
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
STUDY PROTOCOL article
Front. Pediatr. , 30 April 2021
Sec. Pediatric Critical Care
Volume 9 - 2021 | https://doi.org/10.3389/fped.2021.663435
This article is part of the Research Topic Quality of Life After Sepsis View all 10 articles
 Luregn J. Schlapbach1,2*
Luregn J. Schlapbach1,2* Kristen Gibbons1
Kristen Gibbons1 Roberta Ridolfi1
Roberta Ridolfi1 Amanda Harley1,3,4
Amanda Harley1,3,4 Michele Cree1,5
Michele Cree1,5 Debbie Long1,6
Debbie Long1,6 David Buckley7
David Buckley7 Simon Erickson8
Simon Erickson8 Marino Festa9,10
Marino Festa9,10 Shane George1,3,11
Shane George1,3,11 Megan King3
Megan King3 Puneet Singh12
Puneet Singh12 Sainath Raman1
Sainath Raman1 Rinaldo Bellomo13 and the RESPOND PICU study investigators and the Australian New Zealand Intensive Care Society Paediatric Study Group (ANZICS PSG)
Rinaldo Bellomo13 and the RESPOND PICU study investigators and the Australian New Zealand Intensive Care Society Paediatric Study Group (ANZICS PSG)Introduction: Septic shock remains amongst the leading causes of childhood mortality. Therapeutic options to support children with septic shock refractory to initial resuscitation with fluids and inotropes are limited. Recently, the combination of intravenous hydrocortisone with high dose ascorbic acid and thiamine (HAT therapy), postulated to reduce sepsis-related organ dysfunction, has been proposed as a safe approach with potential for mortality benefit, but randomized trials in paediatric patients are lacking. We hypothesize that protocolised early use of HAT therapy (“metabolic resuscitation”) in children with septic shock is feasible and will lead to earlier resolution of organ dysfunction. Here, we describe the protocol of the Resuscitation in Paediatric Sepsis Using Metabolic Resuscitation–A Randomized Controlled Pilot Study in the Paediatric Intensive Care Unit (RESPOND PICU).
Methods and Analysis: The RESPOND PICU study is an open label randomized-controlled, two-sided multicentre pilot study conducted in paediatric intensive care units (PICUs) in Australia and New Zealand. Sixty children aged between 28 days and 18 years treated with inotropes for presumed septic shock will be randomized in a 1:1 ratio to either metabolic resuscitation (1 mg/kg hydrocortisone q6h, 30 mg/kg ascorbic acid q6h, 4 mg/kg thiamine q12h) or standard septic shock management. Main outcomes include feasibility of the study protocol and survival free of organ dysfunction censored at 28 days. The study cohort will be followed up at 28-days and 6-months post enrolment to assess neurodevelopment, quality of life and functional status. Biobanking will allow ancillary studies on sepsis biomarkers.
Ethics and Dissemination: The study received ethical clearance from Children's Health Queensland Human Research Ethics Committee (HREC/18/QCHQ/49168) and commenced enrolment on June 12th, 2019. The primary study findings will be submitted for publication in a peer-reviewed journal.
Trial Registration: Australian and New Zealand Clinical Trials Registry (ACTRN12619000829112). Protocol Version: V1.8 22/7/20.
Sepsis, defined as dysregulated host response to infection leading to life-threatening organ dysfunction, ranks amongst the leading causes of childhood mortality (1–3). Sepsis remains responsible for an estimated three million childhood deaths each year worldwide (4, 5). Despite advances in paediatric intensive care practices, the mortality due to septic shock remains high (around 17%) (6–8). Paediatric sepsis survivors often suffer from long-term sequelae, resulting in a lifelong burden to patients, families, and the society (9, 10).
Paediatric septic shock often is a fulminant disease, and affected children deteriorate rapidly (11). Hence, in order to optimize outcomes and prevent irreversible multi-organ damage, future interventions will need to be applied early after presentation. Therapeutic options for children where septic shock persists despite initial resuscitation remain scarce. Current recommendations for treatment of refractory paediatric septic shock include consideration for intravenous hydrocortisone (12). Several large randomized-controlled trials in septic adults have demonstrated earlier reversal of septic shock using treatment with hydrocortisone (13–15). In children, guidelines explicitly state that clinical trial evidence for the role of hydrocortisone in paediatric septic shock remains unclear, however the use of hydrocortisone appears to be very common (16–18). More recently, ascorbic acid (Vitamin C), has been proposed as a sepsis adjunct therapy. Ascorbic acid carries powerful antioxidant properties and in-vitro and animal model evidence indicates protective effects on endotoxin-related endothelial damage and organ function (19, 20). Given that thiamine deficiency is associated with high lactate (21) and an increased risk of death (21), a protocol combining hydrocortisone, ascorbic acid, and thiamine (HAT therapy, or so called metabolic resuscitation) has been trialled in adult studies with some reporting mortality benefit (22, 23), while other trials did not observe differences in patient-centred outcomes (24–28). Both thiamine and ascorbic acid carry an excellent safety profile (29). A recent systematic review on metabolic resuscitation (30) identified nine registered RCTs on metabolic resuscitation in adult septic shock, but none in children. Children may be more prone to vitamin C deficiency during sepsis due to comorbidities, malnutrition, a higher metabolic rate, and a higher proportion of patients with a rapidly progressive disease course. A propensity-matched cohort study from the USA reported excellent safety and feasibility of metabolic resuscitation in children with septic shock (31). Adjusted analyses showed decreased 30- and 90-day mortality (p < 0.05) in the intervention group compared to hydrocortisone alone and compared to standard care without hydrocortisone.
We therefore designed the pragmatic Resuscitation in Paediatric Sepsis Using Metabolic Resuscitation–A Randomized Controlled Pilot Study in the Paediatric Intensive Care Unit (RESPOND PICU) to test the feasibility of a paediatric RCT comparing metabolic resuscitation vs. standard septic shock management in children aged ≥28 days to <18 years requiring inotropes for suspected septic shock. We hypothesize that a protocol on metabolic resuscitation in children is feasible and that the intervention will lead to faster resolution of shock compared to standard shock management. In this paper, we describe the RESPOND PICU study protocol including the statistical analysis plan.
The RESPOND PICU study is an open label, multicentre, pragmatic randomized controlled pilot trial (RCT) for children aged between 28 days and 18 years who are admitted to PICUs requiring inotrope therapy for presumed septic shock (Figure 1). The study will recruit patients in tertiary PICUs of participating sites in Australia and New Zealand. The trial compares metabolic resuscitation, defined as hydrocortisone, high dose ascorbic acid, and thiamine, with standard care defined as septic shock management according to institutional protocols (12, 32). The study protocol has been approved by the Children's Health Queensland Hospital and Health Service Human Research Ethics Committee (HREC/18/QCHQ/49168) and is registered with the Australian New Zealand Clinical Trials Registry (ACTRN12619000829112).
Children aged between 28 days and 18 years admitted to PICU and receiving inotrope therapy for septic shock for at least 2 h are eligible (Table 1). Inotropes (and vasopressors) include adrenaline, noradrenaline, vasopressin, milrinone, dopamine, and dobutamine. Patients treated with inotropes, independent on the dose or route of administration are eligible. Children not meeting age criteria, those with chronic liver failure, cardiomyopathy, those with conditions affecting steroid or vitamin metabolism, and children under active chemotherapy will be excluded. In addition, children with palliative care orders, and children who suffered a cardiorespiratory arrest in the 2 h preceding enrolment are excluded.
The pragmatic protocol stipulates the use of inotropes as the main feature of shock and does not mandate specific vital signs or laboratory thresholds to qualify for shock.
Screening and recruitment of participants will occur in the PICU (Figure 1). Study staff will approach the parents or guardians for prospective consent where feasible. Consent will include permission to conduct a follow-up assessment by questionnaire at 6 months post randomization (Figure 2). Separate consent will be sought to allow biobanking for ancillary studies on sepsis biomarkers. If timely informed consent is not feasible (for example if parents are not present immediately or if they are too distressed) the study team can employ consent to continue (33), until parents can be approached to seek written consent. Parents have the option to withdraw consent, which will lead to their child's study data being excluded.
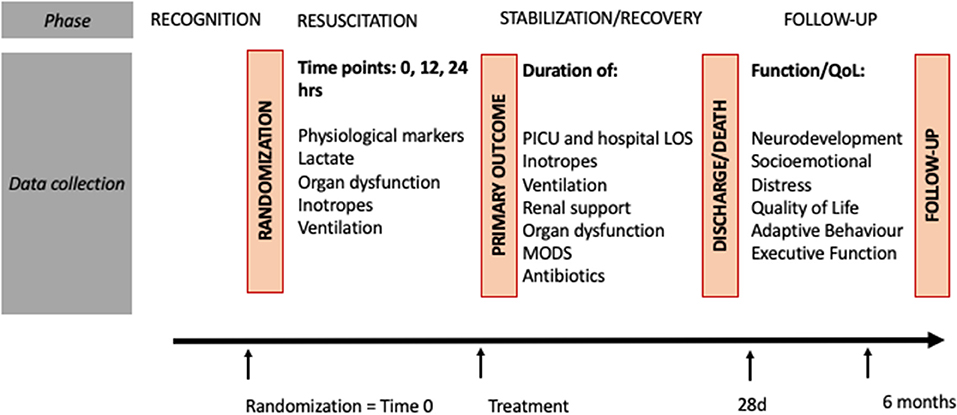
Figure 2. Study procedures. ASQ, ages and stages; BRIEF, paediatric Behavior Rating Inventory of Executive Function; FSS, functional status score; LOS, length of stay; MODS, multiple organ dysfunction syndrome; PICU, paediatric intensive care unit; POPC, Paediatric Overall Performance Category; QoL, quality of life.
Allocation occurs in a 1:1 ratio to the treatment group (receiving metabolic resuscitation vs. standard shock management). We will use a permuted block randomization with variable block sizes of two, four and six stratified by site. Sealed opaque envelopes will be provided using a randomization sequence generated by The University of Queensland, Brisbane, Australia. Once a child meets eligibility criteria, study staff or treating clinicians are advised to open the next sequential sealed envelope to reveal the allocation of the patient.
Due to the challenges in blinding three drugs, and considering the main aim of this study to investigate feasibility of metabolic resuscitation, the study will be open label.
The study treatment should be started immediately after randomization. The RCT compares metabolic resuscitation with standard care.
After initial shock treatment including fluids and intravenous inotrope(s), patients then receive additional treatment with hydrocortisone, ascorbic acid, and thiamine:
The dosing schedule is 30 mg/kg/dose (maximum 1,500 mg per dose) intravenously every 6 h for the duration of study treatment and will be infused over 30–60 min.
The dosing schedule is 4 mg/kg/dose (maximum 200 mg per dose) intravenously every 12 h for the duration of study treatment and will be infused over 30–60 min.
The dosing schedule is 1 mg/kg/dose (maximum 50 mg per dose) intravenously every 6 h for the duration of study treatment given as a slow bolus.
Study drugs will be given through an existing peripheral or central intravenous line. Drug delivery will occur through dose error reduction software for infusion devices to ensure safe delivery of applied standardized drug concentrations. The dosing is aligned with the ADRENAL steroid treatment regimen (13), and with the protocol for the VITAMIN trial (24) and has been shown to restore serum ascorbic acid concentrations in critically ill patients (34). The study treatment will be given for a maximum of 7 days, until resolution of shock (defined as cessation of inotropes for at least 4 h), discharge from PICU, death, or if major adverse events related to the intervention manifest, whichever occurs first.
Patients in the standard treatment arm can receive hydrocortisone only if clinically indicated at the discretion of the attending ICU staff specialist and should be treated as per the standard institutional approach to septic shock management (12, 32).
The study protocol does not prescribe what type, dose, or combination of inotropes should be used. Fluid boluses in all three arms can be balanced or unbalanced crystalloid or colloid fluids. Other care including respiratory management, antimicrobials, glucose and electrolyte control, transfusion, sedation, and extracorporeal life support should be provided at the discretion of the treating physician according to local practice.
In addition to feasibility outcomes, the study will assess clinical outcome measures, and proxy measures of intervention efficacy (Table 2). Feasibility outcomes include consent rates, time to intervention, hydrocortisone administration prior to randomization, and post randomization in the control group, and protocol violations. Survival free of organ dysfunction censored at 28 days is defined as the primary clinical outcome. The pediatric Sequential Organ Failure Assessment (pSOFA) score will be used to assess presence and degree of organ dysfunction (36). All-cause deaths occurring within 28 days of enrolment will be allocated zero organ dysfunction free days. pSOFA scores will be assessed every day during which a patient is admitted in PICU. In children not requiring ongoing respiratory or renal support post discharge from PICU, and not under palliative care, those discharged from PICU will be assumed to have no organ dysfunction which is aligned with the practice at the participating institutions. PICU free survival, survival free of inotrope support, survival free of multi-organ dysfunction, survival free of acute kidney injury (AKI) (37), mortality, PICU and hospital length of stay represent the secondary clinical outcome measures. In addition, study nurses will contact families at 28 days to perform a Pediatric Overall Performance Category (POPC) (38) and Functional Status Score (FSS) assessment (39). As a modification from the POPC “good” category (healthy, alert, and capable of normal age-appropriate activities of daily life; medical and physical problems do not interfere with normal activity), we divided the first POPC category into “good/normal” (no medical conditions), and b) “functionally normal” (requires medication and medical input, normal intellectually and physically, able to do activities without restriction).
Long-term follow-up at 6 months post randomization through questionnaire by proxy on validated domains across quality of life (40) and functional status will be investigated and reported separately from the main analyses.
Furthermore, we will determine proxy measures of intervention efficacy such as shock reversal, normalization of lactate, and time to reversal of tachycardia [defined by age-specific thresholds for Systemic Inflammatory Response Syndrome (35)].
Based on the anticipated severity of the study population informed by recent observational studies in Australian and New Zealand PICUs (7, 11, 41) a proportion of study patients are expected to suffer from complications related to septic shock unrelated to study interventions (42). Major AEs including death, cardiopulmonary arrest, ECMO and amputations will be captured in all patients. In addition, we will report any AE potentially causally related to the study intervention or which is of concern in the investigator's judgement. Specific additional AEs captured include limb ischemia, extravasation injury, hypertension, arrhythmia other than sinus bradycardia or tachycardia, hyperglycaemia, abdominal compartment syndrome, pulmonary edema and confirmed hospital-acquired infection. Adverse events will be assessed routinely until day 28, or until the time of patient discharge from hospital, whichever occurs earlier. The Data and Safety Monitoring Board (DSMB) consists of a statistician, an ICU specialist and an emergency specialist will receive regular DSMB reports and immediate access to any serious adverse event reports. The DSMB, once set up, will meet mid-trial and at the completion of the trial.
The central study coordination will provide a study booklet for site staff, and perform site visits and regular videoconferencing to ensure high standard study conduct and to discuss any challenges in study progress. The REDCap online database (43), hosted by The University of Queensland, has been setup to capture baseline demographic variables, comorbidities, disease and severity features, pre-defined study outcomes, information on study treatments, and adverse events. The staff at each study site will be trained in completing this electronic case report form (eCRF) and in performing the follow-up questionnaires. Study participants alive at day 28 will be contacted by phone (unless still in hospital) to assess POPC and FSS. Subsequently, the study nurses will contact families ~2 months post randomization by phone to perform the questionnaires by proxy on neurodevelopment, quality of life and functional outcomes–details of the follow-up will be published separately.
Physiological parameters and organ support will be collected upon randomization, then at 1, 6, 12, and 24 h subsequently. We will collect individual components of organ dysfunction and organ support every day until discharged from PICU to calculate pSOFA scores.
Where parental consent is available, we will obtain 1–2 mL of EDTA blood, 2.5 mL of PAXgene blood, and 1–2 mL of serum as close to enrolment as possible. The samples will be transferred to a biobank using standard operating procedures and will remain stored for anxillary studies on sepsis markers.
A detailed monitoring plan has been established which guides the data validity checks embedded in REDCap and monitoring by an auditor not involved in entering the study data. Specifically, we will perform primary source data verification in 100% of enrolled patients for key variables such as randomization allocation, commencement of intervention and consent, study treatments, organ support, PICU length of stay, survival status, and reported protocol deviations and adverse events. Furthermore, we will randomly select 10% of patients where a range of other study data will be verified using primary source verification, such as inclusion and exclusion criteria for ineligible patients, baseline data, first 24 h data, demographic data, daily organ dysfunction data, and hospital discharge data. Regular training of study staff and videoconferences with the central study team will be held to observe study conduct.
We define feasibility of recruitment if ≥65% of eligible patients are enrolled. Enrolment of 60 of 80 eligible patients will yield a recruitment rate of 75% with a one-sided lower 95% confidence interval limit of 66%, which would meet our feasibility metric for recruitment rate. We anticipate a recruitment period of 24 months to meet the target sample size of 60 participants; this sample should provide sufficient data on feasibility and safety, and indicate estimates of potential effect size to calculate power for a future full trial.
Using the Consolidated Standards of Reporting Trials (CONSORT) flow chart (Figure 1) (44) we will report on screened, included, and excluded patients, the number consented, and number of those with consent withdrawn. We will report on the number and proportion of children enrolled through prospective consent in comparison to consent to continue, timing of consent, and investigate the investigator-stated reasons if consent to continue was employed. Descriptive statistics will be used to compare demographics, clinical history and baseline clinical characteristics between the study arms (Supplementary Table 1), however statistical comparisons will not be undertaken. The estimate of the difference between the two study groups and the corresponding 95% confidence interval (CI) will be reported for outcomes relating to feasibility and clinical characteristics (Supplementary Tables 2 and 3). Quantile regression will be used to assess the comparison for continuous outcomes and the test of two proportions for binary outcomes. All analyses will be based on the intention-to-treat principle. All patients who underwent randomization and for whom consent is available are included in the ITT analysis. Protocol violations and major adverse events will be listed separately (Supplementary Table 4). A sensitivity analysis will be undertaken for primary and secondary outcomes, separating the standard care arm into two groups–those not receiving hydrocortisone, and those receiving hydrocortisone. Descriptive statistics only will be presented on the sensitivity analysis (Supplementary Table 5). In addition, we will provide graphs to describe temporal changes in pSOFA, physiological measures (heart rate, systolic blood pressure), serum lactate levels, use of fluid bolus volume (in ml/kg), and Vasopressor-Inotrope-Score (45) comparing the intervention group vs. controls, starting at time of randomization. Given the study sample, and that no stratification is performed, we will not perform other pre-planned subgroup analyses.
We anticipate that <30% of RESPOND PICU study patients will be co-enrolled into a concomitant study investigating early inotropes in sepsis, named RESPOND ED (ACTRN12619000828123). Analyses on RESPOND PICU will not be adjusted for co-enrolment in RESPOND ED given that both are pilot feasibility studies.
RESPOND PICU commenced recruiting in June 2019 with a projected completion date of June 2021. Recruitment is live at the central study site Queensland Children's Hospital, at Gold Coast University Hospital, and started recently at Sydney Children‘s Hospital, Westmead Children‘s Hospital, Perth Children‘s Hospital, Australia, and Starship Children‘s Hospital, New Zealand.
This worldwide first trial on metabolic resuscitation in paediatric sepsis will test the feasibility of an intervention showing promise of improving patient-centred outcomes and cost-effective health care delivery. Study sites include several paediatric hospitals across Australia and New Zealand, leveraging off of the ANZICS Paediatric Study Group network.
The study design is informed by the VITAMINS trial (24) and similar several recently published adult RCTs on metabolic resuscitation. While the optimal dosing of metabolic resuscitation, in particular of ascorbic acid, remains uncertain, weight-based dosing schedules were derived from adult studies, considering expert pediatric critical care pharmacist advice. Of note, we recently reviewed the literature on harm related to high dose ascorbic acid in children and identified that the safety of this intervention is very high even at doses superior to those used in this trial (46). Inclusion and exclusion criteria of this pilot study were adapted from adult trials towards pediatric populations, but it is acknowledged that criteria such as cardiomyopathy were not defined in detail to keep the study pragmatic. Limitations include lack of a dedicated hydrocortisone only arm, and hence pre-planned subgroup analyses on children treated with hydrocortisone, vs. controls without hydrocortisone, and vs. intervention with metabolic resuscitation, will be performed.
The interventions are low cost and are based on globally available drugs with excellent safety profiles. The study design is pragmatic, and findings will serve to inform the design of a fully powered trial on high dose ascorbic acid, thiamine, and hydrocortisone in children with septic shock.
The studies involving human participants were reviewed and approved by Children's Health Queensland, Brisbane, HREC/18/QCHQ/49168. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
LS and RB were responsible for the initial protocol development. LS was responsible for subsequent protocol refinement with input from KG, RR, AH, MC, DL, DB, SE, MF, SG, MK, PS, and SR. KG supervised the REDCap setup and contributed to writing the statistical analysis plan. LS refined and developed subsequent manuscript drafts. All authors contributed to final manuscript preparation and approved final submission.
Queensland Children's Hospital: A/Prof Luregn Schlapbach, Ms Amanda Harley, Dr Sainath Raman, Ms Roberta Ridolfi, Ms Natalie Sharp, A/Prof Kristen Gibbons, Ms Renate Le Marsnay, Ms Michele Cree, A/Prof Debbie Long
Austin Hospital and Monash University, Melbourne: Prof. Rinaldo Bellomo
Gold Coast University Hospital: Dr. Megan King, A/Prof. Shane George, Mr Nathan Goddard, Mr Kieran Owen
Perth Children‘s Hospital, Perth: Dr Simon Erickson, Ms Hannah Thomson
Sydney Children‘s Hospital, Sydney: Dr Puneet Singh, Ms Vicki Smith
The Children's Hospital at Westmead, Sydney: Dr Marino Festa, Dr Chong Tien Goh, Ms Gale Harper
Starship Children‘s Hospital, Auckland, NZ: Dr David Buckely, Dr John Beca, Ms Claire Sherring.
Anusha Ganeshalingam, Claire Sherring, Starship Children's Hospital, Auckland, New Zealand; Simon Erickson, Samantha Barr, Perth Children‘s Hospital, Perth, Australia; Sainath Raman, Debbie Long, Luregn Schlapbach (Past Chair), Kristen Gibbons (Vice Chair), Queensland Children's Hospital and The University of Queensland, Brisbane, Australia; Shane George, Gold Coast University Hospital; Puneet Singh, Vicky Smith, Sydney Children's Hospital, Randwick, Australia; Warwick Butt (Chair), Carmel Delzoppo, Johnny Millar (ANZPIC registry lead), Ben Gelbart, Royal Children's Hospital, Melbourne, Australia; Breanna Pellegrini (ANZPIC registry); Felix Oberender, Monash Children‘s Hospital, Melbourne, Australia; Subodh Ganu, Georgia Letton, Women's and Children's Hospital, Adelaide, Australia; Gail Harper, Marino Festa, Westmead Children's Hospital, Sydney, Australia.
LS was supported by a National Health and Medical Research Council (NHMRC) Practitioner Fellowship and by the Children‘s Hospital Foundation, Australia. The study was supported by grants from the Emergency Medicine Foundation Queensland, Financial Markets for Children Australia, the Gold Coast Hospital Foundation, and by a Children‘s Health Queensland SERTA grant.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors thank the children and families participating in this trial. In addition, we would like to express our gratitude to the PICU staff at the participating sites for their help in study conduct.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2021.663435/full#supplementary-material
1. Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet. (2020) 395:200–211. doi: 10.1016/S0140-6736(19)32989-7
2. Fleischmann-Struzek C, Goldfarb DM, Schlattmann P, Schlapbach LJ, Reinhart K, Kissoon N. The global burden of paediatric and neonatal sepsis: a systematic review. Lancet Respir Med. (2018) 6:223–230. doi: 10.1016/S2213-2600(18)30063-8
3. Reinhart K, Daniels R, Kissoon N, Machado FR, Schachter RD, Finfer S. Recognizing Sepsis as a Global Health Priority - A WHO Resolution. N Engl J Med. (2017) 377:414–417. doi: 10.1056/NEJMp1707170
4. Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE, et al. Global, regional, and national causes of child mortality in 2000-13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. (2015) 385:430–40. doi: 10.1016/S0140-6736(14)61698-6
5. Kissoon N, Uyeki TM. Sepsis and the Global Burden of Disease in Children. JAMA Pediatr. (2016) 170:107–8. doi: 10.1001/jamapediatrics.2015.3241
6. Weiss SL, Fitzgerald JC, Pappachan J, Wheeler D, Jaramillo-Bustamante JC, Salloo A, et al. Therapies Study I. Pediatric Acute Lung, and N. Sepsis Investigators, Global epidemiology of pediatric severe sepsis: the sepsis prevalence, outcomes, therapies study. Am J Respir Crit Care Med. (2015) 191:1147–57. doi: 10.1164/rccm.201412-2323OC
7. Schlapbach LJ, Straney L, Alexander J, MacLaren G, Festa M, Schibler A, et al. Mortality related to invasive infections, sepsis, and septic shock in critically ill children in Australia and New Zealand, 2002-13: a multicentre retrospective cohort study. Lancet Infect Dis. (2015) 15:46–54. doi: 10.1016/S1473-3099(14)71003-5
8. Hartman ME, Linde-Zwirble WT, Angus DC, Watson RS. Trends in the epidemiology of pediatric severe sepsis*. Pediatr Crit Care Med. (2013) 14:686–93. doi: 10.1097/PCC.0b013e3182917fad
9. Schlapbach LJ, Aebischer M, Adams M, Natalucci G, Bonhoeffer J, Latzin P, et al. Impact of sepsis on neurodevelopmental outcome in a Swiss National Cohort of extremely premature infants. Pediatrics. (2011) 128:e348–57. doi: 10.1542/peds.2010-3338
10. Schlapbach LJ. Paediatric sepsis. Curr Opin Infect Dis. (2019) 32:497–504. doi: 10.1097/QCO.0000000000000583
11. Schlapbach LJ, MacLaren G, Festa M, Alexander J, Erickson S, Beca J, et al. New Zealand Intensive Care Society Centre for, E. Resource, Australian, and G. New Zealand Intensive Care Society Paediatric Study, Prediction of pediatric sepsis mortality within 1 h of intensive care admission. Intensive Care Med. (2017) 43:1085–96. doi: 10.1007/s00134-017-4701-8
12. Weiss SL, Peters MJ, Alhazzani W, Agus MSD, Flori HR, Inwald DP, et al. Surviving sepsis campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Intensive Care Med. (2020) 46:10–67. doi: 10.1007/s00134-019-05878-6
13. Venkatesh B, Finfer S, Cohen J, Rajbhandari D, Arabi Y, Bellomo R, et al. the Australian-New Zealand intensive care society clinical trials, adjunctive glucocorticoid therapy in patients with septic shock. N Engl J Med. (2018) 378:797–808. doi: 10.1056/NEJMoa1705835
14. Annane D, Renault A, Brun-Buisson C, Megarbane B, Quenot JP, Siami S, et al. Hydrocortisone plus fludrocortisone for adults with septic shock. N Engl J Med. (2018) 378:809–18. doi: 10.1056/NEJMoa1705716
15. Annane D, Sebille V, Charpentier C, Bollaert PE, Francois B, Korach JM, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. (2002) 288:862–71. doi: 10.1001/jama.288.7.862
16. Wong HR, Atkinson SJ, Cvijanovich NZ, Anas N, Allen GL, Thomas NJ, et al. Combining prognostic and predictive enrichment strategies to identify children with septic shock responsive to corticosteroids. Crit Care Med. (2016) 44:e1000–3. doi: 10.1097/CCM.0000000000001833
17. Wong HR, Cvijanovich NZ, Anas N, Allen GL, Thomas NJ, Bigham MT, et al. Developing a clinically feasible personalized medicine approach to pediatric septic shock. Am J Respir Crit Care Med. (2015) 191:309–15. doi: 10.1164/rccm.201410-1864OC
18. Menon K, McNally D, O'Hearn K, Acharya A, Wong HR, Lawson M, et al. Choong, and G. Canadian critical care trials, a randomized controlled trial of corticosteroids in pediatric septic shock: a pilot feasibility study. Pediatr Crit Care Med. (2017) 18:505–12. doi: 10.1097/PCC.0000000000001121
19. Barabutis N, Khangoora V, Marik PE, Catravas JD. Hydrocortisone and ascorbic acid synergistically prevent and repair lipopolysaccharide-induced pulmonary endothelial barrier dysfunction. Chest. (2017) 152:954–62. doi: 10.1016/j.chest.2017.07.014
20. Lankadeva YR, Peiris RM, Okazaki N, Birchall IE, Trask-Marino A, Dornom A, et al. Reversal of the pathophysiological responses to gram-negative sepsis by megadose vitamin C. Crit Care Med. (2020) 49:e179–90. doi: 10.1097/CCM.0000000000004770
21. Donnino MW, Andersen LW, Chase M, Berg KM, Tidswell M, Giberson T, et al. Center for resuscitation science research, randomized, double-blind, placebo-controlled trial of thiamine as a metabolic resuscitator in septic shock: a pilot study. Crit Care Med. (2016) 44:360–7. doi: 10.1097/CCM.0000000000001572
22. Marik PE, Khangoora V, Rivera R, Hooper MH, Catravas J. Hydrocortisone, Vitamin C, and thiamine for the treatment of severe sepsis and septic shock: a retrospective before-after study. Chest. (2017) 151:1229–38. doi: 10.1016/j.chest.2016.11.036
23. Fowler AA 3rd, Truwit JD, Hite RD, Morris PE, DeWilde C, Priday A, et al. Effect of Vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: the CITRIS-ALI randomized clinical trial. JAMA. (2019) 322 1261–70. doi: 10.1001/jama.2019.11825
24. Fujii T, Luethi N, Young PJ, Frei DR, Eastwood GM, French CJ, et al. Effect of Vitamin C, hydrocortisone, and thiamine vs hydrocortisone alone on time alive and free of vasopressor support among patients with septic shock: the VITAMINS randomized clinical trial. JAMA. (2020) 323:423–31. doi: 10.1001/jama.2019.22176
25. Fujii T, Udy AA, Deane AM, Luethi N, Bailey M, Eastwood GM, et al. Vitamin C, hydrocortisone and thiamine in patients with septic shock (VITAMINS) trial: study protocol and statistical analysis plan. Crit Care Resusc. (2019) 21 119–25.
26. Moskowitz Huang DT, Hou PC, Gong J, Doshi PB, Grossestreuer AV, Andersen LW, et al. Effect of ascorbic acid, corticosteroids, and thiamine on organ injury in septic shock: the ACTS randomized clinical trial. JAMA. (2020) 324:642–50. doi: 10.1001/jama.2020.11946
27. Moskowitz Yankama T, Andersen LW, Huang DT, Donnino MW, Grossestreuer AV. Ascorbic Acid, Corticosteroids and Thiamine in Sepsis (ACTS) protocol and statistical analysis plan: a prospective, multicentre, double-blind, randomised, placebo-controlled clinical trial. BMJ Open. (2019) 9:e034406. doi: 10.1136/bmjopen-2019-034406
28. Hwang SY, Ryoo SM, Park JE, Jo YH, Jang DH, Suh GJ, et al. Korean Shock, Combination therapy of vitamin C and thiamine for septic shock: a multi-centre, double-blinded randomized, controlled study. Intensive Care Med. (2020) 46:2015–25. doi: 10.1007/s00134-020-06191-3
29. Yanase F, Fujii T, Naorungroj T, Belletti A, Luethi N, Carr AC, et al. Harm of IV high-dose vitamin C therapy in adult patients: a scoping review. Crit Care Med. (2020) 48:e620–8. doi: 10.1097/CCM.0000000000004396
30. Moskowitz Andersen LW, Huang DT, Berg KM, Grossestreuer AV, Marik PE, Sherwin RL, et al. Ascorbic acid, corticosteroids, and thiamine in sepsis: a review of the biologic rationale and the present state of clinical evaluation. Crit Care. (2018) 22:283. doi: 10.1186/s13054-018-2217-4
31. Wald EL, Sanchez-Pinto LN, Smith CM, Moran T, Badke CM, Barhight MF, et al. Hydrocortisone-ascorbic acid-thiamine use associated with lower mortality in pediatric septic shock. Am J Respir Crit Care Med. (2020) 201:863–7. doi: 10.1164/rccm.201908-1543LE
32. Davis AL, Carcillo JA, Aneja RK, Deymann AJ, Lin JC, Nguyen TC, et al. American College of critical care medicine clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock. Crit Care Med. (2017) 45:1061–93. doi: 10.1097/CCM.0000000000002425
33. Woolfall K, Frith L, Gamble C, Gilbert R, Mok Q, Young B, et al. How parents and practitioners experience research without prior consent (deferred consent) for emergency research involving children with life threatening conditions: a mixed method study. BMJ Open. (2015) 5:e008522. doi: 10.1136/bmjopen-2015-008522
34. Hudson EP, Collie JT, Fujii T, Luethi N, Udy AA, Doherty S, et al. Pharmacokinetic data support 6-hourly dosing of intravenous vitamin C to critically ill patients with septic shock. Crit Care Resusc. (2019) 21:236–42.
35. Goldstein B, Giroir B, Randolph A. International Consensus Conference on Pediatric, International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. (2005) 6:2–8. doi: 10.1097/01.PCC.0000149131.72248.E6
36. Matics TJ, Sanchez-Pinto LN. Adaptation and validation of a pediatric sequential organ failure assessment score and evaluation of the sepsis-3 definitions in critically ill children. JAMA Pediatrics. (2017) 171:e172352. doi: 10.1001/jamapediatrics.2017.2352
37. Khwaja. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. (2012) 120:c179–84. doi: 10.1159/000339789
38. Fiser DH. Assessing the outcome of pediatric intensive care. J Pediatr. (1992) 121:68–74. doi: 10.1016/S0022-3476(05)82544-2
39. Pollack MM, Holubkov R, Funai T, Clark A, Moler F, Shanley T, et al. Relationship between the functional status scale and the pediatric overall performance category and pediatric cerebral performance category scales. JAMA Pediatr. (2014) 168:671–6. doi: 10.1001/jamapediatrics.2013.5316
40. Desai AD, Zhou C, Stanford S, Haaland W, Varni JW, Mangione-Smith RM. Validity and responsiveness of the pediatric quality of life inventory (PedsQL) 4.0 generic core scales in the pediatric inpatient setting. JAMA Pediatr. (2014) 168:1114–21. doi: 10.1001/jamapediatrics.2014.1600
41. Schlapbach LJ, Straney L, Bellomo R, MacLaren G, Pilcher D. Prognostic accuracy of age-adapted SOFA, SIRS, PELOD-2, and qSOFA for in-hospital mortality among children with suspected infection admitted to the intensive care unit. Intensive Care Med. (2018) 44:179–88. doi: 10.1007/s00134-017-5021-8
42. Cook Lauzier F, Rocha MG, Sayles MJ, Finfer S. Serious adverse events in academic critical care research. CMAJ. (2008) 178:1181–4. doi: 10.1503/cmaj.071366
43. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. (2009) 42:377–81. doi: 10.1016/j.jbi.2008.08.010
44. Gamble Krishan A, Stocken D, Lewis S, Juszczak E, Dore C, Williamson PR, et al. Guidelines for the content of statistical analysis plans in clinical trials. JAMA. (2017) 318:2337–43. doi: 10.1001/jama.2017.18556
45. Gaies MG, Jeffries HE, Niebler RA, Pasquali SK, Donohue JE, Yu S, et al. Vasoactive-inotropic score is associated with outcome after infant cardiac surgery: an analysis from the Pediatric Cardiac Critical Care Consortium and Virtual PICU System Registries. Pediatr Crit Care Med. (2014) 15:529–37. doi: 10.1097/PCC.0000000000000153
Keywords: ascorbic acid, child, hydrocortisone, intensive care, sepsis, septic shock, thiamine, vitamin C
Citation: Schlapbach LJ, Gibbons K, Ridolfi R, Harley A, Cree M, Long D, Buckley D, Erickson S, Festa M, George S, King M, Singh P, Raman S, Bellomo R and the RESPOND PICU study investigators and the Australian New Zealand Intensive Care Society Paediatric Study Group (ANZICS PSG) (2021) Resuscitation in Paediatric Sepsis Using Metabolic Resuscitation–A Randomized Controlled Pilot Study in the Paediatric Intensive Care Unit (RESPOND PICU): Study Protocol and Analysis Plan. Front. Pediatr. 9:663435. doi: 10.3389/fped.2021.663435
Received: 02 February 2021; Accepted: 06 April 2021;
Published: 30 April 2021.
Edited by:
Muralidharan Jayashree, Post Graduate Institute of Medical Education and Research (PGIMER), IndiaReviewed by:
Simon Nadel, Imperial College London, United KingdomCopyright © 2021 Schlapbach, Gibbons, Ridolfi, Harley, Cree, Long, Buckley, Erickson, Festa, George, King, Singh, Raman, Bellomo and the RESPOND PICU study investigators and the Australian New Zealand Intensive Care Society Paediatric Study Group (ANZICS PSG). This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luregn J. Schlapbach, bC5zY2hsYXBiYWNoQHVxLmVkdS5hdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.