- 1Department of Pediatrics, West China Second University Hospital, Sichuan University, Chengdu, China
- 2Key Laboratory of Birth Defects and Related Diseases of Women and Children, Ministry of Education, Sichuan University, Chengdu, China
- 3Department of Pediatrics, Zigong Fourth People's Hospital, Zigong, China
Introduction: Necrotizing enterocolitis (NEC) is a fatal condition for very-low-birth-weight infants. Necrotizing enterocolitis is a multi-factor phenomenon that results in intestinal mucosal damage and leads to intestinal necrosis. However, sensitive laboratory indicators for NEC are lacking, making early diagnosis difficult. This study aimed to explore the relationship between the platelet-to-lymphocyte ratio (PLR) and NEC in preterm neonates to enable an earlier diagnosis of the condition.
Methods: This was a retrospective case–control study of preterm neonates diagnosed with NEC between January 2018 and December 2019 in the West China Second University Hospital. Controls were selected from preterm neonatal intensive care unit (NICU) graduates, and they were matched for gestation and year of birth to the preterms diagnosed without NEC. In total, 93 and 107 infants were included in the NEC and control groups, respectively. Empowerstats analysis was used to identify the association between PLR and preterm NEC.
Results: The NEC group had significantly higher PLR levels than the control group. PLR > 100 within 1 week before NEC diagnosis was a risk factor for NEC. There was a positive connection between PLR and preterm NEC. A PLR of >100 was determined as the optimal cutoff for predicting preterm NEC, with patients with PLR >100 having a higher risk of NEC [odds ratio (OR): 18.82 (95% confidence interval (CI): 2.93–120.98), p = 0.002].
Conclusions: A PLR of >100 within 1 week after clinical abnormalities is associated with a high risk of NEC in preterm neonates.
Introduction
Necrotizing enterocolitis (NEC) is one of the most common gastrointestinal emergencies in newborn infants (1). It is a neonatal inflammatory bowel disease characterized by hemorrhagic and necrotizing inflammation of the various layers of the intestinal wall. Premature infants with a birth weight of <750 g have a 42% mortality rate due to NEC, while those with a birth weight of 1,250–1,500 g have a lower mortality rate (2). In 1978, the Bell staging criteria, in which NEC is evaluated according to the development of inflammation, were established (3). In 1986, Walsh and Kliegman modified the staging criteria (4). The modified version has been used till date. The pathophysiological mechanism of NEC is yet to be fully elucidated (5, 6). Further, clinical manifestations at onset are usually non-specific, and conservative or surgical treatment cannot prevent the intramural inflammation. As such, emphasis is placed on prevention.
The platelet-to-lymphocyte ratio (PLR) has attracted increasing research attention as a new potential inflammatory marker. It has been found to predict the risk of cardiovascular disease and tumors and to be also associated with inflammatory diseases. In these conditions, a higher PLR is associated with poorer prognosis (7–11). However, to the best of our knowledge, there has been no research on the relationship between PLR and early NEC. Thus, this study aimed to explore the relationship between PLR and preterm NEC and to determine whether a high PLR is associated with a higher risk of NEC.
Materials and Methods
Study Design and Patients
This was a retrospective case–control study of neonatal intensive care unit (NICU) preterm infants who developed NEC between January 2018 and December 2019. The patients were from the NICU of Sichuan University West China Second University Hospital. Among the 3,527 preterm infants, 133 infants were diagnosed with NEC. After excluding 20 infants due to perforation (n = 4), admission ≥24 h after birth (n = 4), admission <7 days (n = 9), and congenital malformation (n = 3), 93 preterm infants diagnosed with NEC were included in the NEC group. Meanwhile, the control group included 107 preterm infants without NEC matched for gestational age and year of birth (Figure 1). Infants who receive high-dose or exclusive breastfeeding within 14 days of birth have lower risk of NEC (12, 13). Thus, in our study, all infants were on mixed feeding, and there was no significant difference in milk selection and increase rate.
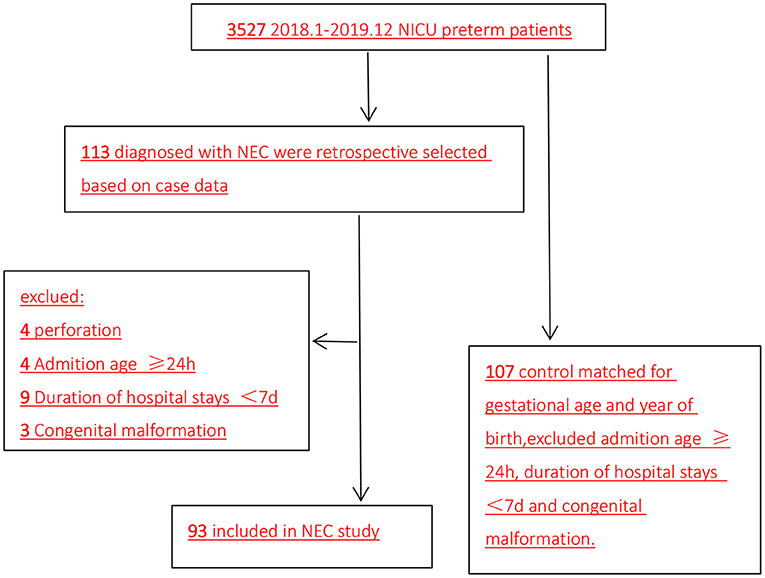
Figure 1. Flow diagram of patient selection (2018.1–2019.12). NICU, neonatal intensive care unit; NEC, necrotizing enterocolitis.
Diagnosis of Necrotizing Enterocolitis
Necrotizing enterocolitis was diagnosed according to the Bell and Walsh grading criteria and revised consensus, including Stage I, Stage II, and Stage IIIA (3, 4).
Calculation of the Platelet-to-Lymphocyte Ratio
Platelet-to-lymphocyte ratio was calculated as the mean ratio of platelet count to lymphocyte count in all blood tests within 1 week before NEC diagnosis.
Statistical Analysis
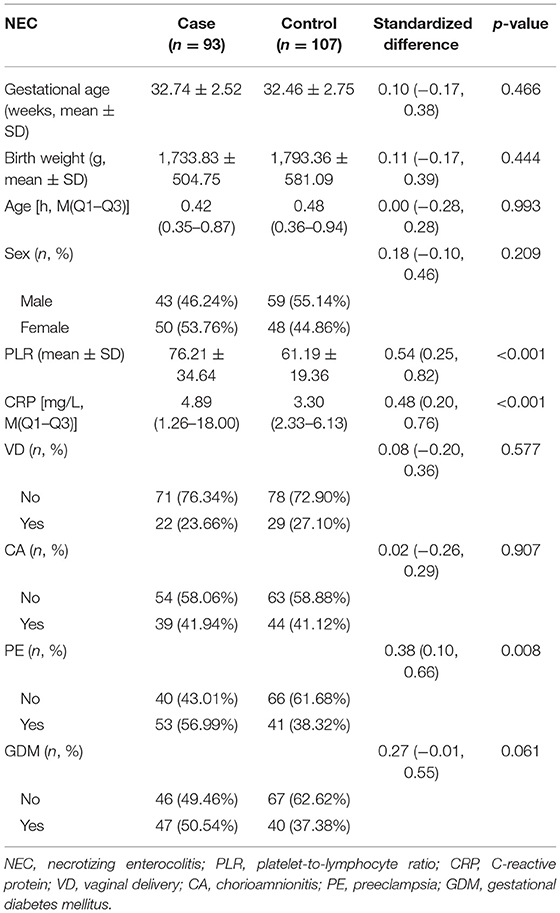
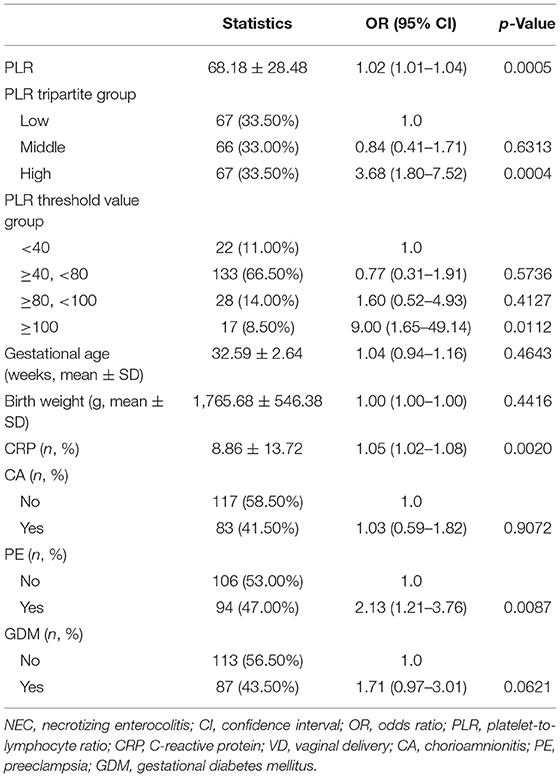
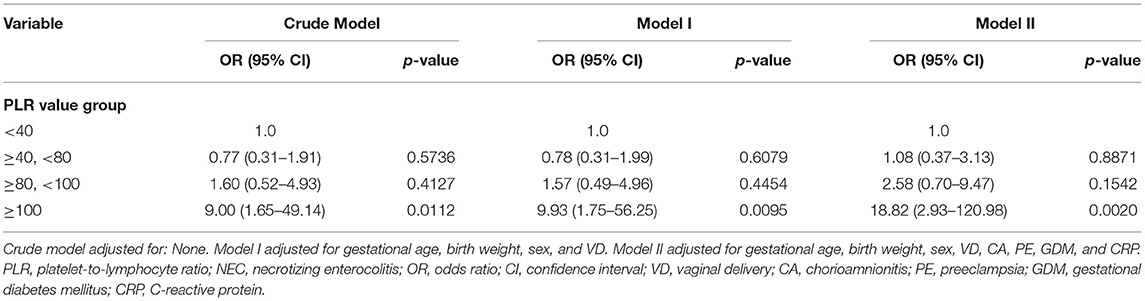
First, normally and non-normally distributed continuous variables were analyzed using the t-test and the Kruskal–Wallis rank sum test. Meanwhile, the χ2-test was used for categorical data to compare the data distribution of each covariate between the exposed and the non-exposed groups (Table 1). Next, generalized additive model curve analysis was done to evaluate the diagnostic capability of PLR for NEC (Figure 2), and univariate (Table 2) and multivariate logistic regression (Table 3) models were performed to examine whether PLR is associated with NEC. A p < 0.05 indicates a statistical difference. All statistical analyses were performed with R (http://www.R-project.org) and Empowerstats software (www.empowerstats.com, X& Y solutions, Inc.).
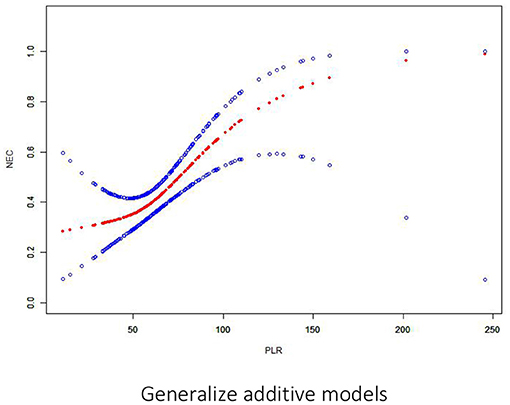
Figure 2. Association between PLR and NEC. There is a threshold and non-linear association between PLR and NEC (p < 0.05) in the generalized additive model (GAM). The smooth curve fit between variables is represented by a solid red line. The 95% confidence interval from the fit is represented by blue bands.
Results
Patient Characteristics
There was no significant difference in patient characteristics with respect to gestational age, birth weight, sex, rate of vaginal delivery (VD), rate of chorioamnionitis, and gestational diabetes mellitus between the two groups (all p > 0.05). Meanwhile, the NEC group had significantly higher PLR levels (76.21 ± 34.64 vs. 61.19 ± 19.36, p < 0.001), C-reactive protein (CRP) levels [4.89 (1.26–18.00) vs. 3.30 (2.33–6.13), p < 0.001], and rate of mothers with pre-eclampsia than the control group (Table 1).
Risk Factors
On univariate analysis, the risk factors for NEC were PLR [odds ratio (OR): 1.02 (95% confidence interval (CI): 1.01–1.04), p = 0.0005], CRP [OR: 1.05 (95% CI: 1.02–1.08), p = 0.002], and pre-eclampsia [OR: 2.13 (95% CI: 1.21–3.76), p = 0.0087] (Table 2).
On multivariate analysis, only PLR > 100 within 1 week before NEC diagnosis was an independent risk factor for NEC [OR: 9.00 (95% CI: 1.65–49.14), p = 0.0112]. There was a positive association between PLR and preterm NEC (Table 3). Generalized additive model curve analysis to evaluate the diagnostic capability of PLR for NEC (Figure 2) showed that PLR > 100 is the optimal predictive cutoff value. In model II, PLR > 100 was closely associated with NEC [OR: 18.82 (95% CI: 2.93–120.98), p = 0.002] (Table 3).
Discussion
Despite the Profound Mortality Risk of Preterm NEC, Reliable Biomarkers for Its Early Diagnosis Are Still Lacking
Necrotizing enterocolitis is one of the main causes of death of premature infants (14). However, the etiology and pathogenesis of NEC remain unclear to date. Some studies have shown that NEC is related to immature intestinal development, ischemia, intestinal flora imbalance, decreased mucin barrier, increased intestinal osmotic pressure, decreased intestinal immunity, and the methods of intestinal feeding. These multifactorial events ultimately lead to oxidative stress, inflammation, and necrosis in the neonatal gut (15–18). Accordingly, the diagnosis of NEC depends on a combination of the clinical symptoms (including vomiting, ventosity, and bloody stool) and radiological features (including gastrointestinal accumulation of gas, portal venous gas, and pneumoperitoneum). The most commonly used clinical grading diagnosis system remains to be the modified Bell's staging criteria. Most cases of NEC are diagnosed in the second or third Bell's stage.
The lack of early diagnostic markers and specific symptoms makes it challenging to distinguish early NEC from sepsis, apnea, and feeding intolerance (19–22). Another challenge is the increasing incidence of non-infectious enteritis [e.g., food protein-induced enterocolitis syndrome, food protein-induced rectal colitis, and food protein-induced enteropathy (23–25)] in recent years, making the selection of appropriate laboratory tests and reasonable antibiotic use difficult. Therefore, exploring the early biomarkers of NEC is particularly important for the early differentiation of NEC from other types of enteritis and to avoid unnecessary antibiotic abuse. However, identifying NEC patients even before making a diagnosis remains a challenge for the neonatologists.
Sepsis influences platelet count (26) as platelet–leukocyte interaction regulates inflammation and hemostasis. Activated platelets can express P-selectin and glycoprotein IIIa on their cell surface. This can in turn interact with the selectin receptor P-selectin glycoprotein ligand-1 (PSGL-1), expressed on leukocytes, and the selectin receptor GpIIb, expressed on neutrophils, to mediate cell extravasation and tissue damage (27–29). An animal study using an infectious mouse model reported that a high PLR is associated with inflammation in the early stages of infection (30). It is inferred that platelet activation and the platelet–leukocyte complex are involved in the early stages of inflammatory response and tissue damage.
Increasing studies have shown that the indicators of platelet count, such as the PLR, can predict acute inflammatory and cardiovascular events, prothrombotic states, and neoplastic and metabolic diseases (31–37). They are also helpful for diagnosing inflammatory rheumatic diseases and predicting inflammation-related morbidities (38). A recent study showed that PLR is positively correlated with early-onset neonatal sepsis (EOS) in preterm neonates, and it can thus be added as an additional diagnostic indicator for EOS (10). Erzat et al. have shown that the risk of preterm premature rupture of the membranes (PPROM) is significantly increased when the PLR is >117.14. A high PLR was found to be independent risk factor of PPROM, mediating adverse maternal and neonatal outcomes (39).
Regarding the association between NEC and PLR, a PLR of >100 within 1 week after clinical abnormalities before NEC diagnosis was found to be a risk factor for NEC. Specifically, there was a positive association between PLR and preterm NEC, with a high PLR of >100 being the optimal cutoff for predicting preterm NEC.
This study has some limitations, including the small sample size and single-center design. The diagnostic grade of NEC is not stratified in great detail. Despite these limitations, our findings may be helpful for lowering unreasonable antibiotic abuse, which can in turn protect the intestinal microbial flora stability in premature infants. Further studies are needed to investigate the relationship between the different levels of NEC and the PLR value and to create NEC predictive models, especially in more preterm neonates.
In conclusion, a PLR > 100 within 1 week after clinical abnormalities is significantly associated with the occurrence of preterm NEC and can thus be used for its early diagnosis. Antibiotic use can be delayed in preterm infants presenting with NEC symptoms (e.g., blood in the stool or abdominal distention) if their PLR is <100. In the future, we will choose antibiotics more carefully while managing NEC according to the ratio of PLR.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Medical Ethics Committee of West China Second Hospital of Sichuan University. Written informed consent from the participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements. Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
JZ contributed to the data analysis and interpretation and prepared the initial draft. HW contributed to the experimental design, data collection, data analysis, result interpretation, and manuscript preparation. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Application Foundation Project of Science and Technology Department of Sichuan Province, 2021YJ0171.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lin PW, Stoll BJ. Necrotising enterocolitis. Lancet. (2006) 368:1271–83. doi: 10.1016/S0140-6736(06)69525-1
2. Zozaya C, García González I, Avila-Alvarez A, Couce ML, Sánchez Tamayo T, Salguero E. Incidence, treatment, and outcome trends of necrotizing enterocolitis in preterm infants: a multicenter cohort study. Front Pediatr. (2020) 8:188. doi: 10.3389/fped.2020.00188
3. Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. (1978) 187:1–7. doi: 10.1097/00000658-197801000-00001
4. Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am. (1986) 33:179–201. doi: 10.1016/S0031-3955(16)34975-6
5. Neu J. Necrotizing enterocolitis: the search for a unifying pathogenic theory leading to prevention. Pediatr Clin North Am. (1996) 43:409–32. doi: 10.1016/S0031-3955(05)70413-2
6. Lim JC, Golden JM, Ford HR. Pathogenesis of neonatal necrotizing enterocolitis. Pediatr Surg Int. (2015) 31:509–18. doi: 10.1007/s00383-015-3697-9
7. Kurtul A, Ornek E. Platelet to lymphocyte ratio in cardiovascular diseases: a systematic review. Angiology. (2019) 70:802–18. doi: 10.1177/0003319719845186
8. Lai Q, Melandro F, Larghi Laureiro Z, Giovanardi F, Ginanni Corradini S, Ferri F, et al. Platelet-to-lymphocyte ratio in the setting of liver transplantation for hepatocellular cancer: a systematic review and meta-analysis. World J Gastroenterol. (2018) 24:1658–65. doi: 10.3748/wjg.v24.i15.1658
9. Diem S, Schmid S, Krapf M, Flatz L, Born D, Jochum W, et al. Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer. (2017) 111:176–81. doi: 10.1016/j.lungcan.2017.07.024
10. Can E, Hamilcikan S, Can C. The value of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio for detecting early-onset neonatal sepsis. J Pediatr Hematol Oncol. (2018) 40:e229–32. doi: 10.1097/MPH.0000000000001059
11. Cho SK, Jung S, Lee KJ, Kim JW. Neutrophil to lymphocyte ratio and platelet to lymphocyte ratio can predict the severity of gallstone pancreatitis. BMC Gastroenterol. (2018) 18:18. doi: 10.1186/s12876-018-0748-4
12. Sisk PM, Lovelady CA, Dillard RG, Gruber KJ, O'Shea TM. Early human milk feeding is associated with a lower risk of necrotizing enterocolitis in very low birth weight infants. J Perinatol. (2007) 27:428–33. doi: 10.1038/sj.jp.7211758
13. Johnson TJ, Patel AL, Bigger HR, Engstrom JL. Meier PP. Cost savings of human milk as a strategy to reduce the incidence of necrotizing enterocolitis in very low birth weight infants. Neonatology. (2015) 107:271–6. doi: 10.1159/000370058
14. Marseglia L, D'Angelo G, Manti S, Aversa S, Reiter RJ, Antonuccio P, et al. Oxidative stress-mediated damage in newborns with necrotizing enterocolitis: a possible role of melatonin. Am J Perinatol. (2015) 32:905–9. doi: 10.1055/s-0035-1547328
15. Morgan JA, Young L, McGuire W. Pathogenesis and prevention of necrotizing enterocolitis. Curr Opin Infect Dis. (2011) 24:183–9. doi: 10.1097/QCO.0b013e328345d5b5
16. Marseglia L, D'Angelo G, Manti M, Aversa S, Fiamingo C, Arrigo T, et al. Visfatin: new marker of oxidative stress in preterm newborns. Int J Immunopathol Pharmacol. (2016) 29:23–9. doi: 10.1177/0394632015607952
17. Torrazza RM, Neu J. The altered gut microbiome and necrotizing enterocolitis. Clin Perinatol. (2013) 40:93–108. doi: 10.1016/j.clp.2012.12.009
18. Martin R, Nauta AJ, Ben Amor K, Knippels LM, Knol J, Garssen J. Early life: gut microbiota and immune development in infancy. Benef Microbes. (2010) 1:367–82. doi: 10.3920/BM2010.0027
19. Gordon PV, Swanson JR, Attridge JT, Clark R. Emerging trends in acquired neonatal intestinal disease: is it time to abandon Bell's criteria? J Perinatol. (2007) 27:661–71. doi: 10.1038/sj.jp.7211782
20. Eaton S, Rees CM, Hall NJ. Current research in necrotizing enterocolitis. Early Hum Dev. (2016) 97:33–9. doi: 10.1016/j.earlhumdev.2016.01.013
21. Lin PW, Nasr TR, Stoll BJ. Necrotizing enterocolitis: recent scientific advances in pathophysiology and prevention. Semin Perinatol. (2008) 32:70–82. doi: 10.1053/j.semperi.2008.01.004
22. Gephart SM, Gordon PV, Penn AH, Gregory KE, Swanson JR, Maheshwari A, et al. Changing the paradigm of defining, detecting, and diagnosing NEC: perspectives on Bell's stages and biomarkers for NEC. Semin Pediatr Surg. (2018) 27:3–10. doi: 10.1053/j.sempedsurg.2017.11.002
23. Nowak-Wegrzyn A, Jarocka-Cyrta E, Moschione Castro A. Food protein-induced enterocolitis syndrome. J Investig Allergol Clin Immunol. (2017) 27:1–18. doi: 10.18176/jiaci.0135
24. Cherian S, Varshney P. Food protein-induced enterocolitis syndrome (FPIES): review of recent guidelines. Curr Allergy Asthma Rep. (2018) 18:28. doi: 10.1007/s11882-018-0767-9
25. Mennini M, Fiocchi AG, Cafarotti A, Montesano M, Mauro A, Villa MP, et al. Food protein-induced allergic proctocolitis in infants: literature review and proposal of a management protocol. World Allergy Organ J. (2020) 13:100471. doi: 10.1016/j.waojou.2020.100471
26. Venkata C, Kashyap R, Farmer JC, Afessa B. Thrombocytopenia in adult patients with sepsis: incidence, risk factors, and its association with clinical outcome. J Intensive Care. (2013) 1:9. doi: 10.1186/2052-0492-1-9
27. Zuchtriegel G, Uhl B, Puhr-Westerheide D, Pörnbacher M, Lauber K, Krombach F, et al. Platelets guide leukocytes to their sites of extravasation. PLoS Biol. (2016) 14:e1002459. doi: 10.1371/journal.pbio.1002459
28. Sreeramkumar V, Adrover JM, Ballesteros I, Cuartero MI, Rossaint J, Bilbao I, et al. Neutrophils scan for activated platelets to initiate inflammation. Science. (2014) 346:1234–8. doi: 10.1126/science.1256478
29. Slaba I, Wang J, Kolaczkowska E, McDonald B, Lee WY, Kubes P. Imaging the dynamic platelet-neutrophil response in sterile liver injury and repair in mice. Hepatology. (2015) 62:1593–605. doi: 10.1002/hep.28003
30. Vardon Bounes F, Mémier V, Marcaud M, Jacquemin A, Hamzeh-Cognasse H, Garcia C, et al. Platelet activation and prothrombotic properties in a mouse model of peritoneal sepsis. Sci Rep. (2018) 8:13536. doi: 10.1038/s41598-018-31910-8
31. Nording HM, Seizer P, Langer HF. Platelets in inflammation and atherogenesis. Front Immunol. (2015) 6:98. doi: 10.3389/fimmu.2015.00098
32. Balta S, Ozturk C. The platelet-lymphocyte ratio: a simple, inexpensive and rapid prognostic marker for cardiovascular events. Platelets. (2015) 26:680–1. doi: 10.3109/09537104.2014.979340
33. Gary T, Pichler M, Belaj K, Hafner F, Gerger A, Froehlich H, et al. Platelet-to-lymphocyte ratio: a novel marker for critical limb ischemia in peripheral arterial occlusive disease patients. PLoS ONE. (2013) 8:e67888. doi: 10.1371/journal.pone.0067688
34. Templeton AJ, Ace O, McNamara MG, Al-Mubarak M, Vera-Badillo FE, Hermanns T, et al. Prognostic role of platelet: lymphocyte ratio in solid tumors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. (2014) 23:1204–12. doi: 10.1158/1055-9965.EPI-14-0146
35. Tan D, Fu Y, Su Q, Wang H. Prognostic role of platelet: lymphocyte ratio in colorectal cancer: a systematic review and meta-analysis. Medicine (Baltimore). (2016) 95:e3837. doi: 10.1097/MD.0000000000003837
36. Li W, Liu Q, Tang Y. Platelet to lymphocyte ratio in the prediction of adverse outcomes after acute coronary syndrome: a meta-analysis. Sci Rep. (2017) 7:40426. doi: 10.1038/srep40426
37. Wang Q, Ma J, Jiang Z, Ming L. Prognostic value of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in acute pulmonary embolism: a systematic review and meta-analysis. Int Angiol. (2018) 37:4–11. doi: 10.23736/S0392-9590.17.03848-2
38. Gasparyan AY, Ayvazyan L, Mukanova U, Yessirkepov M, Kitas GD. The platelet-to-lymphocyte ratio as an inflammatory marker in rheumatic diseases. Ann Lab Med. (2019) 39:345–57. doi: 10.3343/alm.2019.39.4.345
Keywords: NEC, PLR, association, preterm neonates, prediction, generalized additive models
Citation: Zheng J and Wang H (2021) Association Between Platelet-to-Lymphocyte Ratio and Preterm Necrotizing Enterocolitis. Front. Pediatr. 9:686880. doi: 10.3389/fped.2021.686880
Received: 28 March 2021; Accepted: 11 October 2021;
Published: 05 November 2021.
Edited by:
Fernando Cabañas, Quironsalud Madrid University Hospital, SpainReviewed by:
Jianwen Xiao, Children‘s Hospital of Chongqing Medical University, ChinaAna Hou, ShengJing Hospital of China Medical University, China
Copyright © 2021 Zheng and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hua Wang, d2FuZ2h1YUBzY3UuZWR1LmNu
 Juan Zheng
Juan Zheng Hua Wang
Hua Wang