
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr., 04 June 2021
Sec. Pediatric Critical Care
Volume 9 - 2021 | https://doi.org/10.3389/fped.2021.669683
Objective: Brain function monitoring technology for extracorporeal membrane oxygenation (ECMO) support has been developing quite slowly. Our objective was to explore the data distribution, variation trend, and variability of cerebral tissue regional oxygen saturation (CrSO2) in pediatric patients undergoing ECMO.
Methods: Eight patients who received venoarterial ECMO (V-A ECMO) were included in our study. All of them accepted continuous CrSO2 monitoring by near-infrared spectroscopy (NIRS) within 12 h of ECMO initiation until ECMO wean. Differences in the CrSO2 distribution characteristic, the variation trend of daily CrSO2, and the variability of CrSO2 for the first 5 days following ECMO initiation were compared between survivors and non-survivors according to pediatric intensive care unit (PICU) mortality.
Results: The percentage of time of CrSO2 <60% against the whole monitoring time was significantly lower in survivors in both hemispheres {right: 4.34% [interquartile range (IQR) = 0.39–8.55%] vs. 47.45% [IQR = 36.03–64.52%], p = 0.036; left: 0.40% [IQR = 0.01–1.15%] vs. 30.9% [IQR = 26.92–49.62%], p = 0.036}. Survivors had significantly higher CrSO2 on the first 4 days. Root mean of successive squared differences (RMSSD), the variability variable of CrSO2, was significantly lower in survivors (right: 3.29 ± 0.79 vs. 6.16 ± 0.67, p = 0.002; left: 3.56 ± 1.20 vs. 6.04 ± 1.44, p = 0.039).
Conclusion: Lower CrSO2, CrSO2 <60% over a longer period of time, and higher fluctuation of CrSO2 are likely associated with PICU mortality in pediatric patients undergoing V-A ECMO.
Clinical Trial Registry: URL: http://www.chictr.org.cn/showproj.aspx?proj=46639, trial registry number: ChiCTR1900028021.
Extracorporeal membrane oxygenation (ECMO) is an extracorporeal life support technology used for patients with cardiopulmonary failure as a severe refractory to conventional treatment measure (1–3). With the development of ECMO, it has been widely applied to the clinical (4–6); however, monitoring technology for ECMO support, particularly the brain function monitoring, has been developing quite slowly.
Near-infrared spectroscopy (NIRS) can provide useful data on cerebral tissue regional oxygen saturation (CrSO2). Because of its characteristics, such as non-invasiveness, continuousness, and being performed in real time and at the bedside (7–10), NIRS has been suggested as one of the regular monitoring technologies during ECMO support by the Extracorporeal Life Support Organization (ELSO) (11). Although NIRS is extensively used in various fields, there have been only a few studies on NIRS in the pediatric patients with ECMO. Consequently, the aim of this study was to explore the data distribution, variation trend, and variability of CrSO2 in pediatric patients undergoing ECMO so as to provide a relevant reference.
This prospective observational study was conducted between May 24, 2019, and January 18, 2020 in the 32-bed pediatric intensive care unit (PICU) of the Children's Hospital of Chongqing Medical University, China. All patients aged from 28 days to 18 years needing venoarterial ECMO (V-A ECMO) support were included in this study. Exclusion criteria included the following: with open heart surgery or cardiac catheterization procedure; with ECMO support <24 h; with validated cerebral injury before ECMO, including cerebral hemorrhage and ischemia; with serum total bilirubin >7 mg/dL before ECMO (12); with forehead skin damage not fit for NIRS monitoring.
Assessments of neurologic status were mainly based on the medical history and clinical symptoms and signs before ECMO. Patients suspected of having brain injury were examined with brain computed tomography (CT) or magnetic resonance imaging (MRI). Those patients without suspected brain injury and without abnormal neurologic imaging findings were all considered as without brain injury.
This study has been approved by the Institutional Review Board of Children's Hospital of Chongqing Medical University (2019-237) and has been performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards and has been registered in Chinese Clinical Trial Registry (ChiCTR1900028021). Written informed consent for participation was obtained from children's parents or legal guardians.
Baseline information before ECMO initiation was recorded and included: age, gender, weight, diagnosis, the interval of hospital admission to ECMO initiation, the interval of PICU admission to ECMO initiation, the interval of invasive mechanical ventilation begun to ECMO initiation, the use of vasoactive drugs before ECMO, PaO2/FiO2 ratio, and arterial blood gas parameters (pH, PaCO2, PaO2, SaO2, lactate) before ECMO.
Follow-up variables included ECMO duration, ECMO parameters, treatment measures during ECMO, hemodynamic parameters during ECMO, duration of the invasive mechanical ventilation after ECMO wean, length of PICU and hospital stays, and PICU mortality.
All patients receiving ECMO support were managed by our ECMO team. We chose the mode of ECMO support in pediatric respiratory failure according to the patient's hemodynamic status and weight. We have to choose V-A ECMO in infants and low-weight toddlers with respiratory failure purely for a lack of double-lumen venovenous (V-V) cannulas in China. Venoarterial ECMO was surgically implanted using heparin-coated cannulation (Bio-Medicus, Medtronic Inc., Minneapolis, MN, USA) via the right internal jugular vein and the right common carotid artery. The centrifugal pump (Deltastream DP3, Medos, Stolberg, Germany) and membrane oxygenator (Hilite 800 LT, Hilite 2400 LT, or Hilite 7000 LT, Medos, Stolberg, Germany) were used. ECMO-related parameters and hemodynamic parameters were recorded every 2 h during ECMO support.
Monitoring of CrSO2 began within 12 h of ECMO initiation and continued until the weaning of ECMO using NIRS (FORE-SIGHT P/N01-06-2030C, CAS Medical Systems Inc., Branford, CT, USA). Cerebral tissue regional oxygen saturation data were gathered every 2 s and recorded on the device's memory. Our ECMO team did not adjust therapies following CrSO2 data. The daily median CrSO2 values were calculated for the first 5 days following ECMO initiation in both hemispheres and were then used to analyze the variation trend of CrSO2. At the same time, the median CrSO2 values over 4-h time intervals were calculated for the first 5 days and plotted as a time series. The variability of CrSO2 was evaluated using the standard deviation (SD), coefficient of variation (CV), and root mean of successive squared differences (RMSSD).
where xi is the median CrSO2 at interval i (13). The differences of different CrSO2 variables were compared between survivors and non-survivors.
The primary outcome was PICU mortality. All patients weaned off ECMO successfully had accepted brain CT or MRI as soon as the patients' condition relatively stabilized.
Statistical analysis was performed with SPSS version 26 (IBM Corp., Armonk, NY, USA). Continuous variables were confirmed for normal distribution using the Shapiro–Wilk test and are presented either as means ± SD for data with a normal distribution or as median (IQR) for non-normal data. The differences between groups were tested with Student t-test, Satterthwaite t-test, or Mann–Whitney U-test, as appropriate. Categorical variables are presented as counts (percentages). Repeated measurement data were analyzed using the generalized estimating equation. p < 0.05 was considered as statistically significant.
Thirteen patients accepted ECMO support during the study period. Five of them were excluded from the analysis: two had V-V ECMO for respiratory failure, one died within 24 h of ECMO initiation, and two accepted V-A ECMO following open heart surgery. Finally, eight patients undergoing V-A ECMO were included in the study. Patients' demographics and clinical characteristics are summarized in Table 1. Six patients weaned off ECMO successfully, whereas one of them could not detach from the ventilator after ECMO and died of respiratory failure. Two patients had no improvement and died after decannulation. Two patients had abnormal neurologic imaging findings. Patient 4 had intracranial hemorrhage, and patient 8 had cerebral infarction, whereas both of them survived to PICU discharge. For all eight included patients, the PICU length of stay was 20 ± 7 days, and the hospital length of stay was 45 ± 24 days. Arterial blood gas parameters before ECMO were pH 7.40 ± 0.10, PaCO2 = 44.4 ± 7.5 mm Hg, PaO2 = 47.4 ± 13.0 mm Hg, SaO2 = 79.6 ± 12.5%, and lactate = 1.6 ± 0.9 mmol/L. PaO2/FiO2 ratio before ECMO was 48 ± 14.
The centrifugal pump rotating speed was 5,468 ± 347 r/min, and the ECMO flow was 70 ± 10 mL/kg/min for the first 5 days following ECMO initiation. Patients' mean arterial pressure (MAP) was 71 ± 12 mm Hg, SvO2 was 66 ± 3%, and hematocrit was 35 ± 2%. All patients accepted vasoactive drugs during ECMO support, and the duration of vasoactive drugs use was 1.5 days (IQR = 1–3 days). The maximum vasoactive–inotropic score (14, 15) was 17.85 (IQR = 5.36–31.38). Three (37.5%) patients received continuous renal replacement therapy during ECMO support, and all of them suffered PICU mortality.
The duration of CrSO2 monitoring was 79.0 ± 17.0% of the time on the first 5 days of ECMO. The distribution of CrSO2 data for the first 5 days in both hemispheres is shown in Figure 1. The percentage of time of CrSO2 <60% against the whole monitoring time was significantly lower in survivors in both hemispheres [right: 4.34% (IQR = 0.39–8.55%) vs. 47.45% (IQR = 36.03–64.52%), p = 0.036; left: 0.40% (IQR = 0.01–1.15%) vs. 30.9% (IQR = 26.92–49.62%), p = 0.036)].
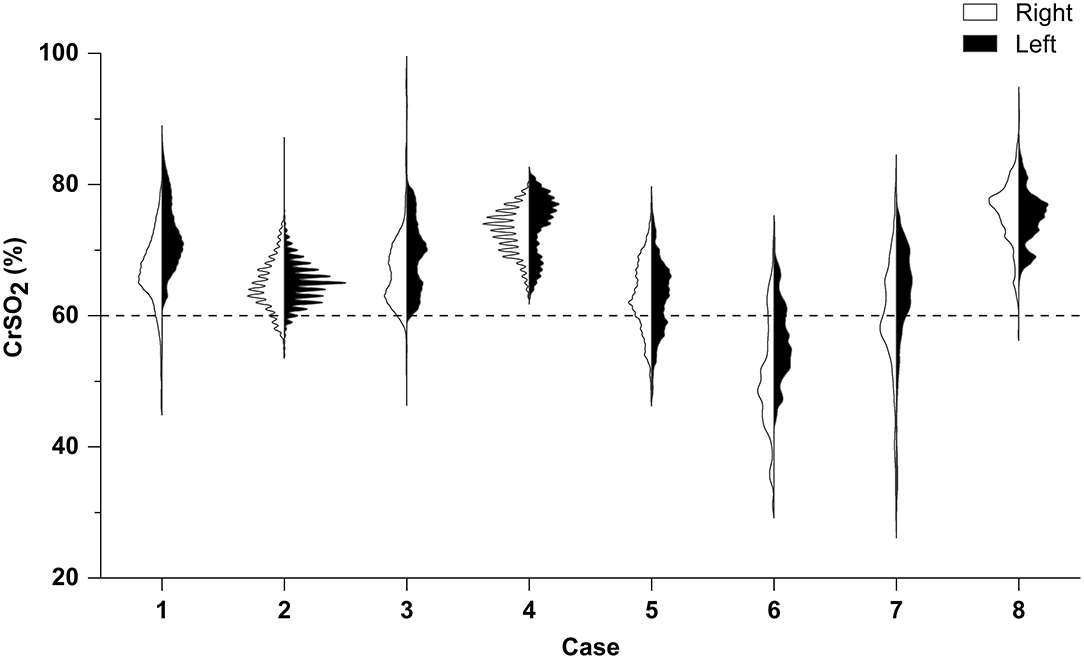
Figure 1. Right–left split violin plot of CrSO2 for the first 5 days following ECMO initiation. According to PICU mortality, patients 5, 6, and 7 suffered death; others survived. Results of the Mann–Whitney U-test indicate that the percentage of time of CrSO2 <60% against the whole monitoring time was significantly lower in survivors in both hemispheres (both p < 0.05).
Table 2 and Figure 2 show CrSO2 variation trends for the first 5 days in both hemispheres. Because of the risk of developing pressure sores, we did not gather CrSO2 signals in the fifth day for patient 1. While the duration of ECMO support was only 41 h for patient 8; thus, the patient did not contribute data to the third, fourth, and fifth day. Survivors had significantly higher CrSO2 for each of the first 4 days in both hemispheres (all p < 0.05), but there were no significant differences between survivors and non-survivors on the fifth day in both hemispheres (right: Wald χ2 = 3.402, p = 0.065, left: Wald χ2 = 1.216, p = 0.270).
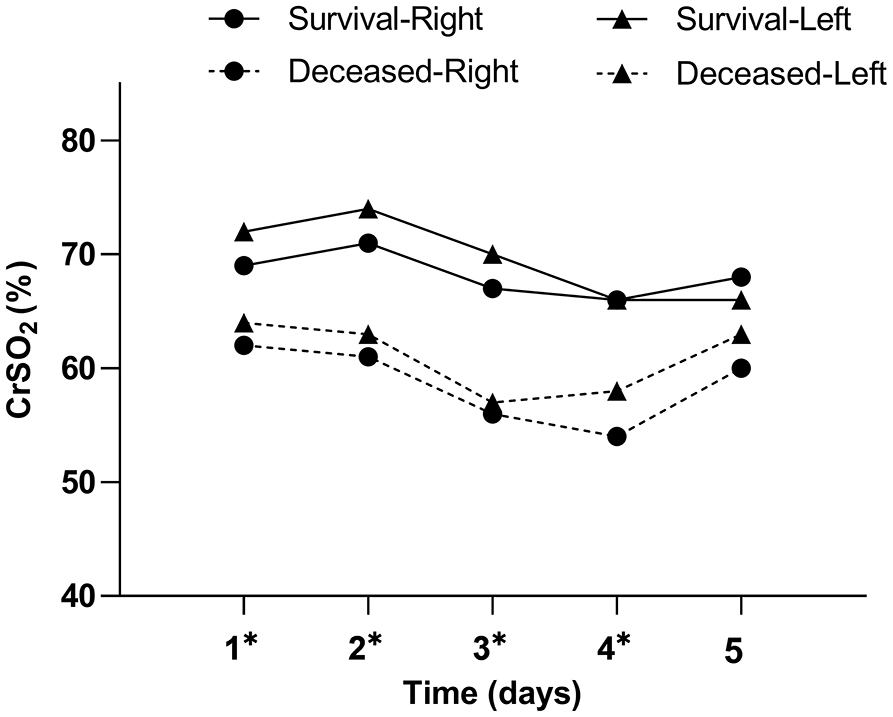
Figure 2. Daily CrSO2 for the first 5 days following ECMO initiation according to PICU mortality. Results of the generalized estimating equation (GEE) indicate significant differences between survivors and non-survivors for each of the first 4 days following ECMO initiation in both hemispheres. *p < 0.05.
The indicators of CrSO2 variability between survivors and non-survivors for the first 5 days following ECMO initiation are shown in Figure 3. Root mean of successive squared differences (RMSSDs) were significantly lower in survivors in both hemispheres (right: 3.29 ± 0.79 vs. 6.16 ± 0.67, p = 0.002; left: 3.56 ± 1.20 vs. 6.04 ± 1.44, p = 0.039). Coefficient of variation was significantly lower in survivors only in the left hemisphere (right: 4.54 ± 0.89 vs. 11.50 ± 3.85, p = 0.084; left: 5.36 ± 1.36 vs. 9.71 ± 2.47, p = 0.016). Standard deviation was seemingly lower in survivors in both hemispheres, but there were no significant differences (right: 3.27 ± 0.47 vs. 6.50 ± 1.83, p = 0.087; left: 3.92 ± 1.03 vs. 5.91 ± 1.71, p = 0.081).
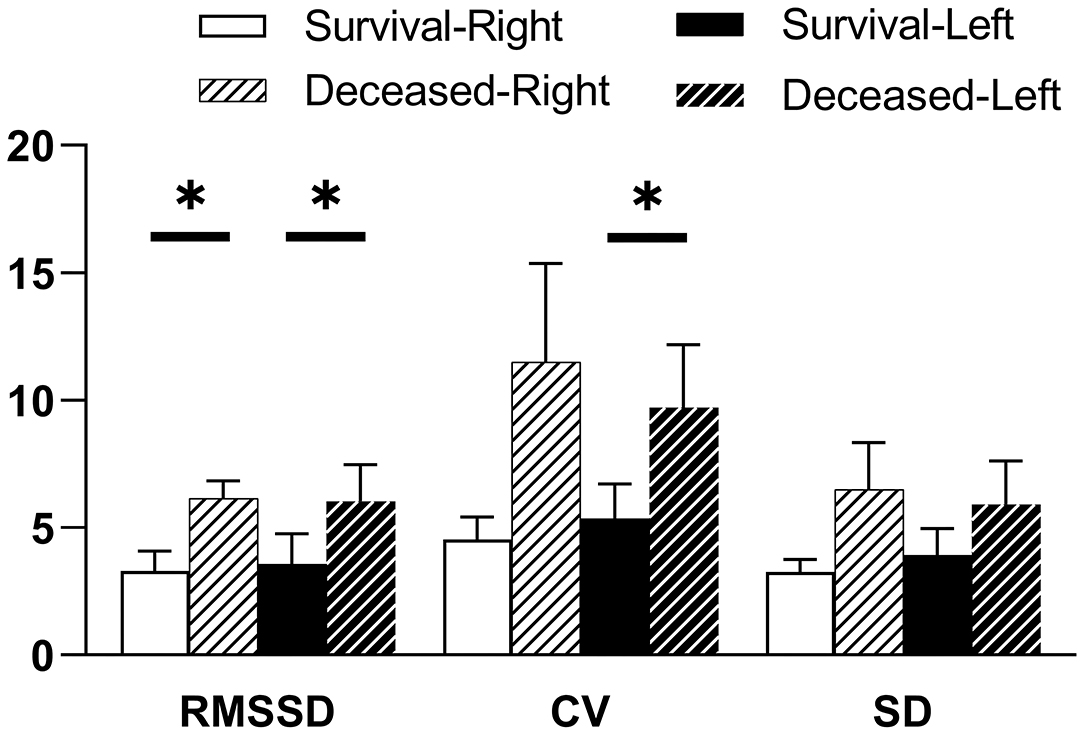
Figure 3. CrSO2 variability for the first 5 days following ECMO initiation according to PICU mortality. Indicators of CrSO2 variability are RMSSD, root mean of successive squared differences; CV, coefficient of variation; SD, standard deviation. Results of the Student t-test indicate significant differences between survivors and non-survivors for right RMSSD, left RMSSD, and left CV, respectively. *p < 0.05.
Monitoring technology has not been developed along with ECMO-related technologies, and monitoring of the central nervous system is particularly challenging. The head ultrasound was used in patients undergoing ECMO with several limitations (16, 17), for example, this approach could not capture the neurologic imaging in pediatric patients with closed cranial fontanels and adult patients (8). Besides, it cannot be used for continuous monitoring. As for electroencephalography, amplitude-integrated electroencephalography, and somatosensory-evoked potentials, a few studies have tested their roles in ECMO support (8, 18). Although head CT and MRI can clearly detect the cerebral hemorrhage or ischemia, the hospital transfer may have potential risks (8). Different from all these monitoring methods, cerebral NIRS can provide a continuous value of CrSO2. Because of its non-invasiveness, continuousness, and being performed in real time and at the bedside (7–10), ELSO suggests regular application of NIRS to patients undergoing ECMO support (11). Nonetheless, there was not a certain method to use NIRS. In our study, we explored the CrSO2 distribution characteristic, the variation trend of daily CrSO2, and the variability of CrSO2 for the first 5 days following ECMO initiation.
In the present study, survivors had higher CrSO2 for the first 4 days following ECMO initiation in both hemispheres, and the percentage of time of CrSO2 <60% against the whole monitoring time was lower in survivors. Previous studies also revealed that survivors had higher CrSO2 than non-survivors (19, 20). And the occurrence of cerebral desaturation, which is defined as CrSO2 <60% for >5% of the entire monitoring period, was independently associated with hospital mortality (21). Wong et al. (22) adjusted therapy measures that could affect the cerebral oxygen balance to improve CrSO2 values. They also found that unilateral or bilateral CrSO2 decreasing persistently was associated with worse outcome. We have similar findings and consider that lower CrSO2 and the longer time of CrSO2 <60% reduce the possibility of survival. However, there was no significant difference for the median CrSO2 of the fifth day among survivors and non-survivors. We guess volume overload may play a role in this phenomenon, because all deceased patients in our study accepted continuous renal replace therapy in the later days of ECMO support. Studies suggested cerebral autoregulation impairment during ECMO was associated with worse outcome (20, 23). Excessive blood would stream into brain circulation without the regulation of cerebral autoregulation, so that the CrSO2 signal gets higher.
Besides, for the indicators of CrSO2 variability, bilateral RMSSD and left CV were significantly higher in non-survivors; meanwhile, bilateral SD and right CV also showed the trend to be higher in non-survivors. Therefore, we speculate that greater fluctuation of CrSO2 is associated with a worse prognosis. Absolute CrSO2 values can be different between different types of NIRS devices, because of different algorithms (24). Different from using absolute CrSO2 value directly, indicators of CrSO2 variability can reduce the error caused by different types of NIRS devices. Using RMSSD to describe the variability of CrSO2 had been studied in perioperative period of cardiac surgery. Spaeder et al. (13) found that lower RMSSD was associated with poor neurodevelopmental outcomes in neonatal survivors of congenital heart disease. Meanwhile, Flechet et al. (25) found that pediatric patients with lower RMSSD had larger probability of developing acute kidney injury after cardiac surgery. This is the first study to explore the CrSO2 variability using RMSSD in patients with ECMO support. However, different from those findings that came from the perioperative period of cardiac surgery, survivors had a lower RMSSD in our study. We consider that there are several potential factors that may cause this difference. First, patients with open heart surgery were excluded in our study, whereas those two previous studies had focused on the perioperative period of cardiac surgery. Second, conclusion of those two previous studies came from CrSO2 data gathered from postoperative period. Cerebral blood flow is pulsatile for most patients in this period, whereas cerebral blood flow in V-A ECMO is non-pulsatile or partial pulsatile, which is associated with endothelial dysfunction, decreased microvascular perfusion, and increased vascular resistance (26). Third, the total monitoring duration is longer in our study. Meanwhile, those two previous studies averaged CrSO2 over 1-min intervals to calculate RMSSD. But we calculated RMSSD using the median CrSO2 over 4-h intervals, because missing data cannot be avoided in such long a time in clinical. We have checked CrSO2 signal every 4–6 h, so that we have enough data in every 4-h intervals. Further studies are needed to confirm our findings and explore underlying mechanisms.
There are some limitations to our study. First, our findings came from a small sample size and single-center study, and most patients in our study had a similar diagnosis of human adenovirus pneumonia. Those make it cautious to interpret the results of our study. Second, only patients receiving V-A ECMO were included in our study; thus, conclusions obtained from the current study may not be extrapolated to those with V-V ECMO. However, Clair et al. (19) reported the mean CrSO2 for the whole time of ECMO support, the duration of CrSO2 below the threshold of 20% from baseline, and the duration of CrSO2 <50% were not different in patients with V-V ECMO compared to V-A ECMO. Third, we only compared CrSO2 values between survivors and non-survivors, as the small sample size is a limitation in adjusting for suspected confounding factors. The relationships between CrSO2 values and hemodynamic parameters were not discussed. At the same time, we did not explore the correlation between CrSO2 and neurologic complications. Studies found that lower CrSO2 and more frequently occurrence of cerebral desaturation in patients with ECMO were more likely related to brain injury (19, 27, 28). However, different studies had different definitions of cerebral desaturation and brain injury. Tian et al. (29) assessed the dynamic relationship between the MAP and CrSO2 fluctuations and found that patients with significant in-phase MAP to CrSO2 coherence always had abnormal neurologic imaging findings. Finally, there are limitations in CrSO2 monitoring. CrSO2 mainly represents the regional oxygen saturation of the forehead. Thus, it may be hard to evaluate the deeper part or other region of the brain. Meanwhile, CrSO2 can be affected by serum bilirubin, skin surface temperature, or scalp blood flow (12, 21, 28).
Cerebral tissue regional oxygen saturation can be used as a regular monitoring parameter for pediatric patients undergoing ECMO. Lower CrSO2, CrSO2 <60% over a longer period of time, and higher fluctuation of CrSO2 are likely associated with PICU mortality in pediatric patients undergoing V-A ECMO. Future multicenter and large-scale studies are needed to mitigate confounding factors and verify our findings and explore the correlation between neurologic complications and CrSO2 variables in our study.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Institutional Review Board of Children's Hospital of Chongqing Medical University. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
SC is the principal author and contributed to data collection, data analysis and interpretation, and drafted the manuscript. SC and FX contributed to the study conception and design. FF, WL, CL, and FX contributed to data analysis and interpretation and revised the manuscript critically for important intellectual content. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank all the medical and nursing staff in our ECMO team. We thank Hui Dong (MD, Department of Gastroenterology, Xinqiao Hospital, Army Medical University, Chongqing, PR China) for his contributions to language instruction.
ECMO, extracorporeal membrane oxygenation; V-A ECMO, venoarterial ECMO; V-V ECMO, venovenous ECMO; PICU, pediatric intensive care unit; NIRS, near-infrared spectroscopy; CrSO2, cerebral tissue regional oxygen saturation; SD, standard deviation; CV, coefficient of variation; RMSSD, root mean of successive squared differences; GEE, the generalized estimating equation.
1. White A, Fan E. What is ECMO? Am J Respir Crit Care Med. (2016) 193:9–10. doi: 10.1164/rccm.1936P9
2. Nasr DM, Rabinstein AA. Neurologic complications of extracorporeal membrane oxygenation. J Clin Neurol. (2015) 11:383–9. doi: 10.3988/jcn.2015.11.4.383
3. Hervey-Jumper SL, Annich GM, Yancon AR, Garton HJL, Muraszko KM, Maher CO. Neurological complications of extracorporeal membrane oxygenation in children. J Neurosurg Pediatr. (2011) 7:338–44. doi: 10.3171/2011.1.Peds10443
4. Combes A, Hajage D, Capellier G, Demoule A, Lavoue S, Guervilly C, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. (2018) 378:1965–75. doi: 10.1056/NEJMoa1800385
5. Lorusso R, Barili F, Di Mauro M, Gelsomino S, Parise O, Rycus PT, et al. In-hospital neurologic complications in adult patients undergoing venoarterial extracorporea l membrane oxygenation: results from the extracorporeal life support organization registry. Crit Care Med. (2016) 44:e964–72. doi: 10.1097/ccm.0000000000001865
6. Lin JC. Extracorporeal membrane oxygenation for severe pediatric respiratory failure. Respir Care. (2017) 62:732–50. doi: 10.4187/respcare.05338
7. Hirsch JC, Charpie JR, Ohye RG, Gurney JG. Near-infrared spectroscopy: what we know and what we need to know-a systematic review of the congenital heart disease literature. J Thorac Cardiovasc Surg. (2009) 137:154–9; 159e1–12. doi: 10.1016/j.jtcvs.2008.08.005
8. Lin N, Flibotte J, Licht DJ. Neuromonitoring in the neonatal ECMO patient. Semin Perinatol. (2018) 42:111–21. doi: 10.1053/j.semperi.2017.12.007
9. Krishnan S, Schmidt GA. Hemodynamic monitoring in the extracorporeal membrane oxygenation patient. Curr Opin Crit Care. (2019) 25:285–91. doi: 10.1097/mcc.0000000000000602
10. Ostadal P, Kruger A, Vondrakova D, Janotka M, Psotova H, Neuzil P. Noninvasive assessment of hemodynamic variables using near-infrared spectroscopy in patients experiencing cardiogenic shock and individuals undergoing venoarterial extracorporeal membrane oxygenation. J Crit Care. (2014) 29:690.e11–5. doi: 10.1016/j.jcrc.2014.02.003
11. Maratta C, Potera RM, van Leeuwen G, Castillo Moya A, Raman L, Annich GM. Extracorporeal Life Support Organization (ELSO): 2020 pediatric respiratory ELSO guideline. ASAIO J. (2020) 66:975–9. doi: 10.1097/mat.0000000000001223
12. Song J-G, Jeong S-M, Shin W-J, Jun I-G, Shin K, Huh I-Y, et al. Laboratory variables associated with low near-infrared cerebral oxygen saturation in icteric patients before liver transplantation surgery. Anesth Analg. (2011) 112:1347–52. doi: 10.1213/ANE.0b013e318214b2b0
13. Spaeder MC, Klugman D, Skurow-Todd K, Glass P, Jonas RA, Donofrio MT. Perioperative near-infrared spectroscopy monitoring in neonates with congenital heart disease: relationship of cerebral tissue oxygenation index variability with neurodevelopmental outcome. Pediatr Crit Care Med. (2017) 18:213–8. doi: 10.1097/pcc.0000000000001056
14. Gaies MG, Gurney JG, Yen AH, Napoli ML, Gajarski RJ, Ohye RG, et al. Vasoactive-inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatr Crit Care Med. (2010) 11:234–8. doi: 10.1097/PCC.0b013e3181b806fc
15. Musick MA, Loftis LL, Kennedy CE. Comparing vasoactive-inotropic score reporting strategies in the picu relative to mortality risk. Pediatr Crit Care Med. (2018) 19:1130–6. doi: 10.1097/pcc.0000000000001738
16. O'Brien NF, Buttram SDW, Maa T, Lovett ME, Reuter-Rice K, LaRovere KL, et al. Cerebrovascular physiology during pediatric extracorporeal membrane oxygenation: a multicenter study using transcranial Doppler ultrasonography. Pediatr Crit Care Med. (2019) 20:178–86. doi: 10.1097/PCC.0000000000001778
17. Zamora CA, Oshmyansky A, Bembea M, Berkowitz I, Alqahtani E, Liu S, et al. Resistive index variability in anterior cerebral artery measurements during daily transcranial duplex sonography a predictor of cerebrovascular complications in infants undergoing extracorporeal membrane oxygenation? J Ultrasound Med. (2016) 35:2459–65. doi: 10.7863/ultra.15.09046
18. Bembea MM, Felling R, Anton B, Salorio CF, Johnston MV. Neuromonitoring during extracorporeal membrane oxygenation: a systematic review of the literature. Pediatr Crit Care Med. (2015) 16:558–64. doi: 10.1097/pcc.0000000000000415
19. Clair MP, Rambaud J, Flahault A, Guedj R, Guilbert J, Guellec I, et al. Prognostic value of cerebral tissue oxygen saturation during neonatal extracorporeal membrane oxygenation. PLoS ONE. (2017) 12:e0172991. doi: 10.1371/journal.pone.0172991
20. Kim HS, Ha SO, Yu KH, Oh MS, Park S, Lee SH, et al. Cerebral oxygenation as a monitoring parameter for mortality during venoarterial extracorporeal membrane oxygenation. ASAIO J. (2019) 65:342–8. doi: 10.1097/MAT.0000000000000827
21. Pozzebon S, Ortiz AB, Franchi F, Cristallini S, Belliato M, Lheureux O, et al. Cerebral near-infrared spectroscopy in adult patients undergoing veno-arterial extracorporeal membrane oxygenation. Neurocrit Care. (2018) 29:94–104. doi: 10.1007/s12028-018-0512-1
22. Wong JK, Smith TN, Pitcher HT, Hirose H, Cavarocchi NC. Cerebral and lower limb near-infrared spectroscopy in adults on extracorporeal membrane oxygenation. Artif Organs. (2012) 36:659–67. doi: 10.1111/j.1525-1594.2012.01496.x
23. Caicedo A, Papademetriou MD, Elwell CE, Hoskote A, Elliott MJ, Van Huffel S, et al. Canonical correlation analysis in the study of cerebral and peripheral haemodynamics interrelations with systemic variables in neonates supported on ECMO. Adv Exp Med Biol. (2013) 765:23–9. doi: 10.1007/978-1-4614-4989-8_4
24. Greenberg S, Murphy G, Shear T, Patel A, Simpson A, Szokol J, et al. Extracranial contamination in the INVOS 5100C versus the FORE-SIGHT ELITE cerebral oximeter: a prospective observational crossover study in volunteers. Can J Anaesth. (2016) 63:24–30. doi: 10.1007/s12630-015-0451-7
25. Flechet M, Guiza F, Scharlaeken I, Vlasselaers D, Desmet L, Van den Berghe G, et al. Near-infrared-based cerebral oximetry for prediction of severe acute kidney injury in critically ill children after cardiac surgery. Crit Care Explor. (2019) 1:e0063. doi: 10.1097/cce.0000000000000063
26. Veraar CM, Rinoesl H, Kuehn K, Skhirtladze-Dworschak K, Felli A, Mouhieddine M, et al. Non-pulsatile blood flow is associated with enhanced cerebrovascular carbon dioxide reactivity and an attenuated relationship between cerebral blood flow and regional brain oxygenation. Crit Care. (2019) 23:426. doi: 10.1186/s13054-019-2671-7
27. Tsou P-Y, Garcia AV, Yiu A, Vaidya DM, Bembea MM. Association of cerebral oximetry with outcomes after extracorporeal membrane oxygenation. Neurocrit Care. (2020) 33:429–37. doi: 10.1007/s12028-019-00892-4
28. Khan I, Rehan M, Parikh G, Zammit C, Badjatia N, Herr D, et al. Regional cerebral oximetry as an indicator of acute brain injury in adults undergoing veno-arterial extracorporeal membrane oxygenation-a prospective pilot study. Front Neurol. (2018) 9:993. doi: 10.3389/fneur.2018.00993
Keywords: mortality, regional oxygen saturation, extracorporeal membrane oxygenation, NIRS, pediatric
Citation: Chen S, Fang F, Liu W, Liu C and Xu F (2021) Cerebral Tissue Regional Oxygen Saturation as a Valuable Monitoring Parameter in Pediatric Patients Undergoing Extracorporeal Membrane Oxygenation. Front. Pediatr. 9:669683. doi: 10.3389/fped.2021.669683
Received: 19 February 2021; Accepted: 04 May 2021;
Published: 04 June 2021.
Edited by:
Giacomo Cavallaro, IRCCS Cà Granda Foundation Maggiore Policlinico Hospital, ItalyReviewed by:
Warwick Wolf Butt, Royal Children's Hospital, AustraliaCopyright © 2021 Chen, Fang, Liu, Liu and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feng Xu, eHVmZW5nOTg5OUAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.