
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 30 September 2021
Sec. Neonatology
Volume 9 - 2021 | https://doi.org/10.3389/fped.2021.711871
This article is part of the Research Topic Extremely Premature Infant Care: Advances in Prevention and Treatment Strategies View all 9 articles
 Wan-Heng Huang1,2
Wan-Heng Huang1,2 De-Ming Li1
De-Ming Li1 Chung-Ting Hsu1*
Chung-Ting Hsu1* Yi-Hsuan Lin1
Yi-Hsuan Lin1 Ya-Chi Hsu1
Ya-Chi Hsu1 Teh-Ming Wang1
Teh-Ming Wang1 Ming-Chih Lin1,3,4,5,6
Ming-Chih Lin1,3,4,5,6Background: In extremely low birth weight (ELBW) infants, the patent ductus arteriosus (PDA) with left-to-right shunt and an increase in systemic artery resistance may cause increasing preload and afterload of the left ventricle. The immature myocardium in ELBW infants has a limited ability to respond to the change, which leads to hemorrhagic complications. In this study, we detected the hemodynamic change of cardiac performance and applied a clinical strategy to prevent PDA-associated hemorrhagic complications in ELBW infants.
Methods: We enrolled ELBW infants at a single medical center in Taiwan. The customized circulatory management was performed by echocardiography after birth until the PDA closed. Inotropic agents were administrated according to the requirements of hemodynamic parameters or clinical conditions. The primary outcomes were hemorrhagic complications including pulmonary hemorrhage and intraventricular hemorrhage (IVH) greater than grade II. The secondary outcomes were the rate of surgical ligation of PDA, mortality, necrotizing enterocolitis, and bronchopulmonary dysplasia.
Results: A total of 20 ELBW infants were evaluated by customized circulatory management from 2019 to 2020. We reviewed 35 ELBW infants born between 2017 and 2018 in our hospital, who served as the non-management group. The management group had a significantly lower incidence rate of IVH greater than grade 2 (p = 0.02). Other outcomes showed no significant differences. Dobutamine was prescribed in 8 cases in the management group, and end-systolic wall stress (ESWS) was significantly decreased after Dobutamine administration (p = 0.017).
Conclusion: The incidence rate of IVH greater than grade II in ELBW infants decreased after use of customized circulatory management in our study. The strategy of customized circulatory management might be an effective “early target therapy” for hemodynamically significant PDA in high-risk ELBW infants. Inotropic therapy with Dobutamine could be a useful medical choice for improving cardiac function to prevent hemorrhagic complications.
In pre-term infants, lower gestational age is related to lower incidence of spontaneous closure of the patent ductus arteriosus (PDA). It is more common in extremely low birth weight (ELBW) infants who have a gestational age of <28 weeks or a birth body weight <1,000 grams. Spontaneous closure of PDA occurs in less than one third of ELBW infants at day 7 after birth (1, 2). The pulmonary vascular resistance declines in the first few days of life, and then the blood flow of PDA shifts to left-to-right shunt. Increased pulmonary blood flow causes pulmonary edema and pulmonary congestion, eventually resulting in respiratory failure (3, 4). The hemodynamic change with increasing pulmonary blood flow and decreasing systemic blood flow also induces numerous clinical symptoms (5–8). The major complications of hemodynamically significant PDA has been reported, such as intraventricular hemorrhage (IVH), pulmonary hemorrhage, necrotizing enterocolitis (NEC), bronchopulmonary dysplasia (BPD), and mortality (3, 4, 6, 8–14).
A number of studies have investigated the hemodynamic change between fetus and newborn. According to previous research, the placenta has the lowest vascular resistance in the fetal stage. The resistance in the systemic artery increases suddenly after birth because of an interruption from the placenta. It causes an increase in the afterload of the left ventricle (15–17). An increase in the pre-load of left ventricle was also observed due to the left-to-right shunt in blood flow of PDA during the decline of pulmonary vascular resistance (7, 18). The immature myocardium, especially in ELBW infants, has a limited response to the increase in preload and afterload. It could induce hemorrhagic complications, including IVH and pulmonary hemorrhage (3, 6). Many echocardiographic parameters have been used to evaluate the hemodynamic change in pre-term infants. However, the stress-velocity relationship is a relatively independent index for evaluation of cardiac function (3, 6, 17, 19–21). Toyoshima et al. used customized circulatory management to evaluate cardiac function based on the stress-velocity relationship aimed at decreasing the mortality rate and incidence of severe IVH in ELBW infants (3). In our study, we used a similar circulatory management and clinical strategy to evaluate the hemodynamic change of PDA, and tried to improve our PDA-associated outcomes in ELBW infants.
This was a prospective cohort study at a single medical center in Taiwan. We enrolled extremely low birth body weight (ELBW) infants with birth weight of <1,000 g born at the neonatal intensive care unit (NICU) of Taichung Veterans General Hospital between 1 January, 2019 and 31 December, 2020. Babies with chromosomal abnormalities, complex congenital heart diseases, or multiple anomalies were excluded. All infants' demographic data including gender, gestational age, birth body weight, Apgar scores at the 1st and 5th min, percentage of body weight loss after birth, Ibuprofen usage, Surfactant usage, inotropes usage, intubation, days of intubation, post-menstrual age (PMA), and body weight at discharge were collected. This study design and protocol were approved by the Institutional Review Board of Taichung Veterans General Hospital. All participants' parents had to sign the informed consent before the study.
We collected the cardiac parameters by echocardiography and clinical data every 12 h after birth until PDA closed. Because the closure of PDA of ELBW infants was variable clinically, we set the endpoint of our study as PDA closure was documented by echocardiography in two consecutive measurements. The measured cardiac parameters in customized circulatory management included left ventricular internal dimension-diastole (LVIDd, cm), left ventricular internal dimension-systole (LVIDs, cm), posterior wall thickness at end-systole (Hes, cm), left ventricular ejection time (ET, sec), RR interval (RR, sec), and end-systolic pressure (Pes, mmHg). The left ventricle rate-corrected mean velocity of circumferential fiber shortening (mVcfc, circ/s) and end-systolic wall stress (ESWS, g/cm2) were calculated using the formulae for these multiple cardiac parameters (3, 22, 23). The mVcfc was an index of left ventricular pumping function. The ESWS was an index of left ventricular afterload or systemic vascular resistance (3, 22, 23). We followed a series of values of mVcfc and ESWS in every infant enrolled in the trial.
In our NICU, general measures provided to the ELBW infants included 90% humidified incubator in the first week, initial daily fluid with 80 ml/kg per day, adjusting amount of intravenous fluid according to body weight every 8 h, and minimal ventilatory support ensuring adequate oxygenation (pulse oximetry saturation between 90 and 95 percent) and allowing permissive hypercapnia (partial pressure of carbon dioxide between 40 and 60 mmHg in arterial blood). According to previous research, appropriate cardiac function in ELBW infants was described as the value of mVcfc above 0.8 circ/s and ESWS was below 40 g/cm2 (3). Inotropic therapy using Dobutamine with an initial dose of 2 μg/kg/min was administrated if mVcfc < 0.8 circ/s, ESWS > 40 g/cm2, or an upward trend of ESWS was observed during measurement. Inotropic therapy using Dopamine was administrated when mean artery pressure was lower than normal for the gestational age.
The hemodynamically significant PDA was defined according to clinical manifestations or echocardiographic parameters in our NICU. The clinical findings included systolic heart murmur, hyperactive precordial pulsation, bounding pulses, persistent tachycardia, persistent tachypnea, cardiomegaly, or hepatomegaly. The echocardiographic findings included left atrial or left ventricular dilatation, reversed diastolic flow in descending aorta, mVcfc < 0.8 circ/s, or ESWS > 40 g/cm2. For those infants who had a hemodynamically significant PDA, pharmacologic intervention with a course of Ibuprofen was given. If the patients failed to respond to an initial course of therapy, administration of the second course of Ibuprofen was considered clinically. The surgical ligation of PDA was prescribed as rescue management if the patients failed to respond to two courses of medical treatment or suffered one of the serious complications, including progressive anuria, progressive hypotension, NEC, severe IVH, or pulmonary hemorrhage.
The primary outcomes were the rates of hemorrhage complications including pulmonary hemorrhage and IVH greater than grade II. The additional outcomes were the rates of surgical ligation of PDA, BPD, definite NEC, and mortality. The BPD was defined as a single entity of oxygen requirement either at 28 postnatal days or 36 weeks postmenstrual age (24). Definite NEC was defined as NEC with modified Bell's stage ≥ IIA clinically (25).
Continuous data were expressed as mean and standard deviation, and categorical data were expressed as frequency and percentage. Continuous data were compared using the Mann-Whitney U-test, and categorical data were compared using Pearson's chi-square test. Fisher's exact test was applied if any cell of categorical data was <5. The Wilcoxon rank-sum test was used to compare the values of mVcfc and ESWS before and after Dobutamine administration. Statistical significance was defined as a p < 0.05. Analyses were performed using statistical software IBM SPSS Statistics for Windows, version 25.0. (Armonk, NY: IBM Corp.).
A total of 22 ELBW infants were admitted to the NICU of Taichung Veterans General Hospital from January 1, 2019 to December 31, 2020. We excluded 2 cases because both of them expired within 48 h after birth. One suffered from septic shock because of chorioamnionitis, and the other suffered tension pneumothorax. The causes of death were not associated with PDA in both cases. A total of 20 cases that received customized circulatory management during the study period were enrolled. We also retrospectively reviewed ELBW infants who were admitted to the NICU of Taichung Veterans General Hospital from January 1, 2017 to December 31, 2018 using the same enrolment criteria and these infants served as the non-management group (the control group). One case was excluded due to congenital heart disease. There were 35 cases in the non-management group, which were included in the final analysis. The study flowchart is shown in Figure 1. The demographic data are shown in Table 1. Dobutamine was administrated in 8 of 20 cases in the management group, and none of the cases in the non-management group. There were no significant differences in gender, Ibuprofen usage, Surfactant usage, Dopamine usage, intubation, days of intubation, gestational age, birth body weight, Apgar scores at the 1st and 5th min, percentage of body weight loss after birth, postmenstrual age, and body weight at discharge between the two groups.
Pulmonary hemorrhage occurred in 4 of 35 cases (11.4%) in the non-management group and 2 of 20 cases (10.0%) in the management group (p = 1.00). Intraventricular hemorrhage greater than grade II was noted in 12 of 35 cases (34.3%) in the non-management group and in 1 of 20 cases (5.0%) in the management group. The rate of IVH higher than grade II decreased significantly in the management group (p = 0.02). The additional outcomes were not significantly different between the two groups in rates of mortality (p = 0.18), surgical ligation of PDA (p = 0.52), NEC greater than stage 2 (p = 1.00), and BPD (p = 0.24; Table 2).
There were 8 cases (8/20) using Dobutamine administration in the management group due to mVcfc < 0.8 circ/s, ESWS > 40 g/cm2, or an upward trend of ESWS during the study period. After initiating Dobutamine, the dosage would be adjusted according to the value of ESWS in the next measurement. If the value of ESWS increased, the dosage would be titrated up by 2 μg/kg/min. If the value of ESWS decreased or remained constant, we would keep the current dosage until PDA closed. Figure 2 showed all longitudinal values of mVcfc in the management group. Almost all values of mVcfc at any time were above 0.8 circ/s whether Dobutamine was administrated or not. Figure 3 showed all longitudinal values of ESWS in the management group. In 8 cases with Dobutamine administration, ESWS > 40 g/cm2 or an upward trend of ESWS was observed before using Dobutamine, and ESWS showed a decreasing trend in almost all cases after using Dobutamine. On the contrary, ESWS < 40 g/cm2 or a downward trend of ESWS was observed as time went on in 12 cases without Dobutamine administration. The value of mVcfc and ESWS were recorded before and after Dobutamine administration in each case. We used the Wilcoxon rank sum test for further analysis. There was no significant difference in value of mVcfc before and after Dobutamine usage (p = 0.26). However, value of ESWS decreased significantly before and after Dobutamine usage (p = 0.017; Figure 4).

Figure 2. Longitudinal values of mVcfc in the management group. Twenty cases were divided into two subgroups: 8 cases with Dobutamine administration and 12 cases without Dobutamine administration. X-axis showed hours after birth, and Y-axis showed the value of mVcfc. ▴ stood for the timing of initiating Dobutamine administration.
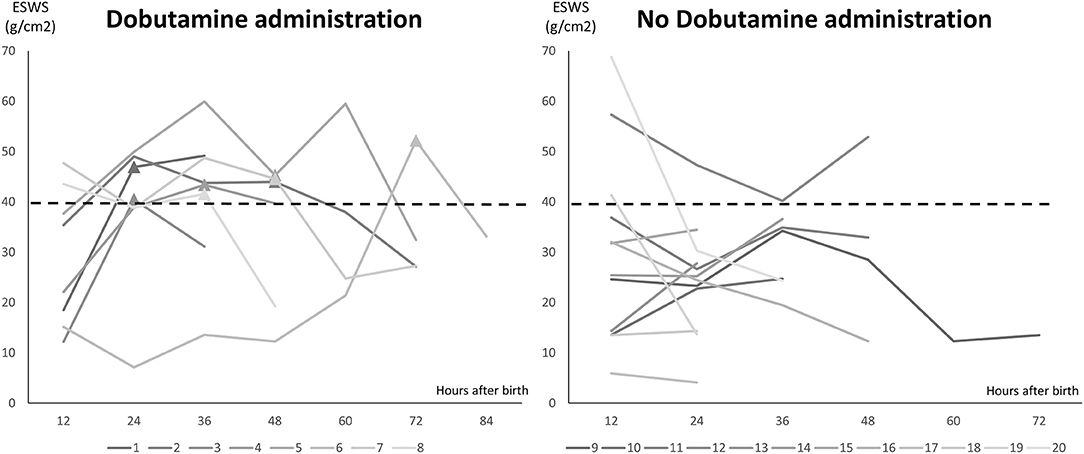
Figure 3. Longitudinal values of ESWS in the management group. Twenty cases were divided into two subgroups: 8 cases with Dobutamine administration and 12 cases without Dobutamine administration. X-axis showed hours after birth, and Y-axis showed the value of ESWS. ▴ stood for the timing of initiating Dobutamine administration.
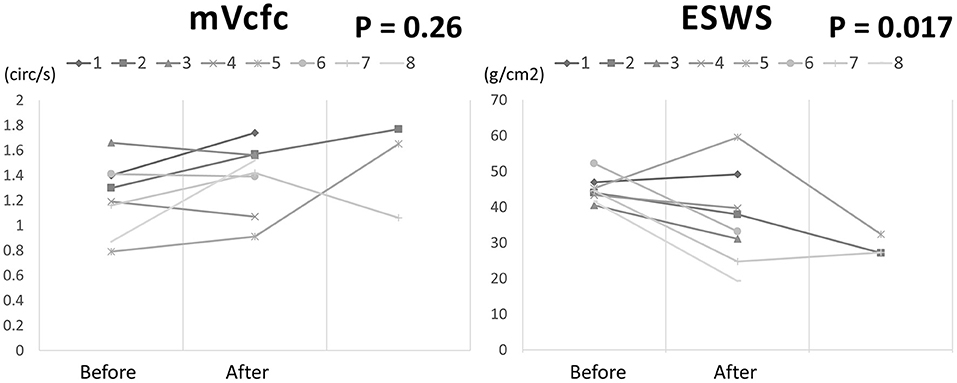
Figure 4. The changing of mVcfc and ESWS before and after Dobutamine administration in 8 cases of the management group.
In our study, we found that the incidence of IVH greater than grade II in ELBW infants declined after implementing customized circulatory management in our NICU. The other outcomes including pulmonary hemorrhage, surgical ligation of PDA, definite NEC, BPD, and mortality showed no difference between the management group and the non-management group. Dobutamine administration might be able to reduce the afterload of the left ventricle, but there was no improvement in the pumping function of the left ventricle in our study. Toyoshima et al. (3) reported introduction of customized circulatory management in ELBW infants could reduce IVH both in incidence and in severity. They found Dobutamine administration might deteriorate cardiac pumping function and even increase systemic vascular resistance in half of their patients. On the contrary, they used intravenous Nitroglycerin to reduce ESWS and increase mVcfc effectively in most patients. The intravenous form of nitroglycerin was not available in our hospital. However, ESWS reduced significantly after Dobutamine administration in our clinical practice. In our opinion, Dobutamine might be another choice for decreasing systemic vascular resistance in ELBW infants.
The recent trend of PDA management in ELBW infants is “early target treatment,” which aims to identify a hemodynamically significant PDA in specific high-risk populations as early as possible (26–30). Our study proved customized circulatory management might be an effective “early target treatment.” In clinical practice, we identified high-risk ELBW infants who had lower mVcfc or higher ESWS, and prescribed inotropic therapy with Dobutamine administration. Using a series of cardiac parameters (mVcfc and ESWS) measured by echocardiography after birth, we were able to determine an adequate timing and indication to intervene with medical treatment for PDA in ELBW infants.
The mechanism of the association between IVH and increased afterload of the left ventricle in ELBW infants was proposed in previous studies (3, 6). To deal with an increased afterload, the preload of the left ventricle is increased due to preload reserve (15, 31). Increased preload of the left ventricle may result in increased preload of the right ventricle because of tight ventricle interaction. An increase in preload of right ventricle leads to elevated central venous pressure and elevated cerebral venous pressure. As a consequence, the fragile vein around the germinal matrix is not able to tolerate the increased cerebral venous pressure, which causes IVH (3, 31–35). In addition, the left ventricle of the pre-term infant has low distensibility (15, 31), which results in increased pulmonary venous pressure and pulmonary congestion during elevation of preload of the left ventricle, ultimately leading to pulmonary hemorrhage (36–38).
A previous study reported grade II IVH in pre-term infants still leads to post-hemorrhagic hydrocephalus (39). The low-grade IVH may also affect brain tissue structure and long-term neurodevelopmental outcomes (40–42). It is for this reason we recognized IVH greater than grade II as one of primary outcomes in our study.
There were certain limitations in the study. In general, echocardiography is a highly operator-dependent skill. A previous study showed the inter-observer repeatability of echocardiographic parameters was poor (43). Operator error may have affected the accuracy of the collected data. Second, this was a single center study. Customized circulatory management was implemented for ELBW infants in our NICU during a 2-year period, which resulted in a small sample size. The effects of this treatment modality on long-term neurodevelopment and growth trends could therefore not be established in our study. Further studies with a larger sample size and a longer follow-up period are necessary.
In conclusion, using customized circulatory management might decrease the incidence of intraventricular hemorrhage in extremely low birth weight infants. Early inotropic therapy with Dobutamine administration might be helpful to reduce the afterload of the left ventricle in extremely low birth weight infants. This strategy of customized circulatory management might be an effective “early target therapy” to avoid serious hemorrhage complications of PDA in the first few days after birth. Because of the small sample size in our study, further research with larger participation is needed in the future.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
M-CL: conceptualization. W-HH and D-ML: data curation. W-HH: formal analysis and writing—original draft. C-TH: methodology and writing—review and editing. All authors contributed to the article and approved the submitted version.
The study was supported by Taichung Veterans General Hospital research fund (TCVGH-110DHA0500728).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2021.711871/full#supplementary-material
1. Clyman RI, Couto J, Murphy GM. Patent ductus arteriosus: are current neonatal treatment options better or worse than no treatment at all? Semin Perinatol. (2012) 36:123–9. doi: 10.1053/j.semperi.2011.09.022
2. Chiruvolu A, Punjwani P, Ramaciotti C. Clinical and echocardiographic diagnosis of patent ductus arteriosus in premature neonates. Early Hum Dev. (2009) 85:147–9. doi: 10.1016/j.earlhumdev.2008.12.008
3. Toyoshima K, Kawataki M, Ohyama M, Shibasaki J, Yamaguchi N, Hoshino R, et al. Tailor-made circulatory management based on the stress-velocity relationship in preterm infants. J Formos Med Assoc. (2013) 112:510–7. doi: 10.1016/j.jfma.2013.02.011
4. Benitz WE, Committee on Fetus and Newborn American Academy of Pediatrics. Patent ductus arteriosus in preterm infants. Pediatrics. (2016) 137:e20153730. doi: 10.1542/peds.2015-3730
5. Zonnenberg I, de Waal K. The definition of a haemodynamic significant duct in randomized controlled trials: a systematic literature review. Acta Paediatr. (2012) 101:247–51. doi: 10.1111/j.1651-2227.2011.02468.x
6. Su BH, Lin HY, Huang FK, Tsai ML, Huang YT. Circulatory management focusing on preventing intraventricular hemorrhage and pulmonary hemorrhage in preterm infants. Pediatr Neonatol. (2016) 57:453–62. doi: 10.1016/j.pedneo.2016.01.001
7. Lee CH, Chen HN, Tsao LY, Hsiao CC, Lee ML. Oral ibuprofen versus intravenous indomethacin for closure of patent ductus arteriosus in very low birth weight infants. Pediatr Neonatol. (2012) 53:346–53. doi: 10.1016/j.pedneo.2012.08.011
8. Benitz WE. Treatment of persistent patent ductus arteriosus in preterm infants: time to accept the null hypothesis? J Perinatol. (2010) 30:241–52. doi: 10.1038/jp.2010.3
9. Clyman RI, Chorne N. Patent ductus arteriosus: evidence for and against treatment. J Pediatr. (2007) 150:216–9. doi: 10.1016/j.jpeds.2006.12.048
10. Dice JE, Bhatia J. Patent ductus arteriosus: an overview. J Pediatr Pharmacol Ther. (2007) 12:138–46. doi: 10.5863/1551-6776-12.3.138
11. Ballabh P. Intraventricular hemorrhage in premature infants: mechanism of disease. Pediatr Res. (2010) 67:1–8. doi: 10.1203/PDR.0b013e3181c1b176
12. Garland J, Buck R, Weinberg M. Pulmonary hemorrhage risk in infants with a clinically diagnosed patent ductus arteriosus: a retrospective cohort study. Pediatrics. (1994) 94:719–23.
13. Cunha GS, Mezzacappa-Filho F, Ribeiro JD. Risk factors for bronchopulmonary dysplasia in very low birth weight newborns treated with mechanical ventilation in the first week of life. J Trop Pediatr. (2005) 51:334–40. doi: 10.1093/tropej/fmi051
14. Dollberg S, Lusky A, Reichman B. Patent ductus arteriosus, indomethacin and necrotizing enterocolitis in very low birth weight infants: a population-based study. J Pediatr Gastroenterol Nutr. (2005) 40:184–8. doi: 10.1097/00005176-200502000-00019
15. Romero TE, Friedman WF. Limited left ventricular response to volume overload in the neonatal period: a comparative study with the adult animal. Pediatr Res. (1979) 13:910–5. doi: 10.1203/00006450-197908000-00008
16. Takahashi Y, Harada K, Kishkurno S, Arai H, Ishida A, Takada G. Postnatal left ventricular contractility in very low birth weight infants. Pediatr Cardiol. (1997) 18:112–7. doi: 10.1007/s002469900127
17. Toyono M, Harada K, Takahashi Y, Takada G. Maturational changes in left ventricular contractile state. Int J Cardiol. (1998) 64:247–52. doi: 10.1016/S0167-5273(98)00047-3
18. Hsiao CC, Wung JT, Tsao LY, Chang WC. Early or late surgical ligation of medical refractory patent ductus arteriosus in premature infants. J Formos Med Assoc. (2009) 108:72–7. doi: 10.1016/S0929-6646(09)60035-8
19. Kimball TR, Daniels SR, Khoury P, Meyer RA. Age-related variation in contractility estimate in patients less than or equal to 20 years of age. Am J Cardiol. (1991) 68:1383–7. doi: 10.1016/0002-9149(91)90250-O
20. Kimball TR, Daniels SR, Loggie JM, Khoury P, Meyer RA. Relation of left ventricular mass, preload, afterload and contractility in pediatric patients with essential hypertension. J Am Coll Cardiol. (1993) 21:997–1001. doi: 10.1016/0735-1097(93)90359-9
21. Colan SD. Nonlinearity of left ventricular end-systolic wall stress-velocity of fiber shortening relation. J Am Coll Cardiol. (1994) 24:1178–80. doi: 10.1016/0735-1097(94)90885-0
22. Colan SD, Borow KM, Neumann A. Left ventricular end-systolic wall stress-velocity of fiber shortening relation: a load-independent index of myocardial contractility. J Am Coll Cardiol. (1984) 4:715–24. doi: 10.1016/S0735-1097(84)80397-6
23. Lang RM, Borow KM, Neumann A, Janzen D. Systemic vascular resistance: an unreliable index of left ventricular afterload. Circulation. (1986) 74:1114–23. doi: 10.1161/01.CIR.74.5.1114
24. Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. (2001) 163:1723–9. doi: 10.1164/ajrccm.163.7.2011060
25. Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am. (1986) 33:179–201. doi: 10.1016/S0031-3955(16)34975-6
26. Su BH, Lin HY, Chiu HY, Tsai ML, Chen YT, Lu IC. Therapeutic strategy of patent ductus arteriosus in extremely preterm infants. Pediatr Neonatol. (2020) 61:133–41. doi: 10.1016/j.pedneo.2019.10.002
27. Clyman RI, Liebowitz M, Kaempf J, Erdeve O, Bulbul A, Hakansson S, et al. PDA-TOLERATE Trial: an exploratory randomized controlled trial of treatment of moderate-to-large patent ductus arteriosus at 1 week of age. J Pediatr. (2019) 205:41–8. doi: 10.1016/j.jpeds.2018.09.012
28. Smith IJ, Goss I, Congdon PJ. Intravenous indomethacin for patent ductus arteriosus. Arch Dis Child. (1984) 59:537–41. doi: 10.1136/adc.59.6.537
29. Clyman RI, Saha S, Jobe A, Oh W. Indomethacin prophylaxis for preterm infants: the impact of 2 multicentered randomized controlled trials on clinical practice. J Pediatr. (2007) 150:46–50. doi: 10.1016/j.jpeds.2006.09.001
30. Mitra S, Scrivens A, von Kursell AM, Disher T. Early treatment versus expectant management of hemodynamically significant patent ductus arteriosus for preterm infants. Cochrane Database Syst Rev. (2020) 12:CD013278. doi: 10.1002/14651858.CD013278.pub2
31. Friedman WF. The intrinsic physiologic properties of the developing heart. Prog Cardiovasc Dis. (1972) 15:87–111. doi: 10.1016/0033-0620(72)90006-0
32. Moody DM, Brown WR, Challa VR, Block SM. Alkaline phosphatase histochemical staining in the study of germinal matrix hemorrhage and brain vascular morphology in a very-low-birth-weight neonate. Pediatr Res. (1994) 35:424–30. doi: 10.1203/00006450-199404000-00008
33. Ghazi-Birry HS, Brown WR, Moody DM, Challa VR, Block SM, Reboussin DM. Human germinal matrix: venous origin of hemorrhage and vascular characteristics. AJNR Am J Neuroradiol. (1997) 18:219–29.
34. Volpe JJ. Brain injury in the premature infant: overview of clinical aspects, neuropathology, and pathogenesis. Semin Pediatr Neurol. (1998) 5:135–51. doi: 10.1016/S1071-9091(98)80030-2
35. Toyoshima K, Isayama T, Kobayashi T, Su C, Mikami M, Yokoyama T, et al. What echocardiographic indices are predictive of patent ductus arteriosus surgical closure in early preterm infants? A prospective multicenter cohort study. J Cardiol. (2019) 74:512–8. doi: 10.1016/j.jjcc.2019.05.004
36. Lin TW, Su BH, Lin HC, Hu PS, Peng CT, Tsai CH, et al. Risk factors of pulmonary hemorrhage in very-low-birth-weight infants: a two-year retrospective study. Acta Paediatr Taiwan. (2000) 41:255–8. doi: 10.7097/APT.200010.0255
37. Su BH, Lin HY, Huang FK, Tsai ML. Pulmonary hemorrhage in very-low-birth-weight infants. Pediatr Neonatol. (2014) 55:326–7. doi: 10.1016/j.pedneo.2014.01.005
38. Scholl JE, Yanowitz TD. Pulmonary hemorrhage in very low birth weight infants: a case-control analysis. J Pediatr. (2015) 166:1083–4. doi: 10.1016/j.jpeds.2014.12.032
39. Krishnamoorthy KS, Kuehnle KJ, Todres ID, DeLong GR. Neurodevelopmental outcome of survivors with posthemorrhagic hydrocephalus following Grade II neonatal intraventricular hemorrhage. Ann Neurol. (1984) 15:201–4. doi: 10.1002/ana.410150215
40. Bolisetty S, Dhawan A, Abdel-Latif M, Bajuk B, Stack J, Lui K, et al. Intraventricular Hemorrhage and neurodevelopmental outcomes in extreme preterm infants. Pediatrics. (2014) 133:55–62. doi: 10.1542/peds.2013-0372
41. Argyropoulou MI, Astrakas LG, Xydis VG, Drougia A, Mouka V, Goel I, et al. Is low-grade intraventricular hemorrhage in very preterm infants an innocent condition? Structural and functional evaluation of the brain reveals regional neurodevelopmental abnormalities. AJNR Am J Neuroradiol. (2020) 41:542–7. doi: 10.3174/ajnr.A6438
42. Pfahl S, Hoehn T, Lohmeier K, Richter-Werkle R, Babor F, Schramm D, et al. Long-term neurodevelopmental outcome following low grade intraventricular hemorrhage in premature infants. Early Hum Dev. (2018) 117:62–7. doi: 10.1016/j.earlhumdev.2017.12.013
Keywords: extremely low birth weight infants (ELBW infants), patent ductus arteriosus (PDA), Intraventricular hemorrhage (IVH), customized circulatory management, dobutamine
Citation: Huang W-H, Li D-M, Hsu C-T, Lin Y-H, Hsu Y-C, Wang T-M and Lin M-C (2021) Decreased Incidence of Intraventricular Hemorrhage in Extremely Low Birth Weight Infants Using Customized Circulatory Management to Evaluate the Hemodynamic Change of Patent Ductus Arteriosus. Front. Pediatr. 9:711871. doi: 10.3389/fped.2021.711871
Received: 19 May 2021; Accepted: 06 September 2021;
Published: 30 September 2021.
Edited by:
Giovanni Vento, Catholic University of the Sacred Heart, ItalyReviewed by:
Jiang-Qin Liu, Shanghai First Maternity and Infant Hospital, ChinaCopyright © 2021 Huang, Li, Hsu, Lin, Hsu, Wang and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chung-Ting Hsu, bG9uZ3R5ZTAxNEBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.