
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Pediatr. , 22 June 2021
Sec. Pediatric Infectious Diseases
Volume 9 - 2021 | https://doi.org/10.3389/fped.2021.686386
 Alastair F. Murray1*
Alastair F. Murray1* Anne Emanuels2
Anne Emanuels2 Caitlin Wolf2
Caitlin Wolf2 Nicholas Franko2
Nicholas Franko2 Lea Starita3
Lea Starita3 Janet A. Englund4
Janet A. Englund4 Helen Y. Chu1
Helen Y. Chu1In order to assess the presence of respiratory pathogens on “high-touch” surfaces and inform sanitation practices at schools, pre-selected surfaces in elementary schools in Seattle, WA, USA were sampled weekly and tested by RT-PCR for 25 viral respiratory pathogens (including SARS-CoV-2 retrospectively) and S. pneumoniae during 2019–2020 winter respiratory illness season. Viral pathogens (rhinovirus, adenovirus, influenza) known to cause respiratory illness were detected on commonly touched surfaces, especially wooden surfaces, and matched the patterns of circulating virus in the community.
The emergence of SARS-CoV-2 has further underscored the importance of detecting and eliminating respiratory pathogens in high-risk congregate settings. The density and proximity of children within school and childcare facilities provides a ready environment for infectious disease transmission (1, 2). Previous environmental surveillance studies for respiratory pathogens have used bioaerosol sampling and surface swabbing techniques to detect spread of respiratory viruses (3, 4). To identify sources of pathogen transmission in the school environment, we sampled “high-touch” surfaces in elementary schools in Seattle, WA, USA and performed weekly air sampling during the winter respiratory illness season of 2019–2020.
Surface swabs were collected weekly at three public elementary schools in Seattle from November 2019 to March 2020. High-touch plastic, wood and metal surfaces including door and fountain handles and handrails were pre-selected and sampled weekly by thoroughly wiping with a synthetic polyester swab in the same time window (5). Additionally, intensive twice daily sampling during the last week of February 2020 was conducted in two schools in mornings and prior to dismissal. Scheduled school cleaning typically took place daily in the afternoons after dismissal. Bioaerosol samples from a cafeteria and hallway were collected during a weekly 90-min period using the SKC QuickTake 30 Air Pump (5). All samples were tested for 25 respiratory viruses including SARS-CoV-2 and S. pneumoniae by multiplex TaqMan real-time PCR on the OpenArray platform (Thermo). Cycle relative threshold (Crt) values were measured for each pathogen and for ribonuclease (RNAse) P, a marker of human cellular material detected on swabs. Prevalence of pathogens was compared to city-wide prevalence of viral infections captured by a related, contemporaneous study (Figure 1) (5).
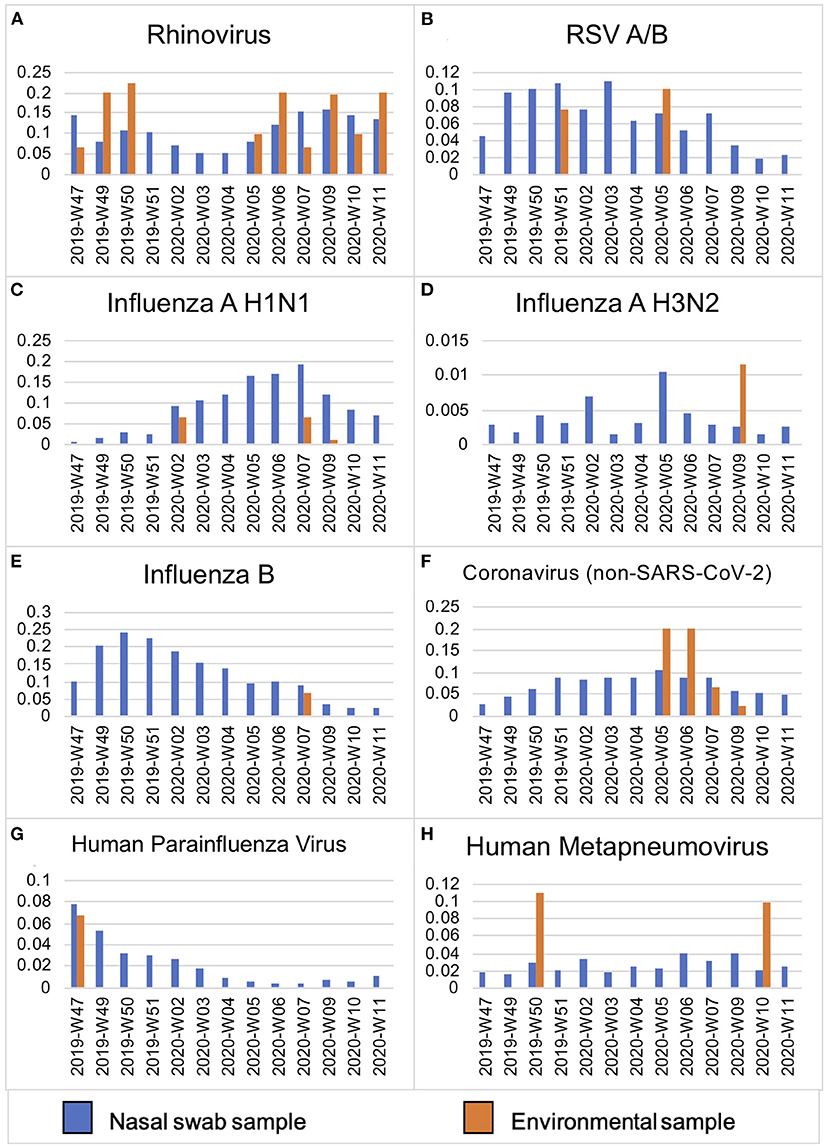
Figure 1. Viral prevalence by week in Seattle, WA from November 2019–March 2020 in symptomatic individuals as compared to environmental surface swabs in schools. Prevalence (y-axis) of viral pathogens (A–H) detected in de-identified residual samples from hospitalized patients and prospective mid-turbinate swabs from symptomatic individuals in Seattle, WA, USA (n = 12,458) as compared to contemporaneous environmental surface swab sampling in Seattle public schools (n = 208) between November 2019–March 2020 (by week, x-axis).
Of samples collected at three schools, 49 (34%) of 208 total samples tested positive for one or more viruses, most commonly human rhinovirus (hRV) (28/208, 13%) and adenovirus (14/208, 6.7%) (Table 1). Overall, 65% of samples were positive for S. pneumoniae. No SARS-CoV-2 was detected on any of the samples tested. Overall mean RNAse P Crt value was 25 (s.d. 2.9) with no significant difference by surface material type (plastic, wood or metal). There was no significant difference in viral and bacterial Crt values across surface material type, although wooden surfaces had the highest quantity of viral detections (average, 1.3 viral detections per sample collected) followed by metal (average, 0.2 viral detections per sample). No virus was detected through bioaerosol sampling. During the week of twice daily intensive sampling, no virus was persistently detected on a surface for more than two consecutive samples. More viruses were found during morning compared to afternoon sampling, but not significantly so (t-test, p = 0.24).
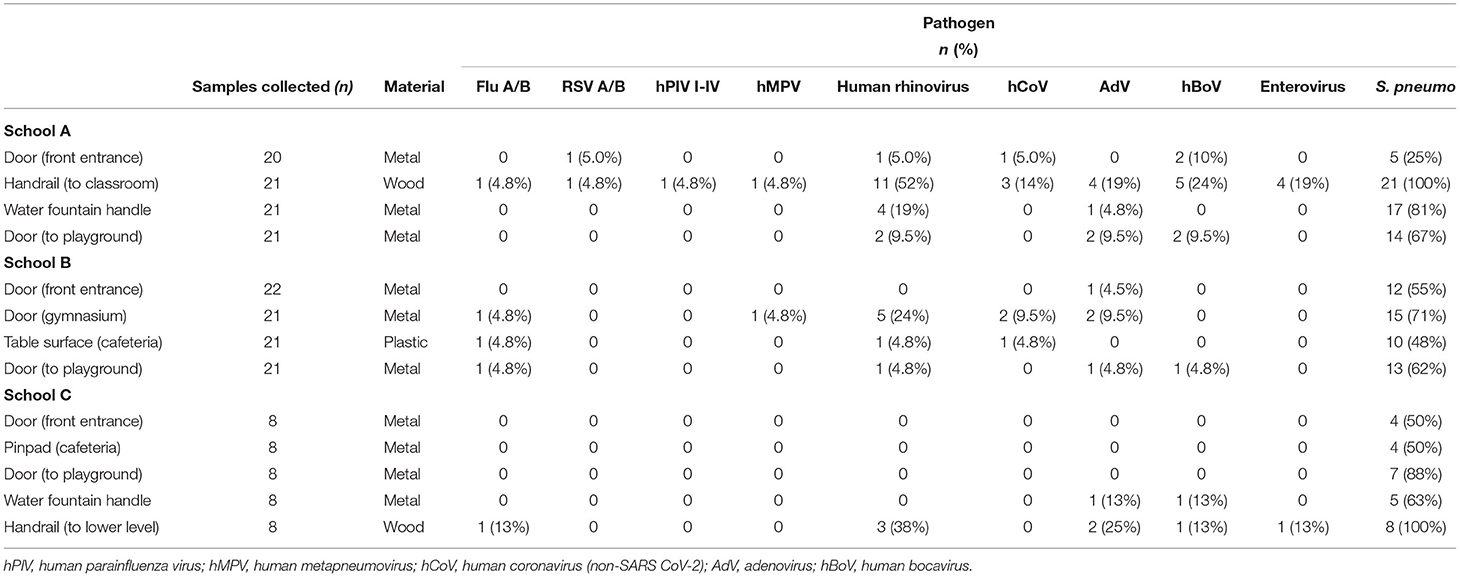
Table 1. Sites, surfaces, and pathogens detected between 13 surfaces sampled across three elementary schools in Seattle, WA.
At the onset of the SARS-CoV-2 pandemic, restrictions were placed on in-person learning in the United States to help decrease viral transmission. Given the prolonged disruption to childhood education, there is an increasing need for evidence to guide safe school reopening policies and inform sanitation practices (6, 7). This school-based surveillance study conducted around the time of United States community transmission of SARS-CoV-2 (November 2019–March 2020) and before school closures, shows that respiratory pathogens known to cause illness were detected on commonly touched surfaces. Risks posed from fomite transmission vs. aerosol transmission are pathogen-specific (8–10).
Nasopharyngeal colonization with S. pneumoniae has been well-described in the pediatric population, and in this study S. pneumoniae was near ubiquitous on surfaces. Previous studies have demonstrated the fomite potential of S. pneumoniae, through tolerance of desiccation and the potential to remain infectious on rehydration (11). It is unlikely that the presence of S. pneumoniae on these surfaces resulted in clinical illness, both in terms of quality of inoculum and serotype in the post-pneumococcal vaccine era (12).
Rhinovirus and adenovirus, both non-enveloped viruses known to persist under harsh environmental conditions, were also the most commonly detected on surfaces at schools in this study (13). It is unclear if the quality or viral load of the inoculum was sufficient to contribute to direct spread of disease, as we did not culture for pathogens. However, the viruses found on surfaces at schools did mirror the prevalence pattern of viral positives in the community from individuals with symptomatic respiratory illness (5) (Figure 1). This finding demonstrates how environmental surface sampling could serve as a non-invasive surveillance tool to detect human viral circulation within a larger community.
Considering how this data may inform school sanitation practices, we found that, across three elementary schools, high-touch wooden surfaces had the highest number of viral pathogen detections. Overall, viral presence on surfaces was transient and did not persist for longer than 1 day (two consecutive samplings) during the intensive week of sampling, either as result of natural degradation or efficacy of standard cleaning practices (14). As schools continue to re-open, frequent targeted cleaning of high-touch surfaces in schools, particularly wooden surfaces, can complement other measures to reduce potential fomite transmission of perennial respiratory pathogens in the context of the global SARS-CoV-2 pandemic.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
AM drafted the manuscript and performed data analysis with assistance from NF and AE. AE and CW contributed to methodology and oversight. LS, HC, and JE obtained funding and conceptualized the study in addition to providing oversight. LS assisted with investigation and data collection. All authors have reviewed and commented on the manuscript.
The Seattle Flu Study was funded by Gates Ventures. The funder was not involved in the design of the study, does not have any ownership over the management and conduct of the study, the data, or the rights to publish.
HC has received research support from Cepheid, and consults for Merck, Pfizer, Gates Foundation, and Ellume. JE has received research support from GlaxoSmithKline, Merck, Pfizer, and AstraZeneca and personal fees from Sanofi Pasteur and Meissa Vaccines.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Lessler J, Reich NG, Cummings DAT. Outbreak of 2009. Pandemic Influenza A (H1N1) at a New York City School. N Engl J Med. (2009) 361:2628–36. doi: 10.1056/nejmoa0906089
2. Fong MW, Leung NHL, Xiao J, Chu DKW, Cheng SMS, So HC, et al. Presence of influenza virus on touch surfaces in kindergartens and primary schools. J Infect Dis. (2020) 222:1329–33. doi: 10.1093/infdis/jiaa114
3. Coleman KK, Sigler WV. Airborne influenza a Virus exposure in an elementary school. Sci Rep. (2020) 10:1859. doi: 10.1038/s41598-020-58588-1
4. Ikonen N, Savolainen-Kopra C, Enstone JE, Kulmala I, Pasanen P, Salmela A, et al. Deposition of respiratory virus pathogens on frequently touched surfaces at airports. BMC Infect Dis. (2018) 18:437. doi: 10.1186/s12879-018-3150-5
5. Chu HY, Boeckh M, Englund JA, Famulare M, Lutz B, Nickerson DA, et al. The seattle flu study: a multiarm community-based prospective study protocol for assessing influenza prevalence, transmission and genomic epidemiology. BMJ Open. (2020) 10:e037295. doi: 10.1136/bmjopen-2020-037295
6. Coronado F, Blough S, Bergeron D, Proia K, Sauber-Schatz E, Beltran M, et al. Implementing mitigation strategies in early care and education settings for prevention of sARS-CoV-2 transmission—eight states, september–October 2020. MMWR Morb Mortal Wkly Rep. (2020) 69:1868–72. doi: 10.15585/mmwr.mm6949e3
7. Sandora TJ, Shih MC, Goldmann DA. Reducing absenteeism from gastrointestinal and respiratory illness in elementary school students: a randomized, controlled trial of an infection-control intervention. Pediatrics. (2008) 121:e1555–62.doi: 10.1542/peds.2007-2597
8. Binder RA, Alarja NA, Robie ER, Kochek KE, Xiu L, Rocha-Melogno L, et al. Environmental and aerosolized sARS-CoV-2 among hospitalized cOVID-19 patients. J Infect Dis. (2020) 222:1798–806. doi: 10.1093/infdis/jiaa575
9. Kraay ANM, Hayashi MAL, Hernandez-Ceron N, SpicknallIH, Eisenberg MC, Meza R, et al. Fomite-mediated transmission as sufficient pathway: a comparative analysis across three viral pathogens. BMC Infect Dis. (2018) 18:540. doi: 10.1186/s12879-018-3425-x
10. van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, et al. Aerosol and surface stability of sARS-CoV-2 as compared with sARS-CoV-1. N Engl J Med. (2020) 382:1564–7. doi: 10.1056/nejmc2004973
11. Walsh RL, Camilli A. Streptococcus pneumoniae Is Desiccation tolerant and Infectious upon Rehydration. MBio. (2011) 2:11. doi: 10.1128/mbio.00092-11
12. Weinberger DM, Malley R, Lipsitch M. Serotype replacement in disease after pneumococcal vaccination. Lancet. (2011) 378:1962–73. doi: 10.1016/s0140-6736(10)62225-8
13. Vasickova P, Pavlik I, Verani M, Carducci A. Issues concerning survival of viruses on surfaces. Food Environ Virol. (2010) 2:24–34. doi: 10.1007/s12560-010-9025-6
Keywords: respiratory virus, school health, influenza, rhinovirus, viral surveillance, fomite surface, adenovirus
Citation: Murray AF, Emanuels A, Wolf C, Franko N, Starita L, Englund JA and Chu HY (2021) School-Based Surveillance of Respiratory Pathogens on “High-Touch” Surfaces. Front. Pediatr. 9:686386. doi: 10.3389/fped.2021.686386
Received: 26 March 2021; Accepted: 17 May 2021;
Published: 22 June 2021.
Edited by:
Robert Cohen, Independent Researcher, Paris, FranceReviewed by:
Josette Raymond, Service de Bactériologie-Hygiène, Hôpital de Bicêtre, FranceCopyright © 2021 Murray, Emanuels, Wolf, Franko, Starita, Englund and Chu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alastair F. Murray, YWZtdXJyYXlAdXcuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.