- Department of Heart Center, Guangzhou Women and Children's Medical Center, Guangzhou Medical University, Guangzhou, China
Background: Acute kidney injury (AKI) is a common and serious complication faced by children following ventricular septal defect (VSD) surgery with cardiopulmonary bypass (CPB). The objective of this study was to explore potential predictors inherent to AKI.
Methods: VSD infants who were scheduled for elective cardiac surgery with CPB from 2017 to 2020 were enrolled in this study. Based on the Pediatric Risk, Injury, Failure, Loss, End-Stage Renal Disease (pRIFLE) criteria, patients were divided into AKI and non-AKI groups. Univariate and multivariate logistic regression analyses were carried out in order to evaluate potential risk factors for AKI. Receiver operating characteristic (ROC) curves were generated to evaluate the predictive probabilities of risk factors for AKI.
Results: Of all the 338 enrolled VSD infants, 49 manifested AKI with an incidence of 14.5% (49/338). The ROC curve indicated that albumin-to-fibrinogen ratio (AFR) during CPB was a significant predictor of AKI [area under the curve (AUC), 0.711; p < 0.001]. Based on the univariate and multivariate logistic analyses, AFR during CPB [odds ratio (OR), 1.89; 95% confidence interval (CI), 1.22–2.76, p = 0.011] was the only independent risk factor for AKI.
Conclusions: This study demonstrated that a low AFR (<9.35) during CPB was an independent risk factor for AKI in VSD infants following cardiac surgery with CPB.
Introduction
Ventricular septal defect (VSD) is the most frequent congenital heart disease (CHD) as seen in both adults and children, which accounts for ~40% of all CHDs (1). Repair surgery with cardiopulmonary bypass (CPB) was a primary therapeutic strategy for infants suffering from VSD (2). CPB is a major contributor to postoperative complications and morbidity, particularly in the case of pediatric cardiac surgery (3). Acute kidney injury (AKI) is well-established as a common and serious complication seen in children following CHD surgery with CPB (4). AKI strongly affects short- and long-term outcomes, and it has been identified as an independent risk factor for a high morbidity and mortality rate in the wake of CPB (5, 6). Therefore, it is crucially important to predict AKI among VSD infants undergoing cardiac surgery with CPB.
The presence of diabetes (7), chronic kidney disease (8), sepsis and septic shock (9), and nephrotoxic agents (10) are independent risk factors with respect to AKI following cardiac surgery. However, comparatively few studies have made an attempt to examined hematological biomarkers as independent predictors of AKI. Albumin (Alb), a critical liver function index, can serve as a useful indicator for inflammatory and nutritional status (11). Fibrinogen (Fib), an essential protein in coagulation, is also widely recognized as a sensitive index for inflammatory status (12). Alb-to-Fib ratio (AFR), which combines Alb and Fib, has been used as prognostic factors for different classes of diseases, including peritonitis-induced sepsis (13), advanced epithelial ovarian cancer (14), untreated chronic lymphocytic leukemia (15), and nonsmall cell lung cancer (16). However, it is unknown whether AFR is able to serve as an independent prognostic marker for AKI in VSD infants after cardiac surgery with CPB. The objective of this study was to explore potential predictors of AKI and provide novel insights into AKI prevention.
Materials and Methods
Patients
The retrospective study protocol was reviewed and approved by the Ethics Committee of our hospital. Infants diagnosed with VSD at the Department of Cardiovascular Center, Guangzhou Women and Children's Medical Center during the period from 2017 to 2020 were enrolled in this study. Inclusion criteria were the following: (1) aged < 1 year, (2) diagnosed with VSD and planned for elective cardiac surgery with CPB, and (3) provided written informed consent with parents' signatures. Exclusion criteria were the following: (1) with renal dysfunction prior to surgery; (2) with the conditions that impact Alb and Fib expressions, e.g., liver function disorder, coagulation/blood dysfunction, etc.; (3) with multiple operations simultaneously or within 14 days; (4) with incomplete clinical data; and (5) followed up <30 days following surgery.
Treatment
All the enrolled infants underwent the same operation and CPB. All surgeries were performed via routine median sternotomy incision under CPB and cardiac incision including right atrial incision, main pulmonary artery incision, or a combination. Extracorporeal circulation was established by conventional cannulation of the ascending aorta and superior–inferior venae cava. CPB was carried out in moderate hypothermia, with the rectal temperature at 32°C. After the ascending aorta being cross clamped, histidine–tryptophan–ketoglutarate cardioplegic solution was perfused through the aortic root. After cardiac arrest, a left ventricular vent was placed through the foramen ovale, and VSD was exposed via the tricuspid valve. VSD repair was performed with intermittent or continuous suture using autologous pericardium as the repair material. Blood samples were harvested during CPB (30 min after CPB initiation) for the subsequent laboratory analyses. The left ventricular vent was then pulled out, and the foramen ovale was sutured. The ascending aorta was opened, and rewarming was started with the satisfied left heart venting. The right atrial incision was closed, and the patients were gradually weaned from CPB.
Data Collection
The clinical data were collected and laid out as below: (1) demographic data, including age, gender, weight, and height; (2) preoperative clinical data, including the Risk Adjustment for Congenital Heart Surgery 1 (RACHS-1) score (17), history of pneumonia and chronic heart failure, and left ventricular ejection fraction (LVEF); (3) intraoperative data, including duration of CPB and aortic cross-clamping (ACC), ultrafiltration, hypothermic circulatory arrest, and urine output; (4) postoperative clinical and outcomes data, including duration of postoperative mechanical ventilation, postoperative hypotension, inputs of blood products, AKI, and duration of cardiac care unit (CCU) and hospital stay; and (5) laboratory variables during CPB, including hemoglobin (Hb), glucose, lactic acid, creatinine, blood urea nitrogen (BUN), total cholesterol, triglycerides, low-density lipoprotein cholesterol (LDL-C), Alb, and Fib.
Definitions
According to the Pediatric Risk, Injury, Failure, Loss, End-Stage Renal Disease (pRIFLE) criteria (18), AKI was defined when the postoperative creatinine clearance (CC) within 48 h was 25% lower than at the preoperative level, or the urine volume <0.5 ml/(k·h) ≥ 8 h.
Statistical Analysis
Data were analyzed using SPSS version 19.0 (SPSS Inc., Chicago, IL, USA) and GraphPad version 8.0 (GraphPad Inc., California, CA, USA). Prior to the study, we performed a simple sample size estimation: (10–15) × the number of covariates (n = 27 in our study) based on the guidance of the statistical textbook. Thus, the minimum required sample size was 270, and 338 infants were included in this study with a calculated power value >0.8 using GPower 3.1. Quantitative variables were presented as mean ± standard deviation (SD) while qualitative variables as number with percentage (n, %). Continuous variables were compared utilizing Student's t-test for normal distributed data or the Mann–Whitney test for non-normal distributed data. Categorical variables were compared by means of a chi-squared test or Fisher's exact test. Receiver operating characteristic (ROC) curves were generated to evaluate the predictive probabilities of risk factors for AKI using the area under the curve (AUC), 95% confidence interval (CI), specificity, and sensitivity. The optimal cutoff value was determined by calculating the Youden index of the ROC curve. Based on the cutoff, continuous data were categorized into high (≥cutoff) and low (<cutoff) expression groups. To analyze possible risk factors of AKI, we applied a binary univariate logistic regression, followed by multivariate logistic regression with the “Enter” method. Only those factors with a p < 0.05 in the univariate logistic model were included in the multivariate logistic model. A two-tailed p < 0.05 was considered statistically different.
Results
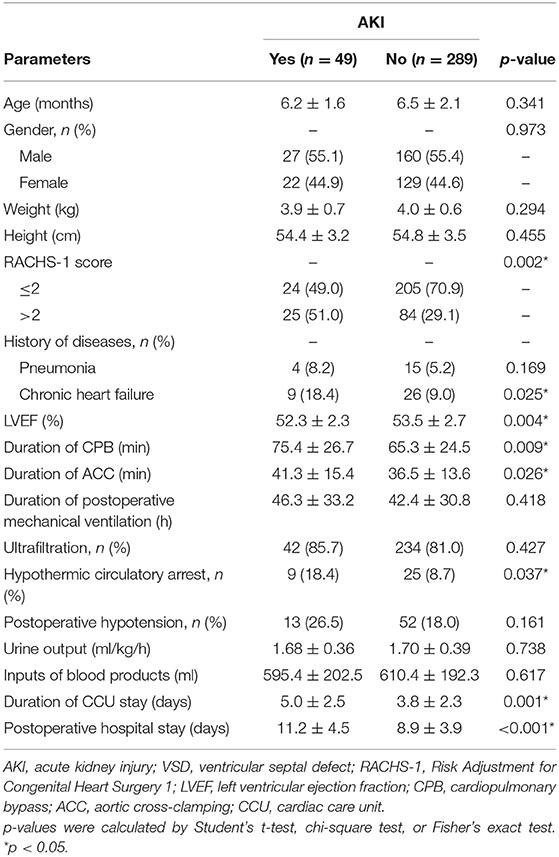
As demonstrated by the flow chart diagram seen in Figure 1, 382 VSD infants were initially enrolled, and 44 were then excluded according to the exclusion criteria. As a result, 338 infants were finally included in the data analysis. Of all the enrolled patients, 49 exhibited AKI with an incidence of 14.5% (49/338). The overall mean age of was 6.5 months (median, 6 months), and the majority were male (55.3%, 187/338). The demographic, preoperative, intraoperative, and postoperative clinical and outcomes data associated with AKI development are summarized in Table 1. The rate of RACHS-1 score >2 was significantly higher for patients with AKI than those without (51.0 vs. 29.1%, p = 0.002). Those infants with a longer duration of CPB and ACC were associated with an increased risk of AKI (p = 0.009 and 0.026, respectively). Patients with the presence of preoperative chronic heart failure (p = 0.025), hypothermic circulatory arrest (p = 0.037), and a lower preoperative LVEF (p = 0.004) were more likely to develop AKI. Moreover, AKI patients were associated with a prolonged CCU (p = 0.001) and hospital stay (p < 0.001). However, no significant differences were observed in terms of age, gender, weight, height, presence of pneumonia, duration of postoperative mechanical ventilation, ultrafiltration, postoperative hypotension, urine output, and the inputs of blood products between AKI and non-AKI groups (p > 0.05).
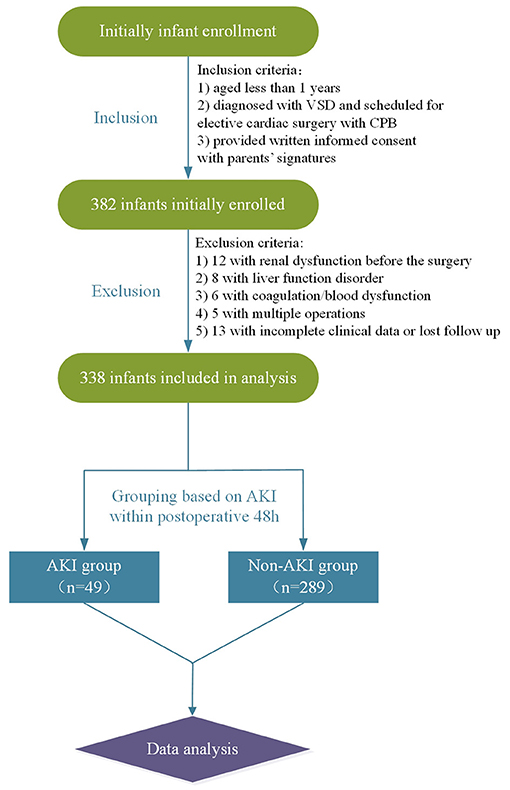
Figure 1. Flow chart. VSD, ventricular septal defect; CPB, cardiopulmonary bypass; AKI, acute kidney injury.
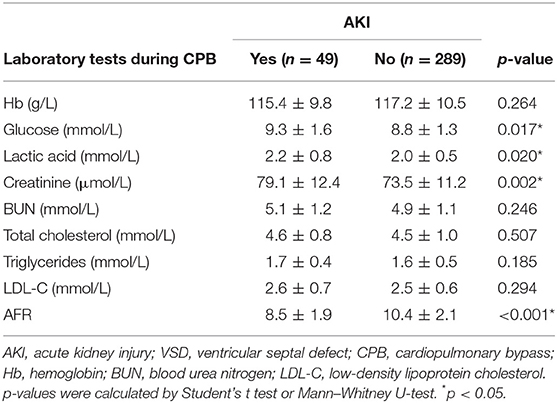
Table 2 compares the laboratory variables between patients with or without AKI. No difference was observed with respect to Hb, BUN, total cholesterol, triglycerides, and LDL-C. Patients with AKI displayed remarkably higher levels of glucose (p = 0.017), lactic acid (p = 0.020), and creatinine (p = 0.002) during CPB when compared with those without AKI. The AFR level in the AKI group was notably lower than that of the non-AKI group (8.5 ± 1.9 vs. 10.4 ± 2.1, p < 0.001).
The ROC curves were used to determine the predictive and cutoff values of continuous variables for AKI. As shown in Figure 2, the duration of CPB (AUC, 0.647; sensitivity, 50.17%; specificity, 71.43%; p = 0.001), ACC (AUC, 0.642; sensitivity, 66.09%; specificity, 59.18%; p = 0.002), glucose (AUC, 0.622; sensitivity, 46.71%; specificity, 73.47%; p = 0.006), creatinine (AUC, 0.641; sensitivity, 49.48%; specificity, 69.39%; p = 0.002), and AFR (AUC, 0.711; sensitivity, 66.78%; specificity, 63.27%; p < 0.001) were significant predictors of AKI. Based on the cutoff values, these continuous variables were categorized into two groups (high vs. low). Twelve potential risk factors (p < 0.05 in Tables 1, 2) were included in the univariate logistic regression model. As illustrated in Figure 3, RCAHS-1 score [odds ratio (OR), 1.89; 95% CI, 1.22–2.76; p = 0.011], duration of CPB (OR, 1.89; 95%CI, 1.22–2.76; p = 0.011), and ACC (OR, 1.89; 95% CI, 1.22–2.76, p = 0.011), hypothermic circulatory arrest (OR, 1.89; 95% CI, 1.22–2.76; p = 0.011), creatinine (OR, 1.89; 95% CI, 1.22–2.76, p = 0.011), and AFR (OR, 1.89; 95% CI, 1.22–2.76; p = 0.011) during CPB were six identifiable risk factors for AKI. These significant factors were further added to the multivariate logistic model. The forest plot in Figure 4 indicated that AFR during CPB (OR, 1.89; 95% CI, 1.22–2.76, p = 0.011) was the only independent risk factor pertinent to AKI in VSD infants with CPB.
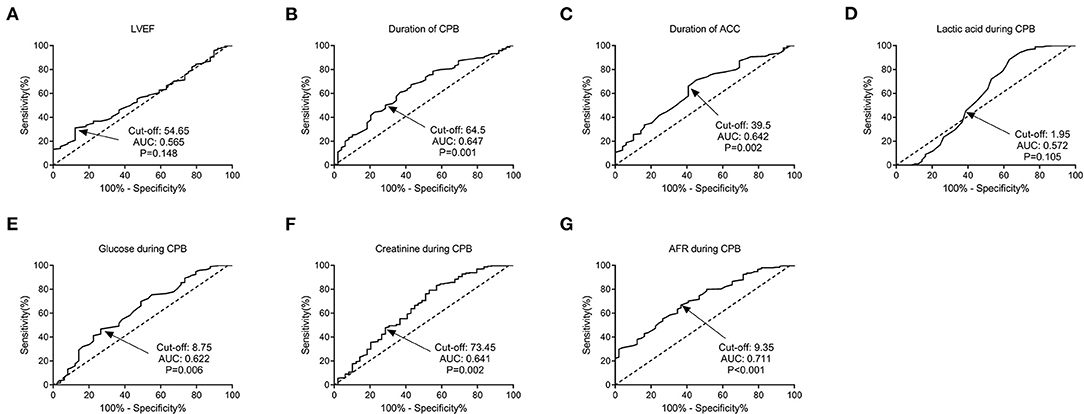
Figure 2. Predictive values of factors for AKI in VSD infants by ROC curve analysis. (A) LVEF; (B) duration of CPB; (C) duration of ACC; (D) lactic acid; (E) glucose; (F) creatinine; and (G) AFR during CPB. AFR was the most significant predictor for AKI, with a cut-off value of 9.35, an AUC of 0.711, a sensitivity of 66.78%, and a specificity of 63.27% (P < 0.001). VSD, ventricular septal defect; CPB, cardiopulmonary bypass; AKI, acute kidney injury; LVEF, left ventricular ejection fraction; ACC, aortic cross-clamping; AFR, albumin-to-fibrinogen ratio; ROC, receiver operating characteristic; AUC, area under the curve.
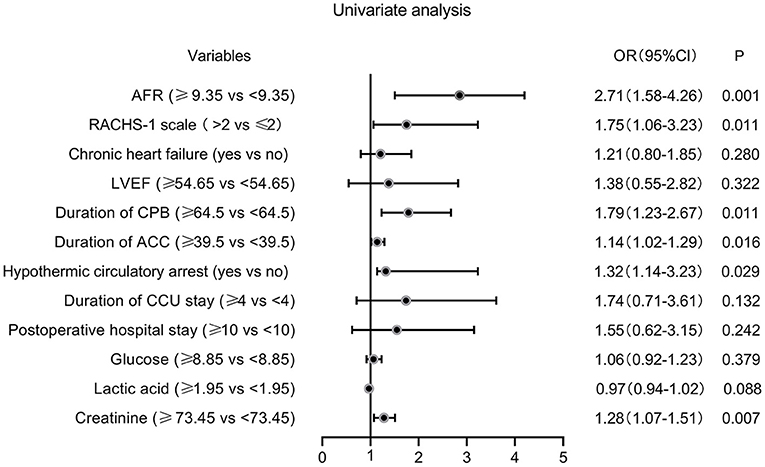
Figure 3. Forest plot of the univariate logistic regression analysis. AFR, albumin-to-fibrinogen ratio; RACHS-1, Risk Adjustment for Congenital Heart Surgery 1; LVEF, left ventricular ejection fraction; CPB, cardiopulmonary bypass; ACC, aortic cross-clamping; CCU, cardiac care unit; OR, odds ratio; CI, confidence interval.
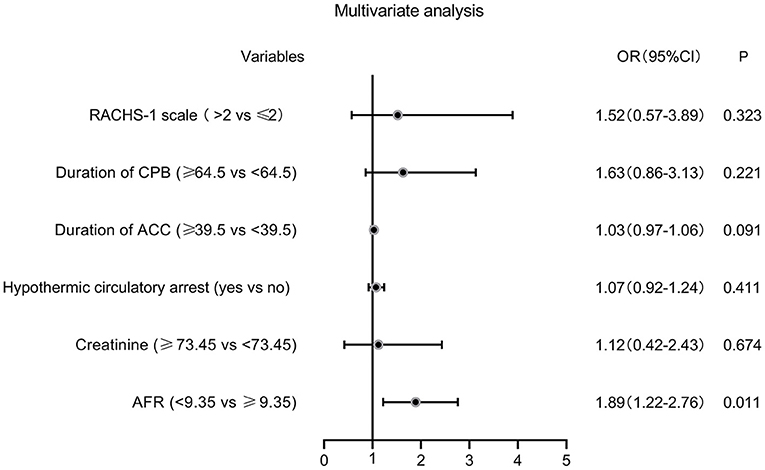
Figure 4. Forest plot of the multivariate logistic regression analysis. AFR, albumin-to-fibrinogen ratio; RACHS-1, Risk Adjustment for Congenital Heart Surgery 1; CPB, cardiopulmonary bypass; ACC, aortic cross-clamping; OR, odds ratio; CI, confidence interval.
Discussion
This study indicated for the first time that a lower AFR during CPB (<9.35) was an independent risk factor for AKI occurrence in VSD infants following operation with CPB. AKI is a common and serious postoperative complication in patients after cardiac surgery (19). The incidence of AKI following pediatric cardiac surgery varies widely across different studies, ranging from 11% (20) to 52% (21). In this present study, the incidence of AKI was calculated as 14.5%, which was quite in accordance with the reported 14.1% by Lee et al. (22) and 13.2% by Fuhrman et al. (23). In our minds, different patient characteristics (e.g., age ranges, races, operation types, etc.), sample sizes, and definitions of AKI may serve to explain the various incidences of AKI in different studies. As indicated by many studies, AKI has been recognized as a risk factor for a prolonged hospitalization, a more complicated clinical course, and increased mortality following cardiac surgery (21). However, the early diagnosis of AKI is quite challenging due to its diverse clinical manifestations, varying from asymptomatic to oliguria, and renal failure. Therefore, it is of great clinical importance to investigate potential risk factors inherent to AKI. Previous reports by Rosner et al. (24) revealed that cardiac-surgery-associated characteristics, including the use of CPB, duration of CPB and ACC, and high levels of vasopressors, were closely correlated with increased risks of AKI occurrence. The altered renal perfusion, ischemia/reperfusion injury, elevated oxidative damages, and inflammation are the potential explanations for the development of AKI (25). Our univariate logistic analysis also supported the potential association between duration of CPB, ACC, and AKI development.
Alb and Fib are two widely reported proteins with inflammatory, nutritional, and hemorheological properties (26). Alb, an important serum component, is a significant regulator of colloid osmotic pressure (27). A low level of Alb is widely observed under inflammatory stimuli, due to decreased synthesis and enhanced catabolism (28). In addition, Alb can act to inhibit the activation and aggregation of platelets (29), and a decreased level will typically lead to increased blood viscosity and impaired endothelial function (30). Fib, an important coagulation factor, is a regulatory component for inflammation via modulating the functions of leukocyte effector and leukocyte transmigration (31). The circulated Fib level is noteworthy for being upregulated in inflammatory status, and it can promote the aggregation of platelets (32, 33). Moreover, significantly increased Fib in kidney tissues is observed following AKI (34). Alb and Fib were both reported to be significant prognostic factors in the case of a variety of diseases (35–38). AFR, an index integrating Alb and Fib, offers a more sensitive reflection of the status of systemic inflammation, blood thrombogenicity, and viscosity when compared with Alb or Fib alone (39). Mechanisms of AKI following cardiac surgery include perioperative renal ischemia, reperfusion injury, inflammation, hemolysis, and pigment nephropathy caused by CPB (25, 40). Previous studies indicate hypoalbuminemia as a modifiable risk factor for AKI in patients with off-pump coronary artery bypass surgery (25). We considered that these may be possible explanations for the predictive role of AFR for AKI occurrence.
Some limitations should be concerned. First, the sample size is not large enough. Second, this is a retrospective study that may harbor potential selection bias. Third, the laboratory variables were detected during CPB without dynamic monitoring. Lastly, whether any benefit can be derived from AFR modulation (e.g., Alb infusion) for AKI prevention remains unclear. Additional prospective multicenter studies addressing larger sample sizes and experimental studies are necessary to verify our results.
Conclusions
In conclusion, this study demonstrated that a low AFR (<9.35) during CPB was an independent risk factor for AKI in VSD infants following cardiac surgery with CPB. In our minds, AFR evaluation will aid early prediction and secondary prevention of AKI. AFR, as an easily available biomarker, has a potential in terms of clinical practice for prognosis improvement.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
This retrospective study protocol was reviewed and approved by the Ethics Committee of our hospital. Written informed consent to participate in this study was provided by the participants or their legal guardian/next of kin.
Author Contributions
FC, MZ, and GH: project development, data collection, data analysis, and manuscript writing. WL, NZ, and HY: data collection, project development. XC: project development and data analysis. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Penny DJ, Vick GW 3rd. Ventricular septal defect. Lancet. (2011) 377:1103–12. doi: 10.1016/S0140-6736(10)61339-6
2. Holzer RJ, Sallehuddin A, Hijazi ZM. Surgical strategies and novel alternatives for the closure of ventricular septal defects. Expert Rev Cardiovasc Ther. (2016) 14:831–41. doi: 10.1586/14779072.2016.1169923
3. Milovanovic V, Bisenic D, Mimic B, Ali B, Cantinotti M, Soldatovic I, et al. Reevaluating the importance of modified ultrafiltration in contemporary pediatric cardiac surgery. J Clin Med. (2018) 7:498. doi: 10.3390/jcm7120498
4. Ricci Z, Di Nardo M, Iacoella C, Netto R, Picca S, Cogo P. Pediatric RIFLE for acute kidney injury diagnosis and prognosis for children undergoing cardiac surgery: a single-center prospective observational study. Pediatr Cardiol. (2013) 34:1404–8. doi: 10.1007/s00246-013-0662-z
5. Ko SF, Yip HK, Zhen YY, Lee CC, Lee CC, Huang SJ, et al. Severe bilateral ischemic-reperfusion renal injury: hyperacute and acute changes in apparent diffusion coefficient, T1, and T2 mapping with immunohistochemical correlations. Sci Rep. (2017) 7:1725. doi: 10.1038/s41598-017-01895-x
6. Wang C, Zhang J, Han J, Yang Q, Liu J, Liang B. The level of urinary IL-18 in acute kidney injury after cardiopulmonary bypass. Exp Ther Med. (2017) 14:6047–51. doi: 10.3892/etm.2017.5317
7. Kim DJ, Kang JM, Park SH, Kwon HK, Song SJ, Moon H, et al. Diabetes aggravates post-ischaemic renal fibrosis through persistent activation of TGF-β1 and Shh signalling. Sci Rep. (2017) 7:16782. doi: 10.1038/s41598-017-16977-z
8. Tsang JY, Blakeman T, Hegarty J, Humphreys J, Harvey G. Understanding the implementation of interventions to improve the management of chronic kidney disease in primary care: a rapid realist review. Implement Sci. (2016) 11:47. doi: 10.1186/s13012-016-0413-7
9. Ping F, Guo Y, Cao Y, Shang J, Yao S, Zhang J, et al. Metabolomics analysis of the renal cortex in rats with acute kidney injury induced by sepsis. Front Mol Biosci. (2019) 6:152. doi: 10.3389/fmolb.2019.00152
10. Goto Y, Koyama K, Katayama S, Tonai K, Shima J, Koinuma T, et al. Influence of contrast media on renal function and outcomes in patients with sepsis-associated acute kidney injury: a propensity-matched cohort study. Crit Care. (2019) 23:249. doi: 10.1186/s13054-019-2517-3
11. Moujaess E, Fakhoury M, Assi T, Elias H, El Karak F, Ghosn M, et al. The Therapeutic use of human albumin in cancer patients' management. Crit Rev Oncol Hematol. (2017) 120:203–9. doi: 10.1016/j.critrevonc.2017.11.008
12. Vilar R, Fish RJ, Casini A, Neerman-Arbez M. Fibrin(ogen) in human disease: both friend and foe. Haematologica. (2020) 105:284–96. doi: 10.3324/haematol.2019.236901
13. Tai H, Zhu Z, Mei H, Sun W, Zhang W. Albumin-to-fibrinogen ratio independently predicts 28-day mortality in patients with peritonitis-induced sepsis. Mediators Inflamm. (2020) 2020:7280708. doi: 10.1155/2020/7280708
14. Yu W, Ye Z, Fang X, Jiang X, Jiang Y. Preoperative albumin-to-fibrinogen ratio predicts chemotherapy resistance and prognosis in patients with advanced epithelial ovarian cancer. J Ovarian Res. (2019) 12:88. doi: 10.1186/s13048-019-0563-8
15. Zou YX, Qiao J, Zhu HY, Lu RN, Xia Y, Cao L, et al. Albumin-to-fibrinogen ratio as an independent prognostic parameter in untreated chronic lymphocytic leukemia: a retrospective study of 191 cases. Cancer Res Treat. (2019) 51:664–71. doi: 10.4143/crt.2018.358
16. Li SQ, Jiang YH, Lin J, Zhang J, Sun F, Gao QF, et al. Albumin-to-fibrinogen ratio as a promising biomarker to predict clinical outcome of non-small cell lung cancer individuals. Cancer Med. (2018) 7:1221–31. doi: 10.1002/cam4.1428
17. Jenkins KJ, Gauvreau K, Newburger JW, Spray TL, Moller JH, Iezzoni LI. Consensus-based method for risk adjustment for surgery for congenital heart disease. J Thorac Cardiovasc Surg. (2002) 123:110–8. doi: 10.1067/mtc.2002.119064
18. Akcan-Arikan A, Zappitelli M, Loftis LL, Washburn KK, Jefferson LS, Goldstein SL. Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int. (2007) 71:1028–35. doi: 10.1038/sj.ki.5002231
19. Dent CL, Ma Q, Dastrala S, Bennett M, Mitsnefes MM, Barasch J, et al. Plasma neutrophil gelatinase-associated lipocalin predicts acute kidney injury, morbidity and mortality after pediatric cardiac surgery: a prospective uncontrolled cohort study. Crit Care. (2007) 11:R127. doi: 10.1186/cc6192
20. Sethi SK, Goyal D, Yadav DK, Shukla U, Kajala PL, Gupta VK, et al. Predictors of acute kidney injury post-cardiopulmonary bypass in children. Clin Exp Nephrol. (2011) 15:529–34. doi: 10.1007/s10157-011-0440-2
21. Blinder JJ, Goldstein SL, Lee VV, Baycroft A, Fraser CD, Nelson D, et al. Congenital heart surgery in infants: effects of acute kidney injury on outcomes. J Thorac Cardiovasc Surg. (2012) 143:368–74. doi: 10.1016/j.jtcvs.2011.06.021
22. Lee SH, Kim SJ, Kim HJ, Son JS, Lee R, Yoon TG. Acute kidney injury following cardiopulmonary bypass in children- risk factors and outcomes. Circ J. (2017) 81:1522–7. doi: 10.1253/circj.CJ-17-0075
23. Fuhrman DY, Nguyen LG, Sanchez-de-Toledo J, Priyanka P, Kellum JA. Postoperative acute kidney injury in young adults with congenital heart disease. Ann Thorac Surg. (2019) 107:1416–20. doi: 10.1016/j.athoracsur.2019.01.017
24. Rosner MH, Okusa MD. Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol. (2006) 1:19–32. doi: 10.2215/CJN.00240605
25. O'Neal JB, Shaw AD, Billings FT. Acute kidney injury following cardiac surgery: current understanding and future directions. Crit Care. (2016) 20:187. doi: 10.1186/s13054-016-1352-z
26. Ozcan Cetin EH, Konte HC, Temizhan A. Blood viscosity should not be overlooked when evaluating the fibrinogen to albumin ratio. Angiology. (2019) 70:465–6. doi: 10.1177/0003319718822244
27. Raposo AC, Lebrilla CB, Portela RW, Goonatilleke E, Neto FAD, Oria AP. The proteomics of roadside hawk (Rupornis magnirostris), broad-snouted caiman (Caiman latirostris) and loggerhead sea turtle (Caretta caretta) tears. BMC Vet Res. (2020) 16:276. doi: 10.1186/s12917-020-02495-0
28. Don BR, Kaysen G. Serum albumin: relationship to inflammation and nutrition. Semin Dial. (2004) 17:432–7. doi: 10.1111/j.0894-0959.2004.17603.x
29. Gresele P, Deckmyn H, Huybrechts E, Vermylen J. Serum albumin enhances the impairment of platelet aggregation with thromboxane synthase inhibition by increasing the formation of prostaglandin D2. Biochem Pharmacol. (1984) 33:2083–8. doi: 10.1016/0006-2952(84)90577-X
30. Joles JA, Willekes-Koolschijn N, Koomans HA. Hypoalbuminemia causes high blood viscosity by increasing red cell lysophosphatidylcholine. Kidney Int. (1997) 52:761–70. doi: 10.1038/ki.1997.393
31. Luyendyk JP, Schoenecker JG, Flick MJ. The multifaceted role of fibrinogen in tissue injury and inflammation. Blood. (2019) 133:511–20. doi: 10.1182/blood-2018-07-818211
32. Davalos D, Akassoglou K. Fibrinogen as a key regulator of inflammation in disease. Semin Immunopathol. (2012) 34:43–62. doi: 10.1007/s00281-011-0290-8
33. Weisel JW. Fibrinogen and fibrin. Adv Protein Chem. (2005) 70:247–99. doi: 10.1016/S0065-3233(05)70008-5
34. Hoffmann D, Bijol V, Krishnamoorthy A, Gonzalez VR, Frendl G, Zhang Q, et al. Fibrinogen excretion in the urine and immunoreactivity in the kidney serves as a translational biomarker for acute kidney injury. Am J Pathol. (2012) 181:818–28. doi: 10.1016/j.ajpath.2012.06.004
35. Onate-Ocana LF, Aiello-Crocifoglio V, Gallardo-Rincon D, Herrera-Goepfert R, Brom-Valladares R, Carrillo JF, et al. Serum albumin as a significant prognostic factor for patients with gastric carcinoma. Ann Surg Oncol. (2007) 14:381–9. doi: 10.1245/s10434-006-9093-x
36. Cuellar LE, Anampa-Guzman A, Holguin AM, Velarde J, Portillo-Alvarez D, Zuniga-Ninaquispe MA, et al. Prognostic factors in HIV-positive patients with non-Hodgkin lymphoma: a Peruvian experience. Infect Agent Cancer. (2018) 13:27. doi: 10.1186/s13027-018-0200-y
37. Yang S, Guan H, Wang S, Wu H, Sun W, Chen Z, et al. Plasma fibrinogen predicts the prognosis of bladder cancer patients after radical cystectomy. Cancer Manag Res. (2020) 12:9303–14. doi: 10.2147/CMAR.S269244
38. He SS, Wang Y, Yang L, Chen HY, Liang SB, Lu LX, et al. Plasma fibrinogen correlates with metastasis and is associated with prognosis in human nasopharyngeal carcinoma. J Cancer. (2017) 8:403–9. doi: 10.7150/jca.17028
39. Wang C, Li G, Liang X, Qin C, Luo Q, Song R, et al. Predictive value of fibrinogen-to-albumin ratio for post-contrast acute kidney injury in patients undergoing elective percutaneous coronary intervention. Med Sci Monit. (2020) 26:e924498. doi: 10.12659/MSM.924498
Keywords: ventricular septal defect, cardiopulmonary bypass, acute kidney injury, albumin-to-fibrinogen ratio, biomarker
Citation: Cao F, Chen X, Huang G, Liu W, Zhou N, Yuan H and Zou M (2021) The Albumin-to-Fibrinogen Ratio Independently Predicts Acute Kidney Injury in Infants With Ventricular Septal Defect Undergoing Cardiac Surgery With Cardiopulmonary Bypass. Front. Pediatr. 9:682839. doi: 10.3389/fped.2021.682839
Received: 19 March 2021; Accepted: 21 June 2021;
Published: 19 July 2021.
Edited by:
Alessandro Inserra, Bambino Gesù Children Hospital (IRCCS), ItalyReviewed by:
Simone Frediani, Bambino Gesù Children Hospital (IRCCS), ItalyAntonio Pellegrino, University of Rome Tor Vergata, Italy
Copyright © 2021 Cao, Chen, Huang, Liu, Zhou, Yuan and Zou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Minghui Zou, em91c2h1Z3VhbmcyMDA4QDE2My5jb20=; Xinxin Chen, ZHJfY2hlbnhpbnhpbkAxMjYuY29t
 Fan Cao
Fan Cao Xinxin Chen
Xinxin Chen Minghui Zou
Minghui Zou