- 1Indiana University School of Medicine, Indianapolis, IN, United States
- 2Academic Model Providing Access to Healthcare, Eldoret, Kenya
- 3Department of Child Health, College of Health Sciences, Moi University, Eldoret, Kenya
- 4Department of Global Health, Icahn School of Medicine at Mount Sinai, New York, NY, United States
- 5Arnhold Institute for Global Health, New York, NY, United States
Introduction: Over 15 million children who were exposed to HIV perinatally but uninfected (HEU) are alive globally, and they are faced with multiple risk factors for poor neurodevelopment. While children who are HIV-infected (HIV+) appear to have worse neurodevelopmental scores compared to children unexposed and uninfected with HIV (HUU), the evidence is mixed in children who are HEU. This small descriptive pilot study aimed to compare neurodevelopmental scores of children who are HIV+, HEU, and HUU in Kenya.
Methods: This cross-sectional pilot study included children ages 18–36 months who were HIV+, HEU, or HUU. Neurodevelopment was assessed, along with sociodemographic, lab, and growth data. Statistical analysis included descriptive statistics, one-way ANOVA, chi-squared, and adjusted linear regression models.
Results: One hundred seventy two were included (n = 24 HIV+; n = 74 HEU; n = 74 HUU). Mothers of children who were HEU experienced more depressive symptoms (p < 0.001). The only neurodevelopmental differences were found among groups was that children who were HIV+ had higher receptive language scores (p = 0.007). Lower height-for-age z-scores and being left home alone were associated with worse neurodevelopmental scores.
Conclusions: Being stunted, left completely alone for at least an hour within the last week, and having higher sociodemographic status were associated with worse neurodevelopmental scores. The higher levels of depressive symptoms within mothers of children who are HEU warrants further investigation.
Introduction
Children born to mothers living with HIV are increasingly able to survive but are at still at higher risk of morbidity and mortality compared to their peers who are unexposed (1). In some countries, >15% of all children are born to mothers living with HIV (2), accounting for many of the estimated 14.8 million children under 15 years of age who are HIV-exposed but uninfected (HEU) worldwide (2). Exposure to HIV or antiretroviral therapy (ART) may impact neurodevelopmental outcomes among children who are HEU. Worse neurodevelopmental scores, particularly in the domains of receptive and expressive language, have been reported in young children who are HEU and exposed to ART compared to HUU (3–6). However, other studies have found no neurodevelopmental differences between children who are HEU and HUU (7, 8). With the current inconclusive evidence, further investigation is required to delineate the neurodevelopmental impact of HIV exposure (1).
This small descriptive cross-sectional pilot study aimed to use a culturally adapted Bayley Scales of Infant and Toddler Development, 3rd Edition (Bayley-3) to measure and compare cognitive, language, and motor domains within Kenyan children ages 18–36 months who are HIV+, HEU, or HUU.
Methods
Study Design and Population
This was a descriptive cross-sectional pilot study assessing neurodevelopment among young children in Kenya as part of the Academic Model Providing Access to Healthcare (AMPATH) consortium. The AMPATH HIV care program represents a 20-year partnership among Indiana University School of Medicine (IUSM), Moi University School of Medicine (MUSM), and the Moi Teaching and Referral Hospital (MTRH) in Eldoret, Kenya. AMPATH has enrolled over 200,000 patients and currently provides care for approximately 15,000 children who are HEU and HIV+ in 65 clinics in western Kenya (9, 10). The children and their caregivers were recruited from the MTRH and AMPATH pediatric HIV clinics between 12/2017 and 9/2019, where care for prevention of mother-to-child transmission (PMTCT) of HIV has been freely provided since 2003 (11). Mixed convenience sampling of potential participants occurred within these clinics, with periods of consecutive sampling by study team members positioned within the clinic along with the clinic staff notifying the study team of eligible participants when the team was not physically present. Inclusion criteria included: (1) child between 18 and 36 months of age; (2) Kiswahili or English are the primary languages; (3) attending the MTRH maternal-child health (MCH) clinic or AMPATH HIV clinics; and (4) primary caregiver ≥18 years of age. Children aged 18–36 months are the population of interest in this study for the following reasons: (1) The young age (≤3 years) promotes early referral to intervention services during a critical period of child neurodevelopment, when intervention is most cost- and time-effective; (2) With 18 months as the lower age limit, cognitive and language domains may be tested with greater rigor and persisting delays will be more perceptible, while still allowing adequate time for intervention during this critical period; (3) From a feasibility standpoint, 18–36 months is the upper age limit of children born to mothers living with HIV routinely attending the MCH clinics in Kenya, due to the timing of their final HIV testing. We aimed to recruit 75 participants from each group (HEU, HIV+, and HUU), with HIV status obtained from maternal report. In total, 187 children were recruited. Twelve children could not complete ≥1 sub-sections of the Bayley-3, despite an additional time allowed to rest; two were excluded due to over-recruitment within the HEU cohort; and one did not return after consenting, resulting in 172 remaining study participants.
Perinatal data were not collected from participants. However, standard PMTCT guidelines at this time included an efavirenz-based first-line ART for adults living with HIV ≥15 years of age (12, 13).
Bayley Scales of Infant and Toddler Development, 3rd Edition (Bayley-3)
The Bayley Scales of Infant and Toddler Development, 3rd edition (Bayley-3) is a neurodevelopment assessment that is internationally known and commonly used in research settings (1, 14). Our research team conducted a psychometric analysis of this culturally-adapted Bayley-3 within this setting in Kenya and found it to be valid and internally reliable (15). The cognitive, language (receptive/expressive), and motor (fine/gross) domains of the Bayley-3 were selected for use within this study, as they were the domains which had been culturally adapted. The Social-Emotional and Adaptive Behavior domains were not adapted due to the cross-cultural challenges in interpreting appropriate emotional status and behavior when compared to the normative population. One research assistant received training and approval to administer the Bayley-3 by a certified trainer and was blinded to study participants' HIV status prior to Bayley-3 administration.
Data Collection
Caregivers completed a questionnaire containing demographic information and basic medical history, including birth history and HIV status. While clinical staff aided in identifying potentially eligible study participants, the HIV status was self-reported by caregivers. The caregiver also provided information on depressive symptoms using the Patient Health Questionnaire (PHQ-9) (16), UNICEF multiple cluster survey questions related to child stimulation (17), and socioeconomic status (SES), measured using the Wealth-Assets-Maternal Education-Income (WAMI index) (18). Anthropometric measurements were obtained at study staff. Each child's body mass and standing height was measured twice and if a discrepancy existed between the two measurements, the average was taken. Standing height was obtained in children <24 months to optimize measurement precision within the cohort. Weight was recorded to 0.1 kg and height was recorded to the 5 mm. Scale used: Salter 9,028 Razor Ultra Slim Technology Electronic Scale. Height measured with Seca 213 Portable Stadiometer Height-Rod. A blood sample was taken at enrollment to measure iron-deficiency anemia (IDA) using hemoglobin and serum ferritin. While the primary data regarding IDA is presented elsewhere (19), this variable was included within the regression analysis, as it is a known risk factor for worse neurodevelopmental outcomes (20). We classified a child as having IDA if (1) hemoglobin concentration was <118 g/L, based on World Health Organization-published algorithm for individuals living ≥2,000 meters in elevation, consist with local elevation (16); and (2) ferritin concentrations <12 μg/L. Study data were managed using REDCap, hosted at IUSM (21).
Sample Size Calculation
At the time of this study's inception, data were limited regarding Bayley-3 scores within an international setting with children who were HEU and HUU. We used differences in rates of developmental delay among children who were HIV+ and HEU (22) to perform sample size calculations. With a margin of error of 5 points and 95% confidence interval, a sample size of 72 individuals per group was needed to determine a difference among groups. We aimed to recruit 75 per group to allow for a number who may not complete all study activities.
Statistical Analysis
Characteristics of children and caregivers were summarized for the total cohort and by HIV status. For continuous variables, the mean and standard deviation (SD) were reported, and differences among HIV groups were assessed using one-way ANOVA. For categorical variables, the frequency and percentage were presented, and proportions were compared using the chi-square test. Anthropometric measurements (height and weight) were converted to height-for-age z-scores, weight-for-age z-scores, and weight-for-height z-scores using the modeling defined by the WHO (23). Multivariable linear regression models were used to examine the associations between scores from neurodevelopmental subtests and predictors. Potential predictors were identified using two methods: (1) a priori using clinical judgement and (2) when corresponding p-values of 0.05 were found among the three groups (HIV+, HEU, and HUU) within univariable analyses. Main effect estimates, standard errors (SE), 95% confidence intervals (CI), and significance tests for the predictors were produced.
Ethics
All caregivers provided written informed consent for their child's participation in this study. The study was approved by Institutional Review Boards at IUSM in Indianapolis, USA and MUSM in Eldoret, Kenya.
Results
Of the 187 children enrolled, 172 (n = 24 HIV+; n = 74 HEU; n = 74 HUU) had Bayley-3 data and were included in the analysis. The mean age was 23.1 (SD: 4.6) months. Children who were HIV+ were older compared to children who were HEU and HUU (26.0 vs. 21.7 and 23.5, respectively; p < 0.001). While not part of the inclusion criteria, all caregivers were mothers. Mothers of children who were HEU had significantly higher levels of depressive symptoms (p = 0.002) compared to their HIV+ or HUU peers (Table 1). Children who were HUU had mothers with higher levels of education (p < 0.001) and household resources (p < 0.001) compared to children who were HEU or HIV+.
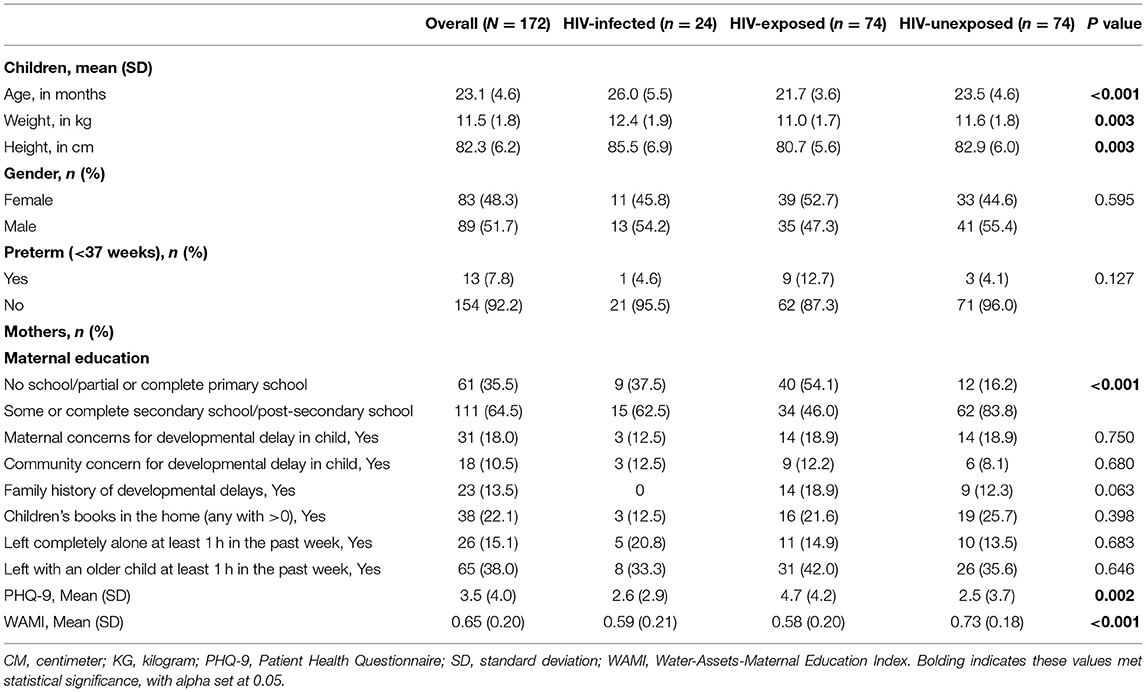
Table 1. Characteristics of study population, recruited from a large referral hospital in western Kenya, at 18–36 months.
No statically significant differences were found among the three groups in cognition, expressive language, fine motor, or gross motor domains, with the exception that children who were HIV+ were found to have higher receptive language scores than others (p = 0.007). Generally, children who were HEU had lower scores than children who were HIV+ or HUU (Table 2).

Table 2. Developmental scores and anthropometrics of study population, aged 18–36 months, by HIV status.
When performing adjusted regression analyses, lower height-for-age z-scores were associated with lower scores in expressive language, fine motor, and gross motor (Table 3). Being left at home alone for at least 1 h within the past week was associated with lower scores in receptive language and expressive language. Higher SES scores were associated with lower fine and gross motor scores.
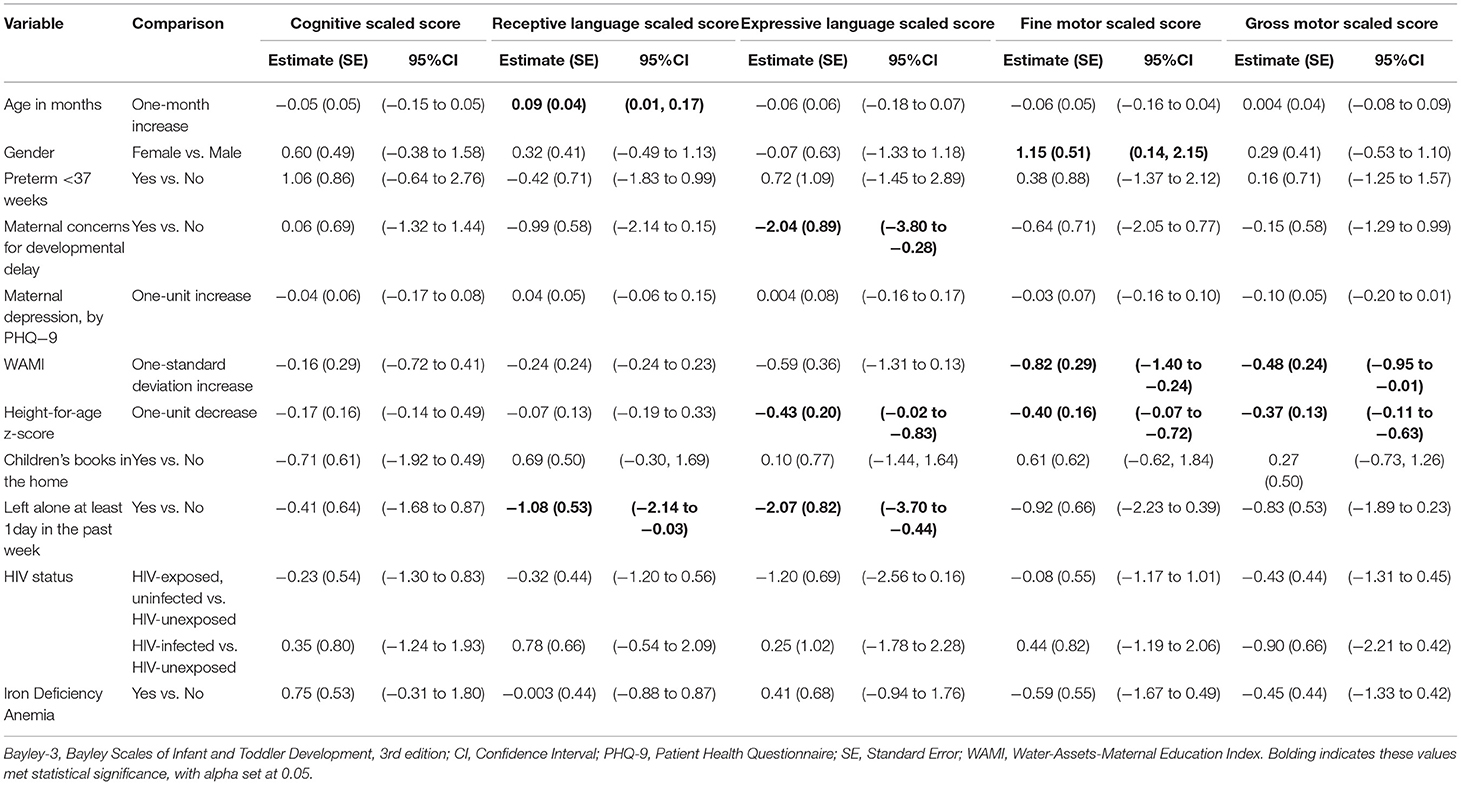
Table 3. Adjusted linear regression analysis of study population, aged 18–36 months, by Bayley-3 subtest.
Discussion
This small cross-sectional pilot study did not show consistent neurodevelopmental differences among children who were HIV+, HEU, and HUU, although children who were HIV+ did have higher receptive language scores. Being stunted, left completely alone for at least an hour within the last week, and having higher SES were associated with worse neurodevelopmental scores. Mothers of children who were HEU reported more symptoms of depression than other mothers. This pilot study provides useful insights on additional variables needed within a larger scale neurodevelopmental study in populations of children who are HEU.
Stunting is a well-known risk factor for poor neurodevelopment (24) and often used as a proxy measure for at-risk development in the absence of more specific data (25). Malnutrition risk is also higher in populations with HIV infection and exposure (26–28). Stunting was associated with worse neurodevelopmental scores within our study cohort, but HIV status was not. In fact, while not statistically significant, children who were HIV+ had the highest mean anthropometric z-scores among the three groups and also had higher scores on receptive language. Due to the small sample of children who are HIV+, these results should be interpreted with caution. However, one may hypothesize that children who are HIV+ at AMPATH were more engaged in care compared to others due to HIV surveillance. This enhanced engagement may have allowed for earlier detection of growth problems and referrals to food programs (29). It is also possible that families may be more concerned for the health of their children who are HIV+ and provide them additional food. We did not collect data on participation in support programs or food availability, which may have differed between groups. Future studies should obtain this data to determine the potentially confounding effect of food supports on neurodevelopment outcomes of populations affected by HIV.
Mothers of children who were HEU expressed significantly more depressive symptoms than those of children who were HIV+ and HUU. While this was not associated with worse neurodevelopmental outcomes in our cohort, maternal depression is a known risk factor for worse development in other populations (30, 31). The lack of information on supports utilized by families affected by HIV limits our understanding of this finding. However, it highlights the issue that siloed care and support systems helping one specific population may unintentionally leave others behind. Any mother living with HIV+ may experience HIV stigma or job discrimination, but in some settings, only those with children who are HIV+ may qualify to access additional resources for their children. More information on the effects of siloed HIV care and depression in mothers of children who are HEU is needed.
In sub-Saharan Africa, an estimated 35% of children <5 years are left at home alone or with a young sibling for >1 h/week (32). Within our study, nearly 15% of children were left completely alone for ≥1 h within the prior week, which was associated with lower receptive and expressive language scores. Poverty, lack of societal support, and local cultural norms are drivers for parental decisions to leave young children home alone (33). While data clearly links lack of supervision with unintentional injury in young children (34), its impact on development in young children has not been well-studied. For school-aged children, being left home alone ≥1 day/week increases conduct problems, hyperactivity and intention symptoms, and peer relationship problems compared to well-supervised peers (35, 36). More research is needed to inform policies and interventions to support families in creating safe, nurturing environments for their children (37).
We found that children with higher SES had lower motor scores. Fine and gross motor scores may be less sensitive to socioeconomic differences (38, 39); however, poverty and lower SES are generally strong risk factors for worse development (24, 40). Within our setting, a few hypotheses may be considered for this finding. Firstly, households with working mothers likely have higher SES, but often necessitate household workers who may provide suboptimal quality childcare. Further, within this context, televisions are more commonly found in households with high SES, and it may be possible that these young children were exposed to substantial television time, which is associated with increased rates of cognitive, language, and motor delays (41). A more comprehensive evaluation of potentially confounding variables, including social supports and household resources, would be helpful in exploring additional hypotheses for this finding in the future.
Our small sample size limited our power to show a difference in neurodevelopment among our three groups, with a particularly small cohort of children who are HIV+. This was a primary limitation of this study. While HIV prevalence among Kenyan women of reproductive age is 6.1% (42), various policy changes and expansion of services have resulted in significant reductions in vertical transmission in Kenya, from 26% in 2009 to 11% in 2018 (13, 43–46). The low numbers of recruited children who are HIV+ is a testament to successful PMTCT in Kenya and within the AMPATH program. However, the high-quality care the children who were HIV+ received through this program may have biased the results toward the null within this study. Furthermore, survival bias may have influenced the number of children who are HIV+ who were recruited to this study and their outcomes, higher anthropometrics and fewer pre-term births, as enrolment did not occur until 18 months of age. Additionally, the inclusion of children who were HUU attending MCH clinic at 18–36 months may also have biased the results toward the null, as many healthy children stop attending the MCH after their 9 month immunizations despite recommendations to attend until 5 years of age (47). Those children who continue coming to the clinic may have more health conscientious caregivers, or perhaps more likely, may have ongoing health issues that require follow up. This is a limitation of this study inherent to its cross-sectional nature and convenience sampling. This pilot study was also limited by the lack of information on in-utero environment, maternal medications, and additional supports offered to families. Also, HIV status was primarily self-reported by mothers, which may have led to mis-classification. Our study team performed careful questioning around HIV status, which ultimately resulted in over recruitment in children who were HEU. During the study visit, two children whose mothers initially self-reported as being HUU were found to be HEU upon further questioning. Further, we did not ask mothers who the primary caregiver was during the day, as house help or family members often provide care while mothers are working. It is critically important that funding of research accommodates prospective data collection, from the period of earliest fetal exposure, namely conception, throughout the period of interest for the child, to ensure that rigorous research is conducted among children who are HEU. Despite these challenges, our study provided high quality data using an adapted Bayley-3.
Of note, the scaled scores within this study were much lower than the normative data for the Bayley-3, with our mean scaled sub-test scores falling >1 SD below the normed scaled mean. Reliance on Bayley-3's U.S. population reference curves has been shown to misclassify children in other cultures (48). In a sample of young Malawian children, raw Bayley-3 scores from the normative data and the Malawian sample were similar until children reached 9–12 months of age, after which the Malawian children's raw scores increased at a slower pace compared to the normative sample (48). A few potential hypotheses are posed for why this may occur. It is possible that the non-U.S. children may not have optimal environments for development. Only a third of mothers within this study completed education beyond secondary school, which is known to be a strong predictor of lower developmental scores (49). Additionally, some necessary alterations made to the test administration during the cultural adaptation process may have negatively impacted scoring when compared to the standard administration (15). A third hypothesis is that certain aspects of development are more adaptive and highly prioritized in settings like Kenya compared to the U.S. but are not measured with assessments like the Bayley-3, thereby resulting in a lower score. As a general example of expectation differences between cultures, we previously found that Kenyan clinical providers were concerned when children were not walking independent by 12 months of age (50), whereas, the U.S. Centers for Disease Control expects that most children will not walk independently until 18 months (51). As with all assessments, the items are evaluated are on the constructs deemed important by the test developers, which may introduce scoring bias. Thus, these results are only to be used to compare scores among our three study groups and not to be directly compared to other populations.
Conclusions
Within our study population, being stunted and left at home alone were associated with worse neurodevelopment. Additional research is needed to explore neurodevelopment in children impacted by HIV, especially the growing population of children who are HEU. A more complete participant history with comprehensive documentation of potentially confounding variables, such as nutritional and household support and ARV exposure, is necessary to determine the true effects of HIV exposure or infection on neurodevelopmental outcomes. Screening mechanisms are needed to detect early risk for worse health and neurodevelopmental outcomes in child who are HEU, so that targeted interventions may be introduced to optimize long-term outcomes.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Institutional Review Boards at Indiana University School of Medicine in Indianapolis, USA and Moi University School of Medicine in Eldoret, Kenya. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author Contributions
MM, EO, and RV designed the research study during MSM's training program. AO and CC performed the research and provided insights for the findings, with supervision by EO, RV, and MM. ZY and MM analyzed the data. MM wrote the paper, with input of all others. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by Indiana University's Morris Green Physician Scientist Development Program and Indiana CTSI at Indiana University School of Medicine. MSM's time during the implementation of this study was funded by a training grant entitled Training in STIs and Other Infections of Global Health Significance (T32AI007637, PI: Dr. Wools Kaloustian) from the National Institute of Allergy and Infectious Diseases, Bethesda, MD, USA.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. McHenry MS, McAteer CI, Oyungu E, McDonald BC, Bosma CB, Mpofu PB, et al. Neurodevelopment in young children born to HIV-infected mothers: a meta-analysis. Pediatrics. (2018) 141:1–12. doi: 10.1542/peds.2017-2888
2. Slogrove AL, Powis KM, Johnson LF, Stover J, Mahy M. Estimates of the global population of children who are HIV-exposed and uninfected, 2000–18: a modelling study. Lancet Global Health. (2020) 8:e67–75. doi: 10.1016/S2214-109X(19)30448-6
3. Van Rie A, Dow A, Mupuala A, Stewart P. Neurodevelopmental trajectory of HIV-infected children accessing care in Kinshasa, Democratic Republic of Congo. J Acquir Immune Defic Syndr. (2009) 52:636–42. doi: 10.1097/QAI.0b013e3181b32646
4. Wedderburn CJ, Yeung S, Rehman AM, Stadler JAM, Nhapi RT, Barnett W, et al. Neurodevelopment of HIV-exposed uninfected children in South Africa: outcomes from an observational birth cohort study. Lancet Child Adolesc Health. (2019) 3:803–13. doi: 10.1016/S2352-4642(19)30250-0
5. Jao J, Kacanek D, Yu W, Williams PL, Patel K, Burchett S, et al. Neurodevelopment of HIV-exposed uninfected infants born to women with perinatally acquired HIV in the United States. J Acquir Immune Defic Syndr. (2020) 84:213–9. doi: 10.1097/QAI.0000000000002318
6. Ntozini R, Chandna J, Evans C, Chasekwa B, Majo FD, Kandawasvika G, et al. Early child development in children who are HIV-exposed uninfected compared to children who are HIV-unexposed: observational sub-study of a cluster-randomized trial in rural Zimbabwe. J Int AIDS Soc. (2020) 23:e25456. doi: 10.1002/jia2.25456
7. Chaudhury S, Williams PL, Mayondi GK, Leidner J, Holding P, Tepper V, et al. Neurodevelopment of HIV-Exposed and HIV-Unexposed Uninfected Children at 24 Months. Pediatrics. (2017) 140:1–10. doi: 10.1542/peds.2017-0988
8. Strehlau R, van Aswegen T, Burke M, Kuhn L, Potterton J. A description of early neurodevelopment in a cohort of HIV-exposed uninfected children. AIDS Care. (2020) 32:1421–8. doi: 10.1080/09540121.2020.1736257
9. Inui TS, Nyandiko WM, Kimaiyo SN, Frankel RM, Muriuki T, Mamlin JJ, et al. AMPATH: living proof that no one has to die from HIV. J Gen Int Med. (2007) 22:1745–50. doi: 10.1007/s11606-007-0437-4
10. Einterz RM, Kimaiyo S, Mengech HN, Khwa-Otsyula BO, Esamai F, Quigley F, et al. Responding to the HIV pandemic: the power of an academic medical partnership. Acad Med. (2007) 82:812–8. doi: 10.1097/ACM.0b013e3180cc29f1
11. Berlacher M, Mercer T, Apondi E, Mwangi W, Were E, McHenry M. Integrating prevention of mother-to-child transmission of HIV care into general maternal child health care in Western Kenya. Int J Mat Child Health AIDS. (2020) 10:19–28. doi: 10.21106/ijma.429
12. WHO. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. Geneva: World Health Organization (2016).
13. National AIDS & STI Control Program 2016. Guidelines on Use of Antiretroviral Drugs for Treating and Preventing HIV Infection in Kenya. National AIDS & STI Control Program 2016 (2016).
15. McHenry MS, Oyungu E, Yang Z, Hines AC, Ombitsa AR, Vreeman RC, et al. Cultural adaptation of the bayley scales of infant and toddler development, 3rd edition for use in Kenyan children aged 18–36 months: a psychometric study. Res Dev Disab. (2021) 110:103837. doi: 10.1016/j.ridd.2020.103837
16. Omoro SA, Fann JR, Weymuller EA, Macharia IM, Yueh B. Swahili translation and validation of the patient health questionnaire-9 depression scale in the Kenyan head and neck cancer patient population. Int J Psychiatry Med. (2006) 36:367–81. doi: 10.2190/8W7Y-0TPM-JVGV-QW6M
17. UNICEF. Multiple Indicator Cluster Surveys: Questionnaire for Children Under Five: UNICEF (2019). Available online at: http://mics.unicef.org/tools (accessed June 17, 2019).
18. Psaki SR, Seidman JC, Miller M, Gottlieb M, Bhutta ZA, Ahmed T, et al. Measuring socioeconomic status in multicountry studies: results from the eight-country MAL-ED study. Popul Health Metr. (2014) 12:8–12. doi: 10.1186/1478-7954-12-8
19. Oyungu E, Roose AW, Ombitsa AR, Yang Z, Vreeman RC, McHenry MS. Anemia and iron-deficiency anemia in children born to mothers with HIV in Western Kenya. Glob Pediatr Health. (2021) 8:1–7. doi: 10.1177/2333794X21991035
20. Grantham-McGregor S, Ani C. A review of studies on the effect of iron deficiency on cognitive development in children. J Nutr. (2001) 131:649S−68S. doi: 10.1093/jn/131.2.649S
21. Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O'Neal L, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. (2019) 95:103208. doi: 10.1016/j.jbi.2019.103208
22. Hutchings J, Potterton J. Developmental delay in HIV-exposed infants in Harare, Zimbabwe. Vulner Child Youth Stud. (2014) 9:43–55. doi: 10.1080/17450128.2013.778440
23. World Health Organization. Global Database on Child Growth and Malnutrition. Geneva: World Health Organization (2018).
24. Walker SP, Wachs TD, Grantham-McGregor S, Black MM, Nelson CA, Huffman SL, et al. Inequality in early childhood: risk and protective factors for early child development. Lancet. (2011) 378:1325–38. doi: 10.1016/S0140-6736(11)60555-2
25. Grantham-McGregor S, Cheung YB, Cueto S, Glewwe P, Richter L, Strupp B. Developmental potential in the first 5 years for children in developing countries. Lancet. (2007) 369:60–70. doi: 10.1016/S0140-6736(07)60032-4
26. Taha TE, Dallabetta GA, Canner JK, Chiphangwi JD, Liomba G, Hoover DR, et al. The effect of human immunodeficiency virus infection on birthweight, and infant and child mortality in urban Malawi. Int J Epidemiol. (1995) 24:1022–9. doi: 10.1093/ije/24.5.1022
27. Ndirangu J, Newell ML, Bland RM, Thorne C. Maternal HIV infection associated with small-for-gestational age infants but not preterm births: evidence from rural South Africa. Hum Reprod. (2012) 27:1846–56. doi: 10.1093/humrep/des090
28. Sint TT, Lovich R, Hammond W, Kim M, Melillo S, Lu L, et al. Challenges in infant and young child nutrition in the context of HIV. AIDS. (2013) 27(Suppl. 2):S169–77. doi: 10.1097/QAD.0000000000000089
29. Mamlin J, Kimaiyo S, Lewis S, Tadayo H, Jerop FK, Gichunge C, et al. Integrating nutrition support for food-insecure patients and their dependents into an HIV care and treatment program in Western Kenya. Am J Public Health. (2009) 99:215–21. doi: 10.2105/AJPH.2008.137174
30. Cummings EM, Davies PT. Maternal depression and child development. J Child Psychol Psychiatry. (1994) 35:73–122. doi: 10.1111/j.1469-7610.1994.tb01133.x
31. Bernard-Bonnin A-C, Society CP, Health M, Committee DD. Maternal depression and child development. Paediatr Child Health. (2004) 9:575–83. doi: 10.1093/pch/9.8.575
32. Percentage of Children 0-59 Months Left Alone or in the Care of Another Child Younger Than 10 Years of Age for More Than One Hour at Least Once in the Past Week (by sex and household wealth quintile). UNICEF (2020). Available online at: https://data.unicef.org/topic/early-childhood-development/home-environment/ (accessed November 23, 2020).
33. Ruiz-Casares M, Heymann J. Children home alone unsupervised: modeling parental decisions and associated factors in Botswana, Mexico, and Vietnam. Child Abuse Negl. (2009) 33:312–23. doi: 10.1016/j.chiabu.2008.09.010
34. Schnitzer PG, Dowd MD, Kruse RL, Morrongiello BA. Supervision and risk of unintentional injury in young children. Inj Prev. (2015) 21(e1):e63–70. doi: 10.1136/injuryprev-2013-041128
35. Doi S, Fujiwara T, Isumi A, Ochi M, Kato T. Relationship between leaving children at home alone and their mental health: results from the a-CHILD study in Japan. Front Psychiatry. (2018) 9:192. doi: 10.3389/fpsyt.2018.00192
36. Colwell MJ, Pettit GS, Meece D, Bates JE, Dodge KA. Cumulative risk and continuity in nonparental care from infancy to early adolescence. Merrill Palmer Q. (2001) 47:207–34. doi: 10.1353/mpq.2001.0009
37. Ruiz-Casares M, Nazif-Muñoz JI, Iwo R, Oulhote Y. Nonadult supervision of children in low- and middle-income countries: results from 61 national population-based surveys. Int J Environ Res Public Health. (2018) 15:1564. doi: 10.3390/ijerph15081564
38. Gottschling-Lang A, Franze M, Hoffmann W. Associations of motor developmental risks with the socioeconomic status of preschool children in north-eastern Germany. Child Dev Res. (2013) 2013:790524. doi: 10.1155/2013/790524
39. Ronfani L, Vecchi Brumatti L, Mariuz M, Tognin V, Bin M, Ferluga V, et al. The complex interaction between home environment, socioeconomic status, maternal IQ and early child neurocognitive development: a multivariate analysis of data collected in a newborn cohort study. PLoS ONE. (2015) 10:e0127052. doi: 10.1371/journal.pone.0127052
40. Venetsanou F, Kambas A. Environmental factors affecting preschoolers' motor development. Early Childhood Educ J. (2010) 37:319–27. doi: 10.1007/s10643-009-0350-z
41. Lin LY, Cherng RJ, Chen YJ, Chen YJ, Yang HM. Effects of television exposure on developmental skills among young children. Infant Behav Dev. (2015) 38:20–6. doi: 10.1016/j.infbeh.2014.12.005
42. UNAIDS. AIDSinfo (2020) Available online at: http://aidsinfo.unaids.org/ (accessed July 21, 2020).
43. National AIDS and STI Control Program. Guidelines on Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Rapid Advice Nairobi, Kenya. (2014). Available online at: http://healthservices.uonbi.ac.ke/sites/default/files/centraladmin/healthservices/Rapid%20Advice%20Booklet%202014%2024%20June%2012%20noon_0.pdf (accessed January 20, 2021).
44. Sirengo M, Muthoni L, Kellogg TA, Kim AA, Katana A, Mwanyumba S, et al. Mother-to-child transmission of HIV in kenya: Results from a nationally representative study. J Acquir Immune Defic Syndr. (2014) 66:S66–S74. doi: 10.1097/QAI.0000000000000115
45. UNAIDS. UNAIDS Data. (2019). Available online at: https://www.unaids.org/en/resources/documents/2019/2019–data (accessed December 04, 2019).
46. Kenya Ministry of Health. Kenya HIV Estimates Report 2018 Nairobi: National AIDS and STI Control Programme. (2018). Available online at: https://nacc.or.ke/wp-content/uploads/2018/11/HIV-estimates-report-Kenya-20182.pdf (accessed December 18, 2018).
47. Mudany MA, Sirengo M, Rutherford GW, Mwangi M, Nganga LW, Gichangi A. Enhancing maternal and child health using a combined mother & child health booklet in Kenya. J Trop Pediatr. (2015) 61:442–7. doi: 10.1093/tropej/fmv055
48. Cromwell EA, Dube Q, Cole SR, Chirambo C, Dow AE, Heyderman RS, et al. Validity of US norms for the bayley scales of infant development-III in malawian children. Eur J Paediatr Neurol. (2014) 18:223–30. doi: 10.1016/j.ejpn.2013.11.011
49. Lennon E, Gardner J, Karmel B, Flory M. Bayley scales of infant development. In: Benson JB, Haith MM, editors. Language, Memory, and Cognition in Infancy and Early Childhood. San Diego, CA; Oxford: Elsevier; Academic Press (2010). p. 37–48.
50. McHenry MS, Oyungu E, McAteer CI, Ombitsa AR, Cheng ER, Ayaya SO, et al. Early childhood development in children born to HIV-infected mothers: perspectives from Kenyan clinical providers and caregivers. Glob Pediatr Health. (2018) 5:1–13. doi: 10.1177/2333794X18811795
51. Centers for Disease Control. Important Milestones: Your Child By Eighteen Months Centers for Disease Control and Prevention website: Centers for Disease Control and Prevention. (2021). Available online at: https://www.cdc.gov/ncbddd/actearly/milestones/milestones-18mo.html (accessed July 29, 2021).
Keywords: child development, HIV, HIV exposure, Kenya, HIV-infected, neurodevelopment
Citation: McHenry MS, Oyungu E, Yang Z, Ombitsa AR, Cherop C and Vreeman RC (2021) Neurodevelopmental Outcomes of Young Children Born to HIV-Infected Mothers: A Pilot Study. Front. Pediatr. 9:697091. doi: 10.3389/fped.2021.697091
Received: 18 April 2021; Accepted: 23 September 2021;
Published: 21 October 2021.
Edited by:
Lindsey Reif, Cornell University, United StatesReviewed by:
Kathleen M. Powis, Massachusetts General Hospital and Harvard Medical School, United StatesMathurin Cyrille Tejiokem, Centre Pasteur du Cameroun, Cameroon
Copyright © 2021 McHenry, Oyungu, Yang, Ombitsa, Cherop and Vreeman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Megan S. McHenry, bXN1aGxAaXUuZWR1
 Megan S. McHenry
Megan S. McHenry Eren Oyungu3
Eren Oyungu3 Ziyi Yang
Ziyi Yang Ananda R. Ombitsa
Ananda R. Ombitsa Cleophas Cherop
Cleophas Cherop Rachel C. Vreeman
Rachel C. Vreeman