, 95%CI: 2.33–12.35, p = 0.001, I2 = 91.3%, pheterogeneity < 0.001), vomiting (OR = 64.30, 95%CI: 5.53–747.80, p < 0.001, I2 = 48.1%, pheterogeneity = 0.103), lethargy (OR = 8.89, 95%CI: 2.55–30.93, p = 0.001, I2 = 84.1%, pheterogeneity < 0.001), leukocytosis (OR = 2.26, 95%CI: 1.42–3.61, p < 0.001, I2 = 87.5%, pheterogeneity = 0.720), tremors/myoclonic jerks (OR = 5.20, 95%CI: 3.14–8.64, p < 0.001, I2 = 5.9%, pheterogeneity < 0.001), and convulsions (OR = 12.64, 95%CI: 1.15–139.00, p = 0.038, I2 = 53.0%, pheterogeneity = 0.145) were each associated with severe HFMD, while rash was not (OR = 1.40, 95%CI: 0.25–7.87, p = 0.704, I2 = 87.3%, pheterogeneity < 0.001).
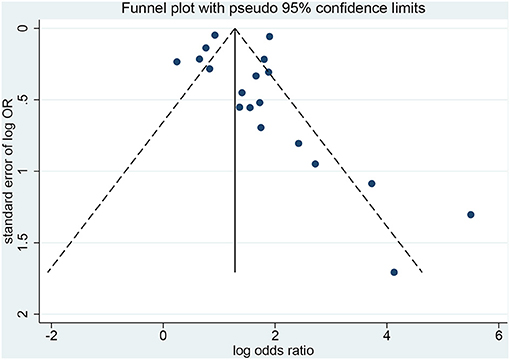
Publication Bias
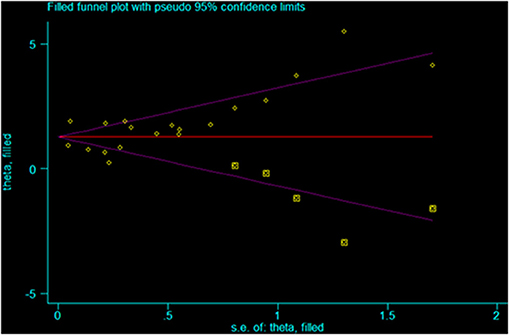
The Egger test (p < 0.001) and the Begg test (p = 0.021) showed that there is publication bias (Figures 6, 7). Five studies would be needed to balance de publication bias (Figure 7).
Discussion
Various risk factors for severe HFMD were reported in the literature (18–20, 61), but there is a lack of consensus. This study aimed to identify potential risk factors for severe HFMD. The results indicate that EV 71, lifestyle habits (rural, being home-raised, a higher number of children in the family, poor hand hygiene, and no breastfeeding), clinical pathway (frequent hospital visits), and symptoms are risk factors for severe HFMD in children, while early diagnosis and admission to higher-level hospitals are protective factors.
Previous meta-analyses examined HFMD (18, 19, 61). Indeed, Sun et al. (18) examined the risk factors for severe HFMD. Peng et al. (19) examined the risk factors for neurogenic pulmonary edema in children with severe HFMD. Fang et al. (62) examined the risk factors for severe HFMD. Ni et al. (61) investigated the association between risk factors and death from HFMD. Therefore, in an innovative view, the present study focused on the risk factors and the changes in disease severity (from mild to severe).
EV 71 is a well-known risk factor for severe HFMD (4, 63–65), and it was confirmed once again. This EV serotype is the cause of HFMD outbreaks in the Asia-Pacific region, although it has been reported to be the cause of outbreaks in Europe and North America in the 1970s (66). This meta-analysis also showed that rural inhabitants are at higher risk of severe HFMD, probably because of poorer hygiene, lower education, lower economic status, and closer proximity to farm animals (e.g., living on a farm) which are supported by previous studies (67–69). In addition, being home-raised, a higher number of children in the family, and poor hand hygiene are already known to be associated with higher risks of transmission of various pediatric diseases, as previously observed (44, 70, 71). On the other hand, a study showed that children attending daycare centers were at higher risk of severe HFMD (72). No breastfeeding is associated with a lower immune status in some children and could lead to more severe HFMD. Some previous studies showed that exclusive breastfeeding protected against HFMD (18, 73), supporting this meta-analysis.
As for any disease, early diagnosis and management are associated with better outcomes, as observed for HFMD (67, 74, 75). Higher-level hospitals are more likely to have experienced physicians with more awareness for the fast and accurate diagnosis of HFMD, as well as for proper management (35). In this study, the diagnosis of HFMD as the first diagnosis and higher-level hospitals were protective factors against severe HFMD. Frequent visits to the hospital might lead to higher nosocomial exposure to hospitals' pathogens, frailer constitution, or decreased awareness from the healthcare professionals toward children and their parents consulting too often. This will have to be examined more closely in future studies.
Symptom severity has already been associated with severe HFMD in previous meta-analyses and reviews. Our previous study illustrated a causal relationship between EV 71 infection and severe HFMD risk, the symptoms of critical illness including autokinetic eyeball, eyeball ataxia, severe coma, respiratory rhythm abnormality, ataxic respiration, absence of pharyngeal reflex, ultrahyperpyrexia (>40°C), excessive tachycardia (>220 bpm), pulmonary edema, and/or hemorrhage and refractory shock; and four indicators with time-correlation with half of the symptoms above, including CRT (capillary refill time) extension, tachycardia, hyperventilation, and nystagmus, which occurred before the above-mentioned critical illness symptoms and also indicated the risk of critical illness (76). Sun et al. (18) showed that fever >3 days and vomiting were associated with severe HFMD. Peng et al. (19) showed that limb tremors, vomiting, abnormal respiration, atypical rash, and drowsiness were associated with HFMD-associated pulmonary edema. The present study also showed that fever, long fever duration, vomiting, lethargy, leukocytosis, tremors/myoclonic jerks, and convulsions were risk factors for severe HFMD. Nevertheless, this study did not examine the risk factors of various complications of HFMD.
This study has limitations that must be considered when analyzing the results. First, although the eligibility criteria did not restrict the country and western databases (PubMed, Embase, and the Cochrane Library) were queried, all included studies were from China. It is probably because HFMD reaches epidemic proportions in some regions of China nearly every year and that EV 71 is particularly present and associated with severe forms of HFMD (4, 63–65). Western countries like the United States of America only have episodic HFMD epidemic events once every few years (3). Furthermore, heterogeneity was observed among the studies because of different study designs, different diagnostic criteria for severe HFMD, and different confounding factors included in the multivariable models. Of note, for the analysis of the association between EV 71 and severe HFMD, excluding Du 2018 (41) decreased heterogeneity. The possible reason is that the sample size of that study is too large compared with the other studies. Finally, publication bias was observed and indicated that positive-findings studies were overrepresented compared with the negative-findings ones. According to Figure 7, five studies would be needed to balance de publication bias.
In conclusion, this meta-analysis indicates that EV 71 infection, lifestyle habits (rural, being home-raised, a higher number of children in the family, poor hand hygiene, and no breastfeeding), clinical pathway (frequent hospital visits), and symptoms are risk factors for severe HFMD in children, while early diagnosis and admission to higher-level hospitals are protective factors. Age, sex, and time from onset to admission are not associated with HFMD severity.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
PL and YH conceived and coordinated the study, designed, performed and analyzed the experiments, and wrote the paper. DZ, DH, and SY carried out the data collection, data analysis, and revised the paper. All authors reviewed the results and approved the final version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81801206), the Medical Science and Technology Research Foundation of Guangdong, China (No. A2019373), Guangdong Natural Science Foundation (No. 2020A1515010014), Science and Technology Key Project for People's Livelihood of Guangzhou, China (No. 201803010026), and the Innovative Project of Children's Research Institute, Guangzhou Women and Children's Medical Center, China (Nos. Pre-NSFC-2018-004, Pre-NSFC-2018-008, Pre-NSFC-2019-002, and Pre-NSFC-2019-007). The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2021.716039/full#supplementary-material
References
1. Sarma N. Hand, foot, and mouth disease: current scenario and Indian perspective. Indian J Dermatol Venereol Leprol. (2013) 79:165–75. doi: 10.4103/0378-6323.107631
2. Aswathyraj S, Arunkumar G, Alidjinou EK, Hober D. Hand, foot and mouth disease (HFMD): emerging epidemiology and the need for a vaccine strategy. Med Microbiol Immunol. (2016) 205:397–407. doi: 10.1007/s00430-016-0465-y
3. Repass GL, Palmer WC, Stancampiano FF. Hand, foot, and mouth disease: identifying and managing an acute viral syndrome. Cleve Clin J Med. (2014) 81:537–43. doi: 10.3949/ccjm.81a.13132
4. World Health Organization. A Guide to Clinical Management and Public Health Response for Hand, Foot and Mouth Disease (HFMD). Geneva: World Health Organization (2018). Available online at: https://iris.wpro.who.int/bitstream/handle/10665.1/5521/9789290615255_eng.pdf
5. Xiao Y, Zhou J, Zhang H, Ding C, Shi P. Epidemiological and aetiological characteristics of hand, foot and mouth disease cases 2011–2017 in Yixing, China. Infect Dis (Lond). (2018) 50:859–61. doi: 10.1080/23744235.2018.1493217
6. Qi L, Tang W, Zhao H, Ling H, Su K, Zhao H, et al. Epidemiological characteristics and spatial-temporal distribution of hand, foot, and mouth disease in Chongqing, China, 2009-2016. Int J Environ Res Public Health. (2018) 15:270. doi: 10.3390/ijerph15020270
7. Chan LG, Parashar UD, Lye MS, Ong FG, Zaki SR, Alexander JP, et al. Deaths of children during an outbreak of hand, foot, and mouth disease in Sarawak, Malaysia: clinical and pathological characteristics of the disease. For the Outbreak Study Group. Clin Infect Dis. (2000) 31:678–83. doi: 10.1086/314032
8. Ho M, Chen ER, Hsu KH, Twu SJ, Chen KT, Tsai SF, et al. An epidemic of enterovirus 71 infection in Taiwan. Taiwan Enterovirus Epidemic Working Group. N Engl J Med. (1999) 341:929–35. doi: 10.1056/NEJM199909233411301
9. Xu W, Jiang L, Thammawijaya P, Thamthitiwat S. Hand, foot and mouth disease in Yunnan Province, China, 2008–2010. Asia Pac J Public Health. (2015) 27:NP769–77. doi: 10.1177/1010539511430523
10. Kit LS. Emerging and re-emerging diseases in Malaysia. Asia Pac J Public Health. (2002) 14:6–8. doi: 10.1177/101053950201400103
11. Messacar K Spence-Davizon E Osborne C Press C Schreiner TL Martin J. Clinical characteristics of enterovirus A71 neurological disease during an outbreak in children in Colorado, USA, in 2018: an observational cohort study. Lancet Infect Dis. (2020) 20:230–9. doi: 10.1016/S1473-3099(19)30632-2
12. Wang Y, He X, Zhao J. Mortality and death cases of hand foot and mouth disease reported in China, 2008–2010. Dis Surveil. (2011) 26:424–6.
13. Esposito S, Principi N. Hand, foot and mouth disease: current knowledge on clinical manifestations, epidemiology, aetiology and prevention. Eur J Clin Microbiol Infect Dis. (2018) 37:391–8. doi: 10.1007/s10096-018-3206-x
14. Hu P, Hou S, Du PF, Li JB, Ye Y. Paroxysmal supraventricular tachycardia in an infant with hand, foot, and mouth disease. Ann Dermatol. (2012) 24:200–2. doi: 10.5021/ad.2012.24.2.200
15. Yang SD, Li PQ, Huang YG, Li W, Ma LZ, Wu L, et al. Factors associated with fatal outcome of children with enterovirus A71 infection: a case series. Epidemiol Infect. (2018) 146:788–98. doi: 10.1017/S0950268818000468
16. Li P, Wang N, Ma W, Gao Y, Yang S. Hand, foot, and mouth disease in mainland China. Lancet Infect Dis. (2014) 14:1042. doi: 10.1016/S1473-3099(14)70974-0
17. Ma H, He F, Wan J, Jin D, Zhu L, Liu X, et al. Glucocorticoid and pyrazolone treatment of acute fever is a risk factor for critical and life-threatening human enterovirus 71 infection during an outbreak in China, 2008. Pediatr Infect Dis J. (2010) 29:524–9. doi: 10.1097/INF.0b013e3181cdd178
18. Sun BJ, Chen HJ, Chen Y, An XD, Zhou BS. The risk factors of acquiring severe hand, foot, and mouth disease: a meta-analysis. Can J Infect Dis Med Microbiol. (2018) 2018:2751457. doi: 10.1155/2018/2751457
19. Peng L, Luo R, Jiang Z. Risk factors for neurogenic pulmonary edema in patients with severe hand, foot, and mouth disease: a meta-analysis. Int J Infect Dis. (2017) 65:37–43. doi: 10.1016/j.ijid.2017.09.020
20. Shi C, Liu J, Shi P, Ji H, Shen Y, Qian YH. Epidemiological characteristics and influential factors of hand, foot, and mouth disease reinfection in Wuxi, China, 2008–2016. BMC Infect Dis. (2018) 18:472. doi: 10.1186/s12879-018-3385-1
21. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
22. Selcuk AA. A guide for systematic reviews: PRISMA. Turk Arch Otorhinolaryngol. (2019) 57:57–8. doi: 10.5152/tao.2019.4058
23. Wang B, Feng H, Huang P, Dang D, Zhao J, Yi J, et al. Developing a nomogram for risk prediction of severe hand-foot-and-mouth disease in children. Indian J Pediatr. (2019) 86:365–70. doi: 10.1007/s12098-019-02898-4
24. National Health Commission of the People's Republic of China. Diagnosis and treatment guideline on hand, foot and mouth disease 2018. Chin J Clin Infect Dis. (2018) 11:161–6.
25. Lo CK, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers' to authors' assessments. BMC Med Res Methodol. (2014) 14:45. doi: 10.1186/1471-2288-14-45
26. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
27. Ades AE, Lu G, Higgins JP. The interpretation of random-effects meta-analysis in decision models. Med Decis Making. (2005) 25:646–54. doi: 10.1177/0272989X05282643
28. Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M. Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). London: Cochrane Collaboration (2019). doi: 10.1002/9781119536604
29. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
30. Tobias A. Assessing the influence of a single study in meta-analysis. Stata Tech Bull. (1999) 47:15–7.
31. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
32. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101. doi: 10.2307/2533446
33. Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. (2000) 56:455–63. doi: 10.1111/j.0006-341X.2000.00455.x
34. Li Y, Xu X. Analysis of risk factors in critical cases of hand-foot-mouth disease. Med Recapitul. (2020). 26:4143–6; 4152.
35. Liu J, Qi J. Prevalence and management of severe hand, foot, and mouth disease in Xiangyang, China, from 2008 to 2013. Front Pediatr. (2020) 8:323. doi: 10.3389/fped.2020.00323
36. Zhou L, Li X. Analysis of risk factors in severe cases of hand foot mouth disease. China Med Eng. (2018) 26.
37. Zhai J, Lin L, Mai L, Fu X. Risk factors of severe hand, foot and mouth disease in Danzhou of China. Chin J Viral Dis. (2018) 8:44–9.
38. Xiong T, Meng Y, Liu J, Huang J, Zhao R, Wu T. Analysis on risk factors of severe cases with hand foot mouth disease infected by Coxsackievirus A6. Jiangsu J Prev Med. (2018). [Epub ahead of print].
39. Xiong T, Meng Y, Yu G, Huang J, Zhao R, Wei S. Risk factors of severe cases with hand-foot-mouth disease infected by EV-A71. China Trop Med. (2018). 18:242–4.
40. Bai T, Wu Z. Analysis on risk factors for severe hand, foot, and mouth disease in a tertiary hospital, Nanyang city 2016 and 2017. Prev Med Trib. (2018) 24:354–6.
41. Du Z, Lawrence WR, Zhang W, Zhang D, Yu S, Hao Y. Bayesian spatiotemporal analysis for association of environmental factors with hand, foot, and mouth disease in Guangdong, China. Sci Rep. (2018) 8:15147. doi: 10.1038/s41598-018-33109-3
42. Liu C, Wang K, Lin N, Cai J, Cui B, Wu B. Risk factors of severe hand, foot and mouth disease in Shantou, China: a case-control study. J Infect Dev Ctries. (2018) 12:359–64. doi: 10.3855/jidc.9845
43. Fu T, Yang J, Lin W, Lie X. Risk factors of severe hand-foot-mouth disease in the southern area of Hainan Province. Acad J Chin PLA Med Sch. (2017) 38:132–5. doi: 10.1177/1010539515579123
44. Zhang D, Li Z, Zhang W, Guo P, Ma Z, Chen Q, et al. Hand-washing: the main strategy for avoiding hand, foot and mouth disease. Int J Environ Res Public Health. (2016) 13:610. doi: 10.3390/ijerph13060610
45. Xuan S, Deng L, Zhao Q, Lu X, Chen L. Analysis for the risk factors of severe hand foot mouth disease cases in Jilin province. Chin J Public Health Eng. (2016) 15:250–2.
46. Luo T, Shi D, Deng M, Shan Z, Liu J. Epidemiological characteristics of severe cases of hand foot mouth disease in Qiandongnan Prefecture in 2014. J Appl Prev Med. (2016) 22.
47. Li J, Gu Y. The epidemiological characteristics and risk factors of severe hand foot- and mouth-disease in Xishan District of Wuxi City between 2011 and 2014. Mod Prevent Med. (2016) 43:972–5.
48. Ying L. HFMD severe cases'risk factors analyses of Lianyungang city. J Med Pest Control. (2015) 31:803–5.
49. Lv B, Mo W, Huang C, Zhang M, Ma A, He J. Analysis of risk factors of severe hand, foot and mouth disease. Chin J Prim Med Pharm. (2015) 22:344–8.
50. Du W, Wang L, Xia Q. Analysis of risk factors in severe cases of hand foot mouth disease. Pract Prev Med. (2015) 22.
51. Mou J, Dawes M, Li Y, He Y, Ma H, Xie X, et al. Severe hand, foot and mouth disease in Shenzhen, South China: what matters most? Epidemiol Infect. (2014) 142:776–88. doi: 10.1017/S0950268813001453
52. Duan G, Yang H, Shi L, Sun W, Sui M, Zhang R, et al. Serum inflammatory cytokine levels correlate with hand-foot-mouth disease severity: a nested serial case-control study. PLoS ONE. (2014) 9:e112676. doi: 10.1371/journal.pone.0112676
54. Zhibing X. Analysis of related risk factors in severe cases of hand, foot and mouth disease in Huaihua City. (2013). doi: 10.3969/j.issn.1671-7171.2013.09.055
55. Li Y, Dang S, Deng H, Wang W, Jia X, Gao N, et al. Breastfeeding, previous Epstein-Barr virus infection, Enterovirus 71 infection, and rural residence are associated with the severity of hand, foot, and mouth disease. Eur J Pediatr. (2013) 172:661–6. doi: 10.1007/s00431-013-1939-1
56. Yang X, Zhou X, Kong D, Yu B. Logistic regression analysis on the influencing factors of severe cases of HFMD in Wuhan City. J Pub Health Prev Med. (2012) 23:15–7.
57. Ruan J, Liang Y, Huang J, Su C. Case-control study of risk factors for severe hand-foot-mouth disease. Chin J Zoon. (2012) 28:857–60.
58. Pan H, Zheng YX, Mao SH, Hu JY, Zheng Y, Li J, et al. [A case-control study on risk factors that associated with severe hand-foot-mouth disease in Shanghai]. Zhonghua Liu Xing Bing Xue Za Zhi. (2012) 33:763–7.
59. Wu H, Zheng Y, Mao S, Gu B, Pan H. Study on risk factors associated with severe case of hand-foot-mouth disease in Shanghai. J Environ Occup Med. (2011) 28:257–61.
60. Cao M, Liu H, Wan J, Zhu L. An case-control study of severe case of Hand-Foot-and-mouth disease(EV71) in Fuyang City, Anhui Province. Anhui J Prev Med. (2010) 16:19–20.
61. Ni XF, Li X, Xu C, Xiong Q, Xie BY, Wang LH, et al. Risk factors for death from hand-foot-mouth disease: a meta-analysis. Epidemiol Infect. (2020) 148:e44. doi: 10.1017/S0950268819002279
62. Xing W, Liao Q, Viboud C, Zhang J, Sun J, Wu JT, et al. Hand, foot, and mouth disease in China, 2008–12: an epidemiological study. Lancet Infect Dis. (2014) 14:308–18. doi: 10.1016/S1473-3099(13)70342-6
63. Fan H, Fu YS, Shan J, Shi C, Zhang XF, Huo X, et al. [Surveillance on the epidemiological and etiological characteristics of hand-foot-mouth disease during the outbreaks in three cities of Jiangsu province, 2012–2015]. Zhonghua Liu Xing Bing Xue Za Zhi. (2016) 37:1608–14. doi: 10.3760/cma.j.issn.0254-6450.2016.12.011
64. Liu MY, Liu J, Lai W, Luo J, Liu Y, Vu GP, et al. Characterization of enterovirus 71 infection and associated outbreak of hand, foot, and mouth disease in Shawo of China in 2012. Sci Rep. (2016) 6:38451. doi: 10.1038/srep38451
65. Yan XF, Gao S, Xia JF, Ye R, Yu H, Long JE. Epidemic characteristics of hand, foot, and mouth disease in Shanghai from 2009 to 2010: enterovirus 71 subgenotype C4 as the primary causative agent and a high incidence of mixed infections with coxsackievirus A16. Scand J Infect Dis. (2012) 44:297–305. doi: 10.3109/00365548.2011.634433
66. Sabanathan S, Tan le V, Thwaites L, Wills B, Qui PT, Rogier van Doorn H. Enterovirus 71 related severe hand, foot and mouth disease outbreaks in South-East Asia: current situation and ongoing challenges. J Epidemiol Community Health. (2014) 68:500–2. doi: 10.1136/jech-2014-203836
67. Wang Y, Zhao H, Ou R, Zhu H, Gan L, Zeng Z, et al. Epidemiological and clinical characteristics of severe hand-foot-and-mouth disease (HFMD) among children: a 6-year population-based study. BMC Public Health. (2020) 20:801. doi: 10.1186/s12889-020-08961-6
68. Qin L, Dang D, Wang X, Zhang R, Feng H, Ren J, et al. Identification of immune and metabolic predictors of severe hand-foot-mouth disease. PLoS ONE. (2019) 14:e0216993. doi: 10.1371/journal.pone.0216993
69. Zhang D, Li R, Zhang W, Li G, Ma Z, Chen X, et al. A case-control study on risk factors for severe hand, foot and mouth disease. Sci Rep. (2017) 7:40282. doi: 10.1038/srep40282
70. Guo N, Ma H, Deng J, Ma Y, Huang L, Guo R, et al. Effect of hand washing and personal hygiene on hand food mouth disease: a community intervention study. Medicine (Baltimore). (2018) 97:e13144. doi: 10.1097/MD.0000000000013144
71. Huang WC, Shih WL, Yang SC, Yen TY, Lee JT, Huang YC, et al. Predicting severe enterovirus 71 infection: age, comorbidity, and parental behavior matter. J Microbiol Immunol Infect. (2017) 50:10–6. doi: 10.1016/j.jmii.2014.11.013
72. Suzuki Y, Taya K, Nakashima K, Ohyama T, Kobayashi JM, Ohkusa Y, et al. Risk factors for severe hand foot and mouth disease. Pediatr Int. (2010) 52:203–7. doi: 10.1111/j.1442-200X.2009.02937.x
73. Lin H, Sun L, Lin J, He J, Deng A, Kang M, et al. Protective effect of exclusive breastfeeding against hand, foot and mouth disease. BMC Infect Dis. (2014) 14:645. doi: 10.1186/s12879-014-0645-6
74. Chen S, Yi K, Chen X, Li L, Tan X. A simple scoring system for quick, accurate, and reliable early diagnosis of hand, foot, and mouth disease. Med Sci Monit. (2018) 24:8627–38. doi: 10.12659/MSM.911736
75. Ooi MH, Wong SC, Mohan A, Podin Y, Perera D, Clear D, et al. Identification and validation of clinical predictors for the risk of neurological involvement in children with hand, foot, and mouth disease in Sarawak. BMC Infect Dis. (2009) 9:3. doi: 10.1186/1471-2334-9-3
Keywords: hand-foot-mouth disease, infection, meta-analysis, risk factors, disease severity
Citation: Li P, Huang Y, Zhu D, Yang S and Hu D (2021) Risk Factors for Severe Hand-Foot-Mouth Disease in China: A Systematic Review and Meta-Analysis. Front. Pediatr. 9:716039. doi: 10.3389/fped.2021.716039
Received: 28 May 2021; Accepted: 13 October 2021;
Published: 10 November 2021.
Edited by:
Roger Frutos, Université de Montpellier, FranceReviewed by:
Jing Yan, Tianjin Medical University, ChinaXing Liao, Institute of Basic Research in Clinical Medicine, China Academy of Chinese Medical Sciences, China
Copyright © 2021 Li, Huang, Zhu, Yang and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sida Yang, eWFuZ3NpZGEyMDEzQDEyNi5jb20=; Dandan Hu, Z3VvaGRkQDEyNi5jb20=
†These authors have contributed equally to this work
 Peiqing Li
Peiqing Li Yuge Huang2†
Yuge Huang2† Danping Zhu
Danping Zhu Sida Yang
Sida Yang