- 1Department of Neonatology, Children's Hospital of Chongqing Medical University, Chongqing, China
- 2National Clinical Research Center for Child Health and Disorders, Chongqing, China
- 3Ministry of Education Key Laboratory of Child Development and Disorders, Chongqing, China
- 4Chongqing Key Laboratory of Pediatrics, Chongqing, China
Objective: The goal of the current study was to assess the associations of typical chest imaging findings of bronchopulmonary dysplasia (BPD) in preterm infants with clinical characteristics and outcomes until 2 years of age.
Method: This retrospective cohort study enrolled 256 preterm infants with BPD who were admitted between 2014 and 2018. A propensity score analysis was used to adjust for confounding factors. The primary outcomes were the severity of BPD, home oxygen therapy (HOT) at discharge and mortality between 28 days after birth and 2 years of age. A multivariate logistic regression analysis was performed to identify related variables of mortality.
Results: Seventy-eight patients with typical chest imaging findings were enrolled, of which 50 (64.1%) were first found by CXR, while 28 (35.9%) were first found by CT. In addition, 85.9% (67/78) were discovered before 36 weeks postmenstrual age (PMA) (gestational age [GA] < 32 weeks) or before 56 days after birth (GA > 32 weeks). After propensity score matching, the matched groups consisted of 58 pairs of patients. Those with typical imaging findings had a remarkably higher mortality rate (29.3 vs. 12.1%, p = 0.022, OR 3.021), higher proportion of severe BPD (32.8 vs. 12.1%, p = 0.003, OR 4.669) and higher rate of HOT at discharge (74.1 vs. 46.6%, p = 0.002, OR 3.291) than those without typical imaging findings. The multivariate logistic regression analysis showed that typical imaging findings ≤ 7 days and typical typical imaging findings >7 days were independent risk factors for mortality in preterm infants with BPD (OR 7.794, p = 0.004; OR 4.533, p = 0.001).
Conclusions: More attention should be given to chest imaging findings of BPD, especially in the early stage (within 7 days). Early recognition of the development of BPD helps early individualized treatment of BPD.
Clinical Trial Registration: www.ClinicalTrials.gov, identifier: NCT04163822.
Introduction
Bronchopulmonary dysplasia (BPD) is a common chronic respiratory disease in preterm infants (1). The increase in the survival rate of premature babies following the improvement of perinatal treatment and care has caused an increase in the incidence of BPD in recent years (2), which has seriously affected the quality of life of preterm infants. According to the consensus reached at the workshop sponsored by the National Institute of Child Health and Human Development (NICHD) in 2001 (3), BPD was clinically defined based on oxygen dependency in preterm infants. However, the refined NICHD definition of BPD in 2018 (4) emphasized imaging findings to support a diagnosis of lung parenchyma disease.
Fibrotic opacities and cystic changes on chest imaging [chest X-ray (CXR) or computed tomography (CT) scan] were considered typical findings in BPD patients (5–7). In patients with severe BPD, the presence of bubbles/cystic appearance on CXR after 28 days of life was reported to be an important factor, and typical imaging findings can predict a poor pulmonary outcome in BPD patients (8). BPD is associated with poor outcomes (9). Although many studies have been conducted on BPD, there are limited reports specifically evaluating the association of typical imaging findings with clinical characteristics and later outcomes in patients with BPD.
We hypothesized that BPD with typical imaging findings was likely to be a particular subgroup of this entity, with a unique etiology, clinical characteristics and prognosis. Therefore, this retrospective study aimed to compare clinical characteristics, short-term outcomes and follow-up data until 2 years of age in preterm infants with or without typical imaging findings of BPD on CXR or CT scan during the entire hospital stay. A propensity score analysis was used to reduce bias between the two groups, and multivariate logistic regression analysis was performed to identify factors related to mortality in preterm infants with BPD.
Methods
Definitions of the Relevant Concepts
BPD was diagnosed and graded according to the 2001 NICHD consensus (3). Oxygen dependence (10) was defined as requiring oxygen supplementation for more than 12 h a day to maintain oxygen saturation ≥92%. Home oxygen therapy (HOT) at discharge (11) was used to maintain oxygen saturation at ≥95% by using short-term oxygen rather than continuous supplemental oxygen.
Typical chest imaging findings include fibrotic opacities and cysts (7, 12, 13) on CXR or CT scans during the entire hospital stay. Fibrotic opacities (14) were defined as an area with diffused and reticular density or triangular subpleural opacities, and cystic appearance (8) was defined as a lucent parenchymal area with a clear boundary. Chorioamnionitis (CAM) was diagnosed based on clinical features, such as maternal fever, localized pain and leukocytosis during pregnancy and delivery. Prenatal glucocorticoid administration was defined as the administration of at least one maternal dose of glucocorticoids between 24 h and 7 days prior to delivery. The meta-analysis by Shah et al. (15) showed that there was no significant difference between infants who received inhaled and systemic steroids in the incidence of death or BPD at 36 weeks PMA. Then, postnatal glucocorticoid administration was defined as the administration of inhaled or systemic glucocorticoids to preterm infants at any time after delivery. Repeated surfactant dose (RSD) (16) was defined as supplementation with at least two doses of pulmonary surfactant (PS).
Sepsis was diagnosed following the Chinese Expert Consensus (version 2019) (17). Patent ductus arteriosus (PDA) was diagnosed by echocardiography if there was a significant left-to-right shunt through the ductus arteriosus. Intraventricular hemorrhage (18) (IVH) was defined according to Papile's classification (stage III to IV). Necrotizing enterocolitis (NEC) (19) was defined as level II or higher according to Bell's classification. Stunting was defined as >2 standard deviations (SD) below the mean length for age, and underweight was defined as >2 SD below the mean weight for age. Weight and length were calculated with Chinese growth reference standards (20). Wheezing disorders were defined as a physician diagnosis of wheezing exposure treated with anti-asthma drugs (bronchodilators and corticosteroids) (21).
Patients
We performed a retrospective cohort study using data from the Department of Neonatology, Children's Hospital of Chongqing Medical University (CHCMU), and the patients were followed by telephone. This study was approved by the Institutional Review Board of CHCMU (2019-209) and was registered on Clinicaltrials.gov (NCT04163822). Eligible patients fulfilled the following three criteria simultaneously: (1) BPD diagnosis according to the 2001 NICHD consensus; (2) chest imaging examination (CXR or CT) in the first week after birth; and (3) hospitalization within the first 7 days after birth.
Patients were excluded from the study if they met one of the following conditions: (1) major congenital malformations or laboratory-confirmed chromosomal abnormalities; (2) inadequate clinical data or missing chest imaging data; or (3) loss to follow-up.
Data Collection
Relevant data were retrospectively collected, including demographics [i.e., sex, gestational age, age at admission, birth weight (BW)], perinatal factors [i.e., pregnancy-induced hypertension (PIH), CAM, cesarean section, prenatal glucocorticoid administration, respiratory distress syndrome (RDS), sepsis, PDA, 5-min Apgar score], management during hospitalization [i.e., surfactant administration, noninvasive and invasive mechanical ventilation (IMV), postnatal glucocorticoid administration, packed red blood cell (PRBC) transfusion], complications during hospitalization [i.e., IVH, retinopathy of prematurity (ROP), NEC], and outcomes.
Primary outcomes were the severity of BPD, HOT at discharge, and mortality between 28 days after birth and 2 years of age. Secondary outcomes were (1) duration of hospital stay; (2) duration of oxygen supplementation; (3) routine physical assessment; (4) wheezing disorders; and (5) clinical visits and rehospitalizations for a respiratory reason between discharge and follow-up. The long-term outcomes were evaluated until 2 years of age.
Statistical Analysis
Statistical analysis was performed using SPSS 26.0. We used the Shapiro-Wilk test to analyze the normality of the data distribution. Variables without normal distributions were analyzed using the Wilcoxon-Mann-Whitney test and are reported as medians [interquartile ranges (IQRs)]. All normally distributed variables were analyzed using a t-test for comparisons of two independent groups and are presented as the means ± standard deviations. Categorical variables were analyzed using the chi-square test or Fisher's exact tests and are reported as the numbers (%) of subjects.
To further evaluate the associations between typical chest imaging findings and the primary and secondary outcomes, a propensity score analysis with 1:1 matching was performed. The following covariates were included based on previous studies (22–26): GA, BW, cesarean section, CAM, PIH, sepsis, RDS, PDA, prenatal glucocorticoid administration, postnatal glucocorticoid administration, NEC, IVH, RSD, IMV ≥ 7 days, ≥2 PRBC transfusions. The nearest neighbor matching method was used to select each matched pair by using calipers with a width equal to 0.01 of the standard deviation of the logit of the propensity score. Typical chest imaging findings and the above 15 covariates were analyzed for collinearity and incorporated into the multivariate binary regression model, and the forward likelihood ratio (LR) was used to select the variables to further determine the independent factors of BPD infant mortality. A p-value < 0.05 was considered statistically significant.
Results
Subjects
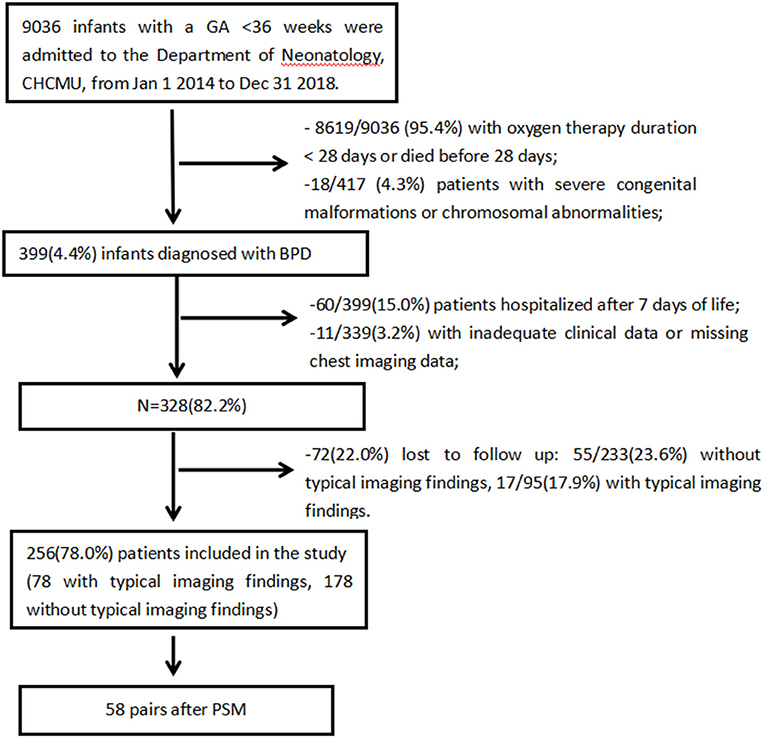
The inclusion process is shown in Figure 1. A total of 9036 preterm infants with GA <36 weeks were admitted to the Department of Neonatology, CHCMU, from January 1, 2014, to December 31, 2018. Among them, 399 (4.4%) infants were diagnosed with BPD. Then, infants who were hospitalized after 7 days of life with missing data and lost to follow-up were excluded. There was no significant difference in baseline characteristics between the included infants and those lost to follow-up, and there was no significant difference in the loss to follow-up rate between the two groups (17.9% (17/95) vs. 23.6% (55/233), p = 0.237). This showed that the loss to follow-up was random and would not affect outcomes significantly.
Ultimately, 256 preterm infants were enrolled; 78 (30.5%) had typical chest imaging findings, whereas 178 (69.5%) did not have typical imaging findings. Propensity score matching (PSM) systematically excluded infants who lacked a control group: those had lower GA, higher rates of sepsis, IMV ≥ 7 days and PRBC transfusion ≥2 times. After PSM, the matched groups consisted of 58 pairs of patients.
Information of Typical Imaging Findings
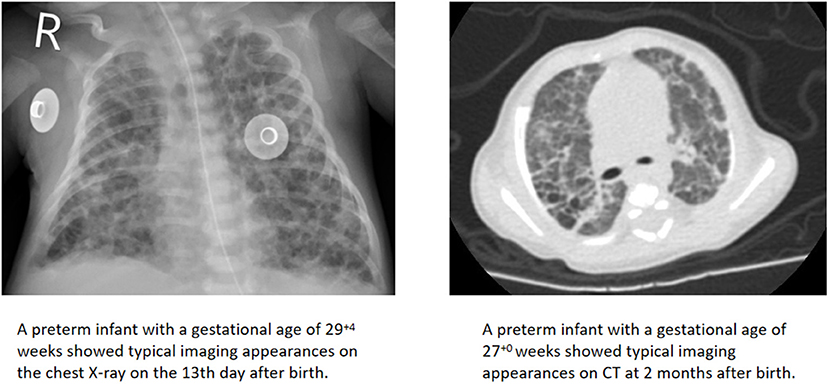
A total of 78 infants had typical imaging findings, of which 50 (64.1%) were first found by CXR, while 28 (35.9%) were first found by CT. In our unit, infants in the neonatal intensive care units (NICUs) had a chest radiograph at the bedside. CT scans required sedation and had the risk of respiratory depression, so they were performed when the child's condition was relatively stable. All chest imaging appearances were jointly assessed by two radiologists to identify whether the typical patterns of BPD were present (Figure 2). If there were disagreements, the decision was made through internal discussion in the department before the clinical report was issued.
This was a retrospective study without intervention and blinding, but radiologists assessing the imaging appearances were basically unaware of the outcomes of interest. As shown in Figure 3, 15.4% (12/78) of the typical imaging patterns were first discovered within 7 days of life, and 57.7% (45/78) were found before 28 days of life, when the diagnostic criteria for BPD had not been met. In addition, 85.9% (67/78) were found before 36 weeks postmenstrual age (PMA) (GA <32 weeks) or before 56 days after birth (GA > 32 weeks), when the severity of BPD was not evaluated. Thus, the radiologists were not aware of the primary outcomes. Only 14.1% (11/78) of the typical patterns were discovered after clinical evaluation of the severity of BPD; not surprisingly, these 11 cases were all CT scans. Radiologists may know the diagnosis, but they were unaware of the mortality. The review of CT appearances was more objective and detailed than CXR.
Clinical Characteristics of BPD Infants
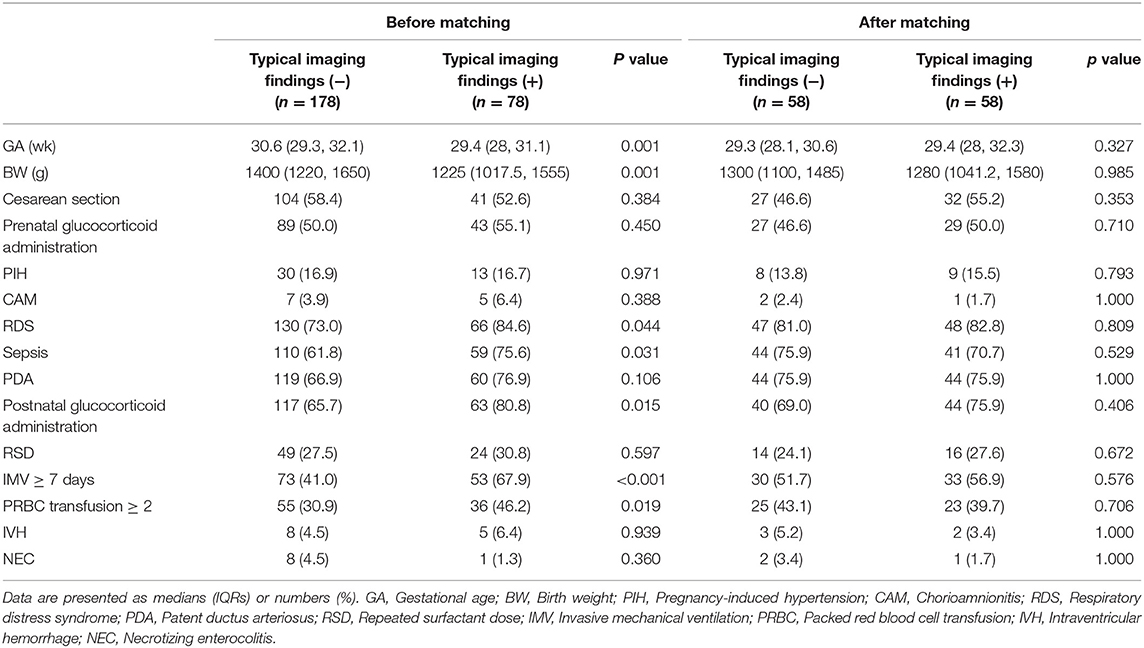
The patient characteristics of the two groups are shown in Table 1. Fifteen covariates were included in the propensity score-matched model. Before matching was implemented, seven out of the 15 covariates were significantly different between the two groups.
The typical imaging finding (+) group had a lower GA (median GA, 29.4 weeks vs. 30.6 weeks, p = 0.001) and BW (median BW, 1225 g vs. 1400 g, p = 0.001) than the typical imaging finding (-) group, which was in agreement with similar research results (27). With regard to perinatal factors, the typical imaging finding (+) group had a higher sepsis rate (75.6 vs. 61.8%, p = 0.031) and higher RDS rate (84.6 vs. 73.0%, p = 0.044) than the typical imaging finding (-) group. In terms of treatment, the typical imaging finding (+) group was more likely to require postnatal glucocorticoid administration (80.8 vs. 65.7%, P = 0.015), long-term IMV (67.9 vs. 41.0%, P < 0.001) and more PRBC transfusions (46.2 vs. 30.9%, P = 0.019) than the typical imaging finding (-) group. After PSM, all 15 covariates were well balanced, and no significant differences were observed between the groups (p > 0.05) (Table 1).
Primary and Secondary Outcomes
Outcomes are shown in Table 2. After PSM, the overall mortality in preterm infants with BPD was 20.7% (24 of 116), the proportion of severe BPD was 19.0% (22 of 116), and the rate of HOT at discharge was 60.3% (70 of 116). Patients with typical imaging findings demonstrated a remarkably higher mortality rate (29.3 vs. 12.1%, p = 0.022, OR 3.021, 95% CI 1.143–7.981). Infants with typical imaging findings differed significantly from the rest of the cohort in that they had a higher proportion of severe BPD (32.8 vs. 12.1%, p = 0.003, OR 4.669, 95% CI 1.723–12.652) and a higher rate of HOT at discharge (74.1 vs. 46.6%, p = 0.002, OR 3.291, 95% CI 1.506–7.195).
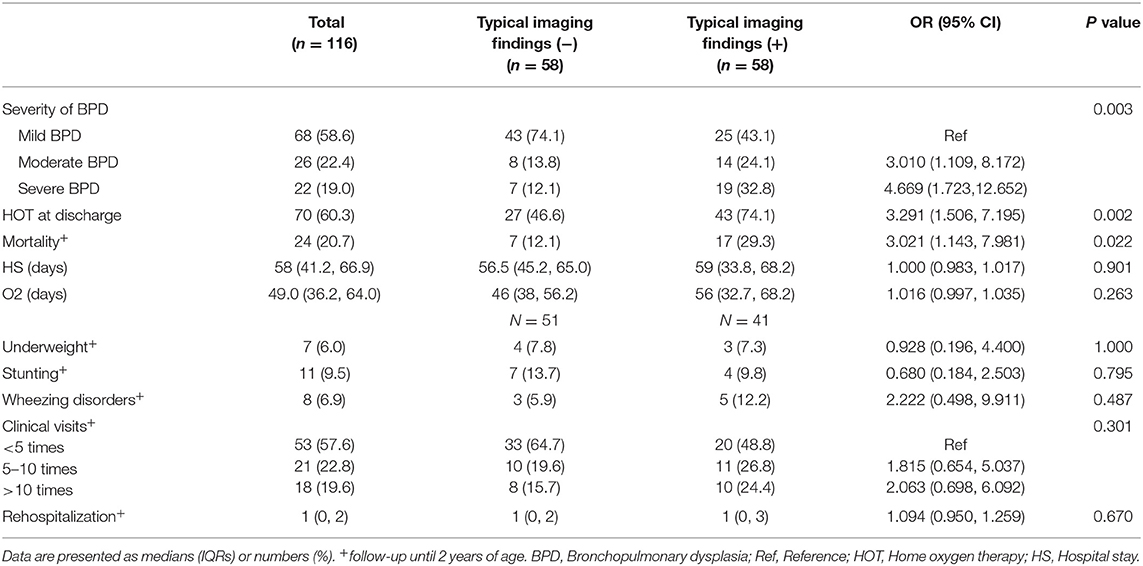
Table 2. Short-term outcomes and follow-up until 2 years of age of preterm infants in the typical imaging finding (±) groups after PSM.
No significant differences were observed between the two groups regarding secondary outcomes after matching. Overall, the study cohort showed a higher rate of stunting and underweight than the general population, but there was no significant difference between the two groups (p = 0.795 and 1.000, respectively). Comparisons of the duration of hospital stay and oxygen therapy as well as the incidence of wheezing disorders also revealed no significant differences between the groups (p = 0.901, 0.263 and 0.285, respectively). Moreover, the number of clinical visits and readmissions due to respiratory causes in the first two years were not significantly different between the matched groups (p = 0.301 and 0.670, respectively).
Independent Factors of Mortality
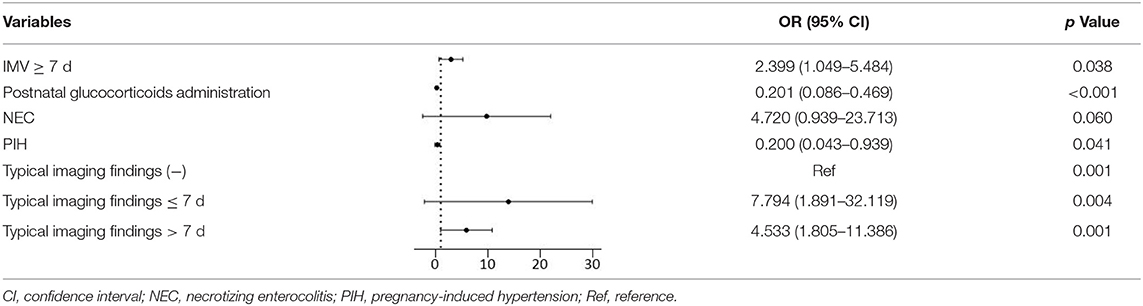
We completed the collinearity analysis and found that the variance inflation factors (VIFs) of all variables were <10, indicating that there was no obvious multicollinearity between the variables. After multivariate binary logistic regression analysis, the following variables were finally selected (Table 3). The factors associated with increased mortality were typical imaging findings found within 7 days and typical imaging findings after 7 days and IMV ≥ 7 days (OR 7.794, p = 0.004; OR 4.533, p = 0.001; OR 2.399, p = 0.038; respectively). NEC could be regarded as a risk factor for mortality with marginal statistical significance (OR 4.720, p = 0.060), whereas postnatal glucocorticoid administration and PIH could be regarded as protective factors against mortality (OR 0.201, p < 0.001; OR 0.200, p = 0.041; respectively).
Discussion
BPD is a prevalent respiratory complication of preterm birth. Radiography plays a critical role in the clinical evaluation of BPD (13, 28, 29). Our data demonstrated that typical imaging findings were associated with the severity of BPD, a higher rate of mortality and HOT at discharge. In particular, the typical imaging findings in the early stage (within 7 days) were closely related to mortality.
In the original definition of BPD proposed by pediatric radiologist Northway in 1967 (30), abnormal chest radiographic findings were classified into four stages according to lung pathology. Serial chest radiographs indicated the evolution of BPD. As the survival rate of early preterm infants increased, the radiologic changes were not as classic as Northway proposed because of changed pathophysiology (31). However, neonatologists still attached great importance to imaging confirmation in BPD (32). Fibrotic opacities/cystic appearances in chest radiographs are recognized as the typical radiographic pattern of BPD (33); these appearances correlate with disease severity and are relatively easy to recognize. The typical imaging findings after 36 weeks may not have an impact on the diagnosis and severity of BPD, but they are closely related to mortality and have certain indications for clinical management and treatment.
The clinical ability to reliably predict the duration of respiratory support, let alone the outcomes of infants with BPD, is not yet sufficient. Exploring additional factors related to BPD is the key to improving its treatment and prognosis. Previous studies have reported that the findings of chest radiographs, such as interstitial shadows, fibrosis or bubbly/cystic appearances, could predict the incidence and severity of BPD (34) and have an association with respiratory outcomes. Some infants with moderate or severe BPD may have typical radiographic changes within the first 7 days of life (35). Ren et al. (36) revealed that infants with BPD-related imaging changes required a longer duration of IMV and parenteral nutrition and were more likely to require HOT. However, these studies contained insufficient clinical data and did not adjust for confounding factors. Most of them only paid attention to the chest X-ray appearance within 7 days or after 28 days. In addition, these studies did not analyze long-term prognosis, such as mortality. Therefore, further studies are needed to identify the association between typical imaging findings and outcomes.
The mortality rate of infants with BPD varies among different NICUs. Our study demonstrated that BPD infants with typical imaging findings had a higher probability of developing moderate to severe BPD, as well as a higher mortality rate, than those without typical imaging findings. The overall mortality rate in infants with BPD was 15.2% (39/256). The relatively high mortality rate of BPD is partially due to deficiencies in my country's medical conditions, which leads to delays in individualized and effective treatment.
The multivariate logistic regression analysis showed that typical imaging findings ≤ 7 days and typical imaging findings >7 days were risk factors for mortality in preterm infants with BPD (OR 7.794, p = 0.004; OR 4.533, p = 0.001, respectively). The significant associations we demonstrated between typical imaging findings and the severity of BPD and mortality are in accordance with previous findings. Infants who had undergone IMV ≥ 7 days had a 2.399 (95% CI 1.049–5.484) times higher risk of mortality than those who had undergone IMV <7 days (37), while postnatal glucocorticoid administration and a maternal PIH history were protective factors against mortality (OR 0.201, p < 0.001; OR 0.200, p = 0.041; respectively). This was consistent with other studies (38, 39). Interestingly, the risk factors for mortality in infants with BPD did not include GA or BW. Further work is needed to determine the clinical reason for this result.
Our study found that BPD infants with typical imaging findings were significantly more likely to require HOT at discharge (74.1 vs. 46.6%). The rates of HOT were influenced by age at discharge and discharge policies (40, 41) and varied widely among hospitals. The higher overall incidence of HOT at discharge in this study may be due to the heavy financial burden on the family and the desire for an earlier discharge to facilitate infant bonding at home. This may be why there was no significant difference in hospital stay or duration of oxygen supplementation between the two groups. The target oxygen saturation of HOT is higher than the oxygen-dependent standard, so the ratio of HOT in our study was higher than the ratio of moderate to severe BPD (42).
Our study indicated that typical imaging findings were associated with a higher rate of wheezing disorders (12.2 vs. 5.9%). However, no significant difference was noted between the groups (p = 0.285). If the follow-up period was longer, it would not be surprising to find a positive conclusion. For example, Arai et al. demonstrated that bubbly/cystic appearance on CXR after 28 days in infants with BPD was a potential risk factor for wheezing disorders at 3 years of age (43). Other studies showed that older children and young adult survivors of BPD had an increased risk of asthma (44–46).
Previous studies (47, 48) have shown that infants with BPD were more than twice as likely to be hospitalized during the first year of life as those without BPD. Our data demonstrated that the presence of typical imaging findings did not significantly increase the rate of rehospitalization or the frequency of clinical visits during the first two years after birth. Compared with infants without typical imaging findings, BPD infants with typical imaging findings did not have a higher proportion of underweight or stunting. This may be reflective of changes and improvements in clinical characteristics, postnatal treatment and admission criteria, which allows determination of and confidence in the clinical treatment of patients with typical imaging findings.
The exact etiologies of fibrotic and cystic changes are unclear and are likely to be associated with intrauterine inflammation. Intrauterine infection and CAM are believed to induce fetal lung inflammation, which could interfere with lung development, resulting in BPD. A meta-analysis confirmed that among preterm infants, exposure to CMA was associated with an increased risk of developing BPD (49). Hirata et al. (22) also reported an association between a cystic/bubbly pattern and intrauterine inflammation. The correlation of typical imaging findings with CAM is clouded by the complexity of fetal exposure to inflammation. Additional studies are needed to show the relationship between typical chest imaging findings and CAM.
Some limitations of the study are as follows: (1) this is a retrospective study, which inevitably leads to loss of follow-up and clinical data. A standardized follow-up program has not been perfected in our unit, especially 3 years ago or even earlier. Poor follow-up compliance was related to different geographical origins of enrolled infants and parents' reluctance to travel a long distance. We routinely asked about the condition of the infant after being discharged from the hospital by phone. Some information was not easy to obtain, there was a certain probability of bias. Nevertheless, we tried our best to verify relevant information and only presented reliable data. (2) This is a single-center study of Chinese premature infants. Our unit did not perform the oxygen reduction test, causing some infants to receive a longer time of supplemental oxygen (50). There are some differences in the incidence and severity of some complications from other international studies (51). (3) We used propensity score matching to balance some of the known confounding variables between the groups, making the outcomes more comparable and the conclusion more reliable and stable. However, it is inevitable to pay the cost of missing samples. (4) In addition, we only evaluated the role of typical imaging findings in preterm infants with BPD. Perhaps some infants with similar chest imaging findings would not even have BPD at all. In the later stage, we could work with the radiology department to conduct a larger population with a wider gestational age study.
Despite these limitations, this study provides a new perspective for understanding chest imaging in BPD. Typical chest imaging findings combined with medical history and clinical characteristics can provide powerful help for early diagnosis and treatment.
Conclusion
This study provides important and fresh information that helps explain the potential harms that might be expected when typical imaging findings are noted in infants with BPD. In summary, our results demonstrated that typical imaging findings were significantly associated with the severity of BPD and a higher rate of mortality in this retrospective study. BPD infants with typical imaging findings also accounted for a higher proportion of infants requiring HOT at discharge.
To identify the influence of chest radiographic findings on long-term respiratory and neurodevelopmental outcomes, further prospective randomized controlled studies with larger samples and strict follow-up are needed.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the Institutional Review Board of Children's Hospital of Chongqing Medical University (2019-209). Written informed consent from the participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author Contributions
QR participated in the design of the study, collected clinical data, conducted statistical analyses, provided interpretation of data, drafted the initial manuscript, and reviewed the final manuscript as submitted. JW conceptualized and designed the study, provided interpretation of data, and revised the article for important intellectual content. YS conceptualized and supervised the design and execution of the study, reviewed analyses, and critically reviewed and revised the manuscript for important intellectual content. All authors contributed to the article and approved the final manuscript as submitted.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank Kaizhen Liu and Long Chen, Department of Neonatology, CHCMU, for their valuable expertise and their advice on data analysis.
Abbreviations
GA, Gestational age; BW, Birth weight; PIH, Pregnancy-induced hypertension; CAM, Chorioamnionitis; RDS, Respiratory distress syndrome; PDA, Patent ductus arteriosus; RSD, Repeated surfactant dose; IMV, Invasive mechanical ventilation; PRBC, Packed red blood cell; IVH, Intraventricular hemorrhage; NEC, Necrotizing enterocolitis; CI, Confidence interval; HOT, Home oxygen therapy; BPD, Bronchopulmonary dysplasia; ROP, Retinopathy of prematurity.
References
1. Stoecklin B, Simpson SJ, Pillow JJ. Bronchopulmonary dysplasia: rationale for a pathophysiological rather than treatment based approach to diagnosis. Paediatr Respir Rev. (2019) 32:91–7. doi: 10.1016/j.prrv.2018.12.002
2. Healy H, Croonen L, Onland W, van Kaam AH, Gupta M. A systematic review of reports of quality improvement for bronchopulmonary dysplasia. Semin Fetal Neonatal Med. (2021) 26:101201. doi: 10.1016/j.siny.2021.101201
3. Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. (2001) 163:1723–9. doi: 10.1164/ajrccm.163.7.2011060
4. Higgins RD, Jobe AH, Koso-Thomas M, Bancalari E, Viscardi RM, Hartert TV, et al. Bronchopulmonary dysplasia: executive summary of a workshop. J Pediatr. (2018) 197:300–8. doi: 10.1016/j.jpeds.2018.01.043
5. Moya MP, Bisset GS III, Auten RL Jr, Miller C, Hollingworth C, Frush DP. Reliability of CXR for the diagnosis of bronchopulmonary dysplasia. Pediatr Radiol. (2001) 31:339–42. doi: 10.1007/s002470000420
6. Wilson AC. What does imaging the chest tell us about bronchopulmonary dysplasia. Paediatr Respir Rev. (2010) 11:158–61. doi: 10.1016/j.prrv.2010.05.005
7. Walkup LL, Woods JC. Newer imaging techniques for bronchopulmonary dysplasia. Clin Perinatol. (2015) 42:871–87. doi: 10.1016/j.clp.2015.08.012
8. Arai H, Ito T, Ito M, Ota S, Takahashi T. Impact of chest radiography-based definition of bronchopulmonary dysplasia. Pediatr Int. (2019) 61:258–63. doi: 10.1111/ped.13786
9. Collaco JM, McGrath-Morrow SA. Bronchopulmonary dysplasia as a determinant of respiratory outcomes in adult life. Pediatr Pulmonol. (2021). doi: 10.1002/ppul.25301. [Epub ahead of print].
10. Meyer S, Franz AR, Bay J, Gortner L. Developing a better and practical definition of bronchopulmonary dysplasia. Acta Paediatr. (2017) 106:842. doi: 10.1111/apa.13783
11. Pirr S, Peter C. Home oxygen therapy after hospital discharge. Semin Fetal Neonatal Med. (2020) 25:101082. doi: 10.1016/j.siny.2020.101082
12. Shin SM, Kim WS, Cheon JE, Kim HS, Lee W, Jung AY, et al. Bronchopulmonary dysplasia: new high resolution computed tomography scoring system and correlation between the high resolution computed tomography score and clinical severity. Korean J Radiol. (2013) 14:350–60. doi: 10.3348/kjr.2013.14.2.350
13. Sung TJ, Hwang SM, Kim MY, Park SG, Choi KY. Relationship between clinical severity of “new” bronchopulmonary dysplasia and HRCT abnormalities in VLBW infants. Pediatr Pulmonol. (2018) 53:1391–8. doi: 10.1002/ppul.24121
14. Philip AG. Bronchopulmonary dysplasia: then and now. Neonatology. (2012) 102:1–8. doi: 10.1159/000336030
15. Shah SS, Ohlsson A, Halliday HL, Shah VS. Inhaled versus systemic corticosteroids for preventing bronchopulmonary dysplasia in ventilated very low birth weight preterm neonates. Cochrane Database Syst Rev. (2017) 10:CD002058. doi: 10.1002/14651858.CD002058.pub3
16. Kallio M, van der Zwaag AS, Waldmann AD, Rahtu M, Miedema M, Papadouri T, et al. Initial observations on the effect of repeated surfactant dose on lung volume and ventilation in neonatal respiratory distress syndrome. Neonatology. (2019) 116:385–9. doi: 10.1159/000502612
17. Subspecialty Group of Neonatology The Society of Pediatric Chinese Medical Association. Expert consensus on the diagnosis and management of neonatal sepsis (version 2019). Zhonghua Er Ke Za Zhi. (2019) 57:252–7. doi: 10.3760/cma.j.issn.0578-1310.2019.04.005
18. Chevallier M, Debillon T, Pierrat V, Delorme P, Kayem G, Durox M, et al. Leading causes of preterm delivery as risk factors for intraventricular hemorrhage in very preterm infants: results of the EPIPAGE 2 cohort study. Am J Obstet Gynecol. (2017) 216:518.e1–518.e12. doi: 10.1016/j.ajog.2017.01.002
19. Patel RM, Ferguson J, McElroy SJ, Khashu M, Caplan MS. Defining necrotizing enterocolitis: current difficulties and future opportunities. Pediatr Res. (2020) 88:10–5. doi: 10.1038/s41390-020-1074-4
20. Capital Institute of Pediatrics The Coordinating Study Group of Nine Cities on the Physical Growth and Development of Children. A national survey on physical growth and development of children under seven years of age in nine cities of China in 2015. Zhonghua Er Ke Za Zhi. (2018) 56:192–9. doi: 10.3760/cma.j.issn.0578-1310.2018.03.008
21. Wai KC, Hibbs AM, Steurer MA, Black DM, Asselin JM, Eichenwald EC, et al. Maternal black race and persistent wheezing illness in former extremely low gestational age newborns: secondary analysis of a randomized trial. J Pediatr. (2018) 198:201–8.e3. doi: 10.1016/j.jpeds.2018.02.032
22. Hirata K, Nishihara M, Shiraishi J, Hirano S, Matsunami K, Sumi K, et al. Perinatal factors associated with long-term respiratory sequelae in extremely low birthweight infants. Arch Dis Child Fetal Neonatal Ed. (2015) 100:F314–9. doi: 10.1136/archdischild-2014-306931
23. Lapcharoensap W, Gage SC, Kan P, Profit J, Shaw GM, Gould JB, et al. Hospital variation and risk factors for bronchopulmonary dysplasia in a population-based cohort. JAMA Pediatr. (2015) 169:e143676. doi: 10.1001/jamapediatrics.2014.3676
24. Principi N, Di Pietro GM, Esposito S. Bronchopulmonary dysplasia: clinical aspects and preventive and therapeutic strategies. J Transl Med. (2018) 16:36. doi: 10.1186/s12967-018-1417-7
25. Taglauer E, Abman SH, Keller RL. Recent advances in antenatal factors predisposing to bronchopulmonary dysplasia. Semin Perinatol. (2018) 42:413–24. doi: 10.1053/j.semperi.2018.09.002
26. Villeneuve A, Arsenault V, Lacroix J, Tucci M. Neonatal red blood cell transfusion. Vox Sang. (2021) 116:366–78. doi: 10.1111/vox.13036
27. Morita M, Tanaka K, Matsumura S, Tamura M, Namba F. Perinatal factors associated with bubbly/cystic appearance in bronchopulmonary dysplasia: a nationwide, population-based cohort study in Japan. J Matern Fetal Neonatal Med. (2021) 34:1221–6. doi: 10.1080/14767058.2019.1628945
28. Breysem L, Smet MH, Van Lierde S, Devlieger H, De Boeck K. Bronchopulmonary dysplasia: correlation of radiographic and clinical findings. Pediatr Radiol. (1997) 27:642–6. doi: 10.1007/s002470050203
29. Vanhaverbeke K, Van Eyck A, Van Hoorenbeeck K, De Winter B, Snoeckx A, Mulder T, et al. Lung imaging in bronchopulmonary dysplasia: a systematic review, Respir Med. (2020) 171:106101. doi: 10.1016/j.rmed.2020.106101
30. Northway WH Jr, Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N Engl J Med. (1967) 276:357–68. doi: 10.1056/NEJM196702162760701
31. Lignelli E, Palumbo F, Myti D, Morty RE. Recent advances in our understanding of the mechanisms of lung alveolarization and bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. (2019) 317:L832–887. doi: 10.1152/ajplung.00369.2019
32. Jensen EA, Wright CJ. Bronchopulmonary dysplasia: the ongoing search for one definition to rule them all. J Pediatr. (2018) 197:8–10. doi: 10.1016/j.jpeds.2018.02.047
33. Greenough A, Thomas M, Dimitriou G, Williams O, Johnson A, Limb E, et al. Prediction of outcome from the chest radiograph appearance on day 7 of very prematurely born infants. Eur J Pediatr. (2004) 163:14–8. doi: 10.1007/s00431-003-1332-6
34. Li R, Zhang J. Diagnostic value of chest CT combined with x-ray for premature infants with bronchopulmonary dysplasia. Medicine. (2018) 97:e9723. doi: 10.1097/MD.0000000000009723
35. Kim HR, Kim JY, Yun B, Lee B, Choi CW, Kim BI. Interstitial pneumonia pattern on day 7 chest radiograph predicts bronchopulmonary dysplasia in preterm infants. BMC Pediatr. (2017) 17:125. doi: 10.1186/s12887-017-0881-1
36. Ren YL, Kong XY, Du ZF, Feng ZC, Huang JJ, Chen J, et al. Clinical and imaging features of premature infants with different degrees of bronchopulmonary dysplasia. Zhongguo Dang Dai Er Ke Za Zhi. (2015) 17:440–4
37. Ramaswamy VV, Bandyopadhyay T, Nanda D, Bandiya P, Ahmed J, Garg A, et al. Assessment of postnatal corticosteroids for the prevention of bronchopulmonary dysplasia in preterm neonates: a systematic review and network meta-analysis. JAMA Pediatr. (2021) 175:e206826. doi: 10.1001/jamapediatrics.2020.6826
38. Doyle LW, Cheong JL, Ehrenkranz RA, Halliday HL. Early (<8 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst Rev. (2017) 10:CD001146. doi: 10.1002/14651858.CD001145.pub4
39. Razak A, Florendo-Chin A, Banfield L, Abdul Wahab MG, McDonald S, Shah PS, et al. Pregnancy-induced hypertension and neonatal outcomes: a systematic review and meta-analysis. J Perinatol. (2018) 38:46–53. doi: 10.1038/jp.2017.162
40. Ejiawoko A, Lee HC, Lu T, Lagatta J. Home oxygen use for preterm infants with bronchopulmonary dysplasia in California. J Pediatr. (2019) 210:55–62.e1. doi: 10.1016/j.jpeds.2019.03.021
41. Lau R, Crump RT, Brousseau DC, Panepinto JA, Nicholson M, Engel J, et al. Parent preferences regarding home oxygen use for infants with bronchopulmonary dysplasia. J Pediatr. (2019) 213:30–7.e3. doi: 10.1016/j.jpeds.2019.05.069
42. Jensen EA, Edwards EM, Greenberg LT, Soll RF, Ehret D, Horbar JD. Severity of Bronchopulmonary dysplasia among very preterm infants in the United States. Pediatrics. (2021) 148:e2020030007. doi: 10.1542/peds.2020-030007
43. Arai H, Ito M, Ito T, Ota S, Takahashi T. Bubbly and cystic appearance on chest radiograph of extremely preterm infants with bronchopulmonary dysplasia is associated with wheezing disorder. Acta Paediatr. (2020) 109:711–9. doi: 10.1111/apa.15008
44. Gough A, Linden M, Spence D, Patterson CC, Halliday HL, McGarvey LP. Impaired lung function and health status in adult survivors of bronchopulmonary dysplasia. Eur Respir J. (2014) 43:808–16. doi: 10.1183/09031936.00039513
45. Vom Hove M, Prenzel F, Uhlig HH, Robel-Tillig E. Pulmonary outcome in former preterm, very low birth weight children with bronchopulmonary dysplasia: a case-control follow-up at school age. J Pediatr. (2014) 164:40–5.e4. doi: 10.1016/j.jpeds.2013.07.045
46. Cheong J, Doyle LW. An update on pulmonary and neurodevelopmental outcomes of bronchopulmonary dysplasia. Semin Perinatol. (2018) 42:478–84. doi: 10.1053/j.semperi.2018.09.013
47. DeMauro SB, Jensen EA, Bann CM, Bell EF, Hibbs AM, Hintz SR, et al. Home oxygen and 2-year outcomes of preterm infants with bronchopulmonary dysplasia. Pediatrics. (2019) 143:e20182956. doi: 10.1542/peds.2018-2956
48. Lapcharoensap W, Bennett MV, Xu X, Lee HC, Dukhovny D. Hospitalization costs associated with bronchopulmonary dysplasia in the first year of life. J Perinatol. (2020) 40:130–7. doi: 10.1038/s41372-019-0548-x
49. Villamor-Martinez E, Álvarez-Fuente M, Ghazi A, Degraeuwe P, Zimmermann L, Kramer BW, et al. Association of chorioamnionitis with bronchopulmonary dysplasia among preterm infants: a systematic review, meta-analysis, and metaregression. JAMA Netw Open. (2019) 2:e1914611. doi: 10.1001/jamanetworkopen.2019.14611
50. Bamat N, Ghavam S, Liu Y, DeMauro SB, Jensen EA, Roberts R, et al. Reliability of a noninvasive measure of V./Q. Mismatch for bronchopulmonary dysplasia. Ann Am Thorac Soc. (2015) 12:727–33. doi: 10.1513/AnnalsATS.201410-462OC
Keywords: bronchopulmonary dysplasia, typical imaging findings, clinical characteristics, outcomes, preterm infants, propensity score
Citation: Ruan Q, Wang J and Shi Y (2021) Clinical Characteristics and Outcomes Until 2 Years of Age in Preterm Infants With Typical Chest Imaging Findings of Bronchopulmonary Dysplasia: A Propensity Score Analysis. Front. Pediatr. 9:712516. doi: 10.3389/fped.2021.712516
Received: 20 May 2021; Accepted: 30 July 2021;
Published: 23 August 2021.
Edited by:
Georg Schmölzer, University of Alberta, CanadaReviewed by:
Kenneth Tan, Monash University, AustraliaWes Onland, Amsterdam University Medical Center, Netherlands
Copyright © 2021 Ruan, Wang and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuan Shi, c2hpeXVhbkBob3NwaXRhbC5jcW11LmVkdS5jbg==
 Qiqi Ruan
Qiqi Ruan Jianhui Wang
Jianhui Wang Yuan Shi
Yuan Shi