- 1Department of Pediatrics, Peking University People's Hospital, Beijing, China
- 2Department of Pediatrics, Peking University First Hospital, Beijing, China
Autism spectrum disorder (ASD) is characterized by stereotyped behavior and deficits in communication and social interaction. There are no curative treatments for children with ASD. The ketogenic diet (KD) is a high-fat, appropriate-protein, and low-carbohydrate diet that mimics the fasting state of the body and is proven beneficial in drug-resistant epilepsy and some other brain diseases. An increasing number of studies demonstrated that a KD improved autistic behavior, but the underlying mechanisms are not known. We reviewed the neuroprotective role of a KD in ASD, which is likely mediated via improvements in energy metabolism, reductions in antioxidative stress levels, control of neurotransmitters, inhibition of the mammalian target of rapamycin (mTOR) signaling pathway, and modulation of the gut microbiota. A KD is likely a safe and effective treatment for ASD.
Introduction
Autism spectrum disorder (ASD) is a lifelong neurodevelopmental disorder that is characterized by stereotyped behavior and deficits in communication and social interaction. ASD affects 3.4–6.7 per 1,000 children (1). Boys are four times more likely than girls to have ASD (1). The core features of ASD patients are social communication deficits and repetitive sensory–motor behaviors (2). According to the Diagnostic and Statistical Manual of Mental Disorders (DSM) 5, patients who are diagnosed with ASD must have persistent deficits in social communication and repetitive and unusual sensory–motor behaviors (2). Comorbidities in ASD are common and include epilepsy, sleep disorders, gastrointestinal (GI) symptoms, and psychopathologies such as anxiety, depression, attention deficit hyperactivity disorder, and intellectual disability (3). Sleep disturbances occur in 50–80% ASD children, and sleep disorder is associated with behavioral dysregulation (4). Epilepsy is also one of the most common comorbidities in ASD children, and the average prevalence reaches 26% (5). ASD individuals who have epilepsy are likely to exhibit more severe autism-related symptoms (6). GI symptoms, which range from 23 to 70% in ASD children, are related to the severity of ASD (7). Approximately 31% of children with ASD have intelligence quotient scores below 70 (7). The cost of raising a child with ASD is 1.4–3.6 million dollars according to the level of intellectual disability, and the largest expenses are special education costs and the loss of parental productivity. When children grow up, supportive living accommodations and the loss of individual productivity become the highest costs (8). Therefore, ASD places a large burden on society and the affected families. There are no effective drugs for ASD. Several interventions, such as special education and behavioral interventions, provide some benefits, but these interventions do not improve all core symptoms of ASD and have less effects on comorbidities, including epilepsy. Therefore, new therapies are urgently needed to broaden the management options and improve the prognosis of these patients. Lower levels of disaccharidases and hexose transporters were found in ASD patients with GI symptoms (9), which suggests carbohydrate digestion disorders as a physiopathological mechanism in ASD patients. Therefore, a low-carbohydrate diet, such as a ketogenic diet (KD), is likely suitable for ASD patients.
A KD is a high-fat, appropriate-protein, and low-carbohydrate diet that has a positive effect on energy metabolism. For example, a KD increases the levels of adenosine triphosphate (ATP) and enzymes associated with mitochondrial metabolic pathways and enhances mitochondrial biogenesis (10–12). Acetyl-CoA is converted to ketone bodies under the effects of D-β-hydroxybutyrate dehydrogenase, acetoacetate succinyl-CoA transferase, and acetoacetyl-CoA-thiolase (13). Ketone bodies, including β-hydroxybutyrate, acetoacetate, and acetone, function as fuels under fasting or starvation conditions and cross the blood–brain barrier (BBB) to feed the brain. These molecules also prevent mitochondrial permeability transition and attenuate reactive oxygen species (ROS) (14, 15). Therefore, ketone bodies have neuroprotective effects in the central nervous system (CNS). A KD is a significantly effective treatment for epilepsy. Refractory epilepsy patients aged 1–18 years treated with a KD for 4 months had a 56% reduction in mean seizure frequency (16). A KD may also improve some core autistic features and comorbidities of ASD, but data of clinical studies of a KD as a treatment for ASD are very limited. The present review examined the role of a KD in ASD treatment and discussed the underlying mechanisms.
A Ketogenic Diet and Autism Spectrum Disorder
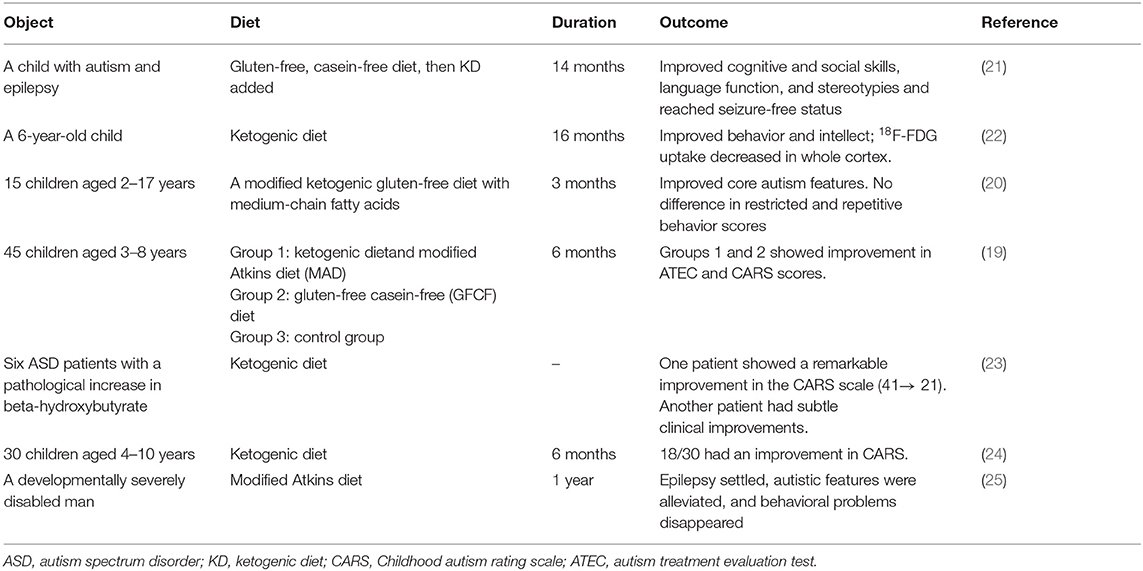
A KD is a dietary intervention therapy in neurological disorders such as epilepsy and ASD (17, 18). A KD may be an effective therapy for ASD because it might improve ASD core symptoms and could benefit its comorbidities, including seizures. The efficiency of a KD must be monitored using urinary ketones and serum beta-hydroxybutyrate (BHB) (19, 20). Some evidence showed that a KD improved the core features of ASD patients (Table 1). El-Rashidy et al. showed that a KD improved autistic manifestations, which was demonstrated as improved scores on the Autism Treatment Evaluation Test (ATEC) scales and the Childhood Autism Rating Scale (CARS), especially sociability improvement (19). Lee et al. also reported that a modified ketogenic gluten-free diet with supplemental medium-chain triglycerides (MCTs) improved the social affect subdomain and total autism diagnostic observation schedule, 2nd edition (ADOS-2) scores, but it did not affect the restricted and repetitive behavior scores (20). A KD improved social exploration and social interactions in an animal model of ASD (26, 27). It also ameliorates the comorbidities of ASD more efficiently than the core symptoms of ASD. A KD improved the social communication of one of six ASD patients, but it reduced the comorbidities of all six ASD patients, including attention deficit hyperactivity disorder (ADHD), compulsive behavior, preoccupation with parts of objects, and abnormal sleep (23). It also decreased the frequency of seizures (28). A case report of an ASD child found that a KD improved the electroencephalogram results and increased the child's intelligence quotient (21). Although a KD-induced decrease in seizures will lead to a better quality of life in patients with epilepsy, including ASD, it is not associated with improvements in behaviors in ASD patients. Antiepileptic drugs do not have a large effect on the behavioral symptoms in ASD (29).
After KD treatment, some blood parameters changed, such as an upregulation of beta-hydroxybutyrate, high-density lipoprotein (HDL), low-density lipoprotein, and cholesterol and a downregulation of eosinophils (20). Only the HDL increase, eosinophil percentage, and white blood cell count decrease predicted the effects of a modified KD treatment in ASD patients (20). Why the effects of KD vary so much from patient to patient in ASD is not clear. The pro-inflammatory condition at baseline was associated with an effective KD treatment, which was demonstrated by a reduction in eosinophils (20).
A KD seems effective in ASD patients, but all of these clinical studies had small sample sizes, which is likely due to the difficulty of setting up randomized trials in ASD children. ASD patients also eat a narrower range of foods and exhibit more feeding problems. They refuse some food because of the presentation or the need for certain utensils (30). Therefore, it is difficult to introduce the KD to ASD children. The duration of these studies was 3–16 months, which is not sufficient to assess the side effects of KD. In summary, more studies are needed to verify the precise role of KD in ASD patients.
The Neuroprotective Role of a KD in the Central Nervous System
A KD contains abundant fat and induces the generation of acetyl-CoA in the mitochondria of the liver via fatty acid oxidation. Therefore, abundant acetyl-CoA is shunted to the formation of ketone bodies (acetoacetate, β-hydroxybutyrate, and acetone) in the liver. These ketone bodies enter into the circulation and are used to produce energy. One of the main ketones is acetone, which increases the seizure threshold and potentiates the anticonvulsant activity of some antiepileptic drugs (31). Therefore, fatty acids and ketone bodies exert neuroprotective effects in the brain. For example, ketone bodies improve the energy metabolism by enhancing ATP production and normalizing mitochondrial function via the stimulation of mitochondrial biogenesis and the reduction of oxidative stress, which reduces neuronal death. Ketone bodies regulate neurotransmitters, increase γ-aminobutyric acid (GABA) levels, and inhibit the activation of the mammalian target of rapamycin (mTOR) signaling pathway (32–36) (Figure 1). However, the exact neuroprotective mechanisms of a KD are not fully understood.
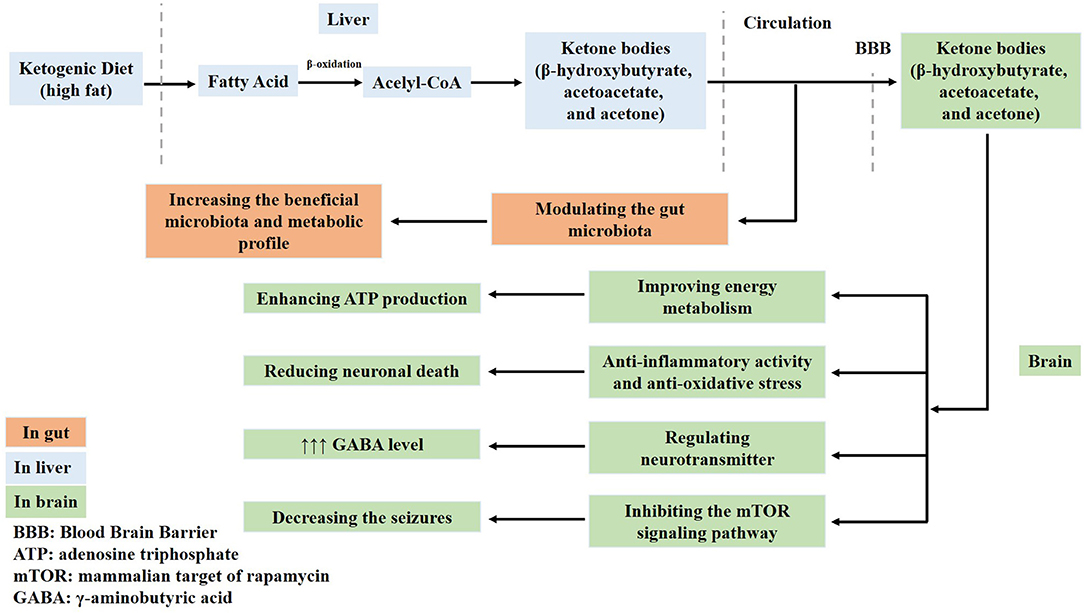
Figure 1. Ketone bodies have neuroprotective effects in the brain. Ketone bodies containing acetoacetate, β-hydroxybutyrate, and acetone generated from a ketogenic diet cross the blood–brain barrier. Firstly, ketone bodies improve the energy metabolism and enhance adenosine triphosphate (ATP) production. Secondly, they normalize mitochondrial function by stimulating mitochondrial biogenesis and reducing oxidative stress, which reduces neuronal death. Thirdly, ketone bodies regulate neurotransmitters and increase γ-aminobutyric acid (GABA) levels. Fourthly, they also inhibit the activation of the mammalian target of rapamycin (mTOR) signaling pathway and decrease seizures. Lastly, ketone bodies modulate the gut microbiota.
The Improvement of Energy Metabolism in the CNS
ASD individuals have impaired mitochondrial energy production due to the presence of abnormal mitochondrial markers in their plasma, such as elevated levels of lactic acid and pyruvate (37). Weissman et al. showed that ASD patients had mitochondrial electron transport chain dysfunction, including complex I and complex III deficiencies (38). A KD provides fuel sources in the human body, and the ketone bodies, including β-hydroxybutyrate, cross the BBB and replace glucose as fuel for the brain. This molecule crosses the BBB via proton symporters and a sodium-dependent monocarboxylate transporter, which is located in the BBB, neurons, and astrocytes (39). Ketone bodies enhance adenosine triphosphate (ATP) production via the Krebs cycle to generate energy and balance metabolism (39). A KD reduced seizures by enhancing brain metabolism via the regulation of transcripts encoding energy metabolism enzymes or mitochondrial proteins in rats with seizures (10).
Anti-inflammatory Activity and Antioxidative Stress
One of the risk factors for ASD in children is abnormal maternal immune activation. For example, pregnant mice were injected with double-stranded RNA (dsRNA) poly (I: C) to mimic a viral infection, and the offspring of these mice had ASD-like behaviors (40). ASD patients also experience aberrant inflammation. Some cytokines and chemokines, such as interleukin 6 (IL-6), tumor necrosis factor alpha (TNF-α), and monocyte chemotactic protein-1 (MCP-1), are found at abnormal levels in brain samples and cerebrospinal fluid (CSF) in ASD patients (41). These cytokines activate the immune response via the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling pathway (42). Young and colleagues found that the NF-κB protein was hyper-expressed in mature microglia in brain samples in ASD patients, which indicates that immunity is activated in the brains of ASD patients (43). ASD patients also had higher levels of eight cytokines in plasma compared to control individuals (44). ASD individuals also have associated oxidative stress level upregulation and antioxidant ability downregulation. Evidence showed that antioxidant enzymes, including superoxide dismutase and glutathione peroxidase, are altered in ASD patients, which increases inflammation (45). In summary, there is a consensus that ASD individuals have immune dysfunction.
A KD has powerful anti-inflammatory activity and antioxidative stress effects in the brain. Jeong et al. found that a KD reduced neuroinflammation via the activation of the peroxisome proliferator-activated receptor gamma (PPARγ) and protected against excitotoxicity-induced neuronal cell death (46). Fatty acids activate PPARs and are critical regulators of lipid metabolism (47). Greco et al. found that ketone bodies decreased oxidative stress and improved the mitochondrial respiratory complex activity in a traumatic brain injury animal model (48). A KD likely normalizes mitochondrial function by stimulating mitochondrial biogenesis, decreasing oxidative stress and the levels of pro-apoptotic factors, preventing changes in mitochondrial permeability, and decreasing the mitochondrial ROS production in neocortical neurons (14, 49, 50). Mirza et al. showed that rats treated with propionic acid exhibited social impairment and repetitive behavior. The cerebellum, brainstem, and prefrontal cortex of these rats had high levels of oxidative stress and inflammation, with increased IL-6 and TNF-α levels and decreased IL-10 levels. They also found that decreased levels of oxidative stress and inflammation improved neurobehavioral disorders in rats (51).
The Regulation of Neurotransmitters in the Brain
GABA is the main inhibitory neurotransmitter in the brain, and it originates from glutamate decarboxylation. Glutamate is the primary excitatory neurotransmitter in the CNS. Patients with autism exhibited abnormal levels of proteins and messenger RNAs (mRNAs) associated with the glutamate system in the cerebellum (52). The medium-chain fatty acids that are present during the consumption of a KD directly inhibit glutamate receptors and reduce seizures (53). Other studies found that beta-hydroxybutyrate, which is produced from a KD, inhibited GABA degradation in astrocytes (54). One study showed that children with ASD had reduced GABA levels in sensorimotor function, and this phenomenon was associated with poor tactile performance compared to healthy children (55). ASD patients had significantly lower GABA concentrations in the auditory cortex. Patients with ASD also had abnormal maturation of the neuronal circuitry on magnetoencephalography (MEG) and edited magnetic resonance spectroscopy (MRS) (56). In summary, a KD may ameliorate ASD behaviors via the modulation of neurotransmitters, such as increasing GABA levels.
Inhibition of the mTOR Signaling Pathway
The mTOR signaling pathway is associated with protein synthesis, cell growth, cell proliferation, and axonal sprouting (57). This pathway plays a role in ASD (58–60). Ketone bodies may inhibit the mTOR pathway and exert an anticonvulsant effect (36). Tang et al. found that the mTOR signaling pathway was overactive both in postmortem ASD samples and TSC2+/− mice, and the mTOR inhibitor rapamycin corrected ASD-like behaviors (59). However, eIF4E, which is downstream of mTOR, was associated with ASD-like phenotypes in mice (61). A KD played an anticonvulsant role by decreasing downstream mTOR signaling in the hippocampus of rats with kainate-induced epilepsy (36).
Modulation of the Gut Microbiota
GI symptoms, including constipation and diarrhea, are common in ASD individuals and are associated with the severity of ASD symptoms (7, 62). Gorrindo et al. reported that constipation was associated with increased social impairment and language disorders (62). Patients with ASD have different gut microbiome components and metabolic products (7). The gut microbiota communicates with the brain via the neuroendocrine, neuroimmune, and autonomic nervous systems, which is the so-called microbiota–gut–brain axis (7, 63). Porphyromonadaceae, Prevotellaceae, Bacteroidales, and Lachnospiraceae were more abundant in the ASD animal model (40). An increasing number of studies showed that gut microbiota disturbances were associated with ASD patients, and modulation of the gut microbiota improved symptoms in ASD patients (7, 64, 65).
A KD restored gut microbial composition (66) and improved ASD core features, including social communication and repetitive behaviors, in an ASD animal model (26, 67). A KD increased the levels of Akkermansia, Parabacteroides, Bacteroides, and Desulfovibrio spp. in animal models of seizure, glucose transporter 1 deficiency syndrome, and ASD (66, 68–70). A KD did not change locomotor activity, anxiety-related behaviors, recognition memory, or sociability in young male rats, which suggests that a KD may be more effective in females in some cases (71, 72). As mentioned above, a KD likely modifies the composition of the gut microbiota in different animal disease models, including ASD. However, there are few studies on alterations of the gut microbiota in humans treated with a KD. In summary, modulation of the gut microbiota may be a new target for therapy in ASD patients.
The Side Effects of a Ketogenic Diet
ASD children have feeding problems because most of them are selective eaters (30). Therefore, it is difficult to apply a KD to children with ASD. There are also some side effects of the long-term implementation of a KD in children. The main side effects of KD treatment in children are constipation, vomiting, lack of energy, and hunger (73). Late-onset adverse effects include hyperuricemia, hyperlipidemia, and kidney stones (16). One serious side effect of a KD in children is the suppression of physical development (74). Long-term KD administration decreased growth, as indicated by height z-scores, but growth, as indicated by weight z-scores, did not change. A KD may cause height deceleration (74). However, these side effects do not occur often, and a KD has been widely used in children with refractory epilepsy. In summary, although studies with larger samples of ASD patients are lacking, KD is a safe and effective treatment in people with ASD.
Conclusions
ASD is a neurodevelopmental disease, and timely and effective treatment help improve the prognosis. There is no effective treatment for ASD children. Many treatments are used for ASD, but there are no curative treatments for all of the core features of ASD. A KD may improve social behavior in ASD via normalizing GABA, improving mitochondrial function, ameliorating inflammatory activity and oxidative stress in the brain, inhibiting the mTOR signaling pathway, and modulating the gut microbiota. However, the effects of KD vary widely between ASD patients, and the underlying mechanisms are not known. ASD children may also reject KD food because of their selective eating habits, which complicates the introduction of a KD to ASD patients. Some studies showed that ASD children have nutrient deficiencies, including vitamin D and folic acid (75). Long-term treatment of a KD likely aggravates the nutritional deficiency. The benefits of a long-term KD treatment in ASD children are not known. Therefore, more studies with larger samples and long-term KD treatment are needed to demonstrate the beneficial effects of a KD and its side effects in children with ASD.
Author Contributions
QL wrote the manuscript. JL and NF reviewed the literature and contributed to writing the manuscript. YH and JQ conceived the review and provided final approval of the version to be published. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Foundation of 2018 Beijing Key Clinical Specialty Construction Project-Pediatrics (2199000726).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Centers for Disease Control and Prevention (CDC). Mental health in the United States: parental report of diagnosed autism in children aged 4-17 years–United States, 2003-2004. Morb Mortal Wkly Rep. (2006) 55:481–6.
2. Lord C, Elsabbagh M, Baird G, Veenstra-Vanderweele J. Autism spectrum disorder. Lancet. (2018) 392:508–20. doi: 10.1016/S0140-6736(18)31129-2
3. Masi A, DeMayo MM, Glozier N, Guastella AJ. An overview of autism spectrum disorder, heterogeneity and treatment options. Neurosci Bull. (2017) 33:183–93. doi: 10.1007/s12264-017-0100-y
4. Wang GF, Li WL, Han Y, Gao L, Dai W, Su YY, et al. Sensory processing problems and comorbidities in chinese preschool children with autism spectrum disorders. J Autism Dev Disord. (2019) 49:4097–108. doi: 10.1007/s10803-019-04125-7
5. Viscidi EW, Triche EW, Pescosolido MF, McLean RL, Joseph RM, Spence SJ, et al. Clinical characteristics of children with autism spectrum disorder and co-occurring epilepsy. PLoS ONE. (2013) 8:e67797. doi: 10.1371/journal.pone.0067797
6. Ko C, Kim N, Kim E, Song DH, Cheon KA. The effect of epilepsy on autistic symptom severity assessed by the social responsiveness scale in children with autism spectrum disorder. Behav Brain Funct. (2016) 12:20. doi: 10.1186/s12993-016-0105-0
7. Li Q, Han Y, Dy ABC, Hagerman RJ. The gut microbiota and autism spectrum disorders. Front Cell Neurosci. (2017) 11:120. doi: 10.3389/fncel.2017.00120
8. Buescher AV, Cidav Z, Knapp M, Mandell DS. Costs of autism spectrum disorders in the United Kingdom and the United States. JAMA Pediatr. (2014) 168:721–8. doi: 10.1001/jamapediatrics.2014.210
9. Williams BL, Hornig M, Buie T, Bauman ML, Cho Paik M, Wick I, et al. Impaired carbohydrate digestion and transport and mucosal dysbiosis in the intestines of children with autism and gastrointestinal disturbances. PLoS ONE. (2011) 6:e24585. doi: 10.1371/journal.pone.0024585
10. Bough KJ, Wetherington J, Hassel B, Pare JF, Gawryluk JW, Greene JG, et al. Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet. Ann Neurol. (2006) 60:223–35. doi: 10.1002/ana.20899
11. Gano LB, Patel M, Rho JM. Ketogenic diets, mitochondria, and neurological diseases. J Lipid Res. (2014) 55:2211–28. doi: 10.1194/jlr.R048975
12. Marti LF. Dietary interventions in children with autism spectrum disorders - an updated review of the research evidence. Curr Clin Pharmacol. (2014) 9:335–49. doi: 10.2174/15748847113086660074
13. Hartman AL, Gasior M, Vining EP, Rogawski MA. The neuropharmacology of the ketogenic diet. Pediatr Neurol. (2007) 36:281–92. doi: 10.1016/j.pediatrneurol.2007.02.008
14. Kim DY, Davis LM, Sullivan PG, Maalouf M, Simeone TA, van Brederode J, et al. Ketone bodies are protective against oxidative stress in neocortical neurons. J Neurochem. (2007) 101:1316–26. doi: 10.1111/j.1471-4159.2007.04483.x
15. Kim DY, Simeone KA, Simeone TA, Pandya JD, Wilke JC, Ahn Y, et al. Ketone bodies mediate antiseizure effects through mitochondrial permeability transition. Ann Neurol. (2015) 78:77–87. doi: 10.1002/ana.24424
16. Lambrechts DA, de Kinderen RJ, Vles JS, de Louw AJ, Aldenkamp AP, Majoie HJ. A randomized controlled trial of the ketogenic diet in refractory childhood epilepsy. Acta Neurol Scand. (2017) 135:231–9. doi: 10.1111/ane.12592
17. Gogou M, Kolios G. Are therapeutic diets an emerging additional choice in autism spectrum disorder management? World J Pediatr. (2018) 14:215–23. doi: 10.1007/s12519-018-0164-4
18. Verrotti A, Iapadre G, Pisano S, Coppola G. Ketogenic diet and childhood neurological disorders other than epilepsy: an overview. Expert Rev Neurother. (2017) 17:461–73. doi: 10.1080/14737175.2017.1260004
19. El-Rashidy O, El-Baz F, El-Gendy Y, Khalaf R, Reda D, Saad K. Ketogenic diet versus gluten free casein free diet in autistic children: a case-control study. Metab Brain Dis. (2017) 32:1935–41. doi: 10.1007/s11011-017-0088-z
20. Lee RWY, Corley MJ, Pang A, Arakaki G, Abbott L, Nishimoto M, et al. A modified ketogenic gluten-free diet with MCT improves behavior in children with autism spectrum disorder. Physiol Behav. (2018) 188:205–11. doi: 10.1016/j.physbeh.2018.02.006
21. Herbert MR, Buckley JA. Autism and dietary therapy: case report and review of the literature. J Child Neurol. (2013) 28:975–82. doi: 10.1177/0883073813488668
22. Żarnowska I, Chrapko B, Gwizda G, Nocuń A, Mitosek-Szewczyk K, Gasior M. Therapeutic use of carbohydrate-restricted diets in an autistic child; a case report of clinical and 18FDG PET findings. Metab Brain Dis. (2018) 33:1187–92. doi: 10.1007/s11011-018-0219-1
23. Spilioti M, Evangeliou AE, Tramma D, Theodoridou Z, Metaxas S, Michailidi E, et al. Evidence for treatable inborn errors of metabolism in a cohort of 187 greek patients with autism spectrum disorder (ASD). Front Hum Neurosci. (2013) 7:858. doi: 10.3389/fnhum.2013.00858
24. Evangeliou A, Vlachonikolis I, Mihailidou H, Spilioti M, Skarpalezou A, Makaronas N, et al. Application of a ketogenic diet in children with autistic behavior: pilot study. J Child Neurol. (2003) 18:113–8. doi: 10.1177/08830738030180020501
25. Arvio M, Kuisma L, Pontinen M. Modified Atkins diet brought back the joy of life to a developmentally severely disabled youth. Duodecim. (2010) 126:557–60.
26. Castro K, Baronio D, Perry IS, Riesgo RDS, Gottfried C. The effect of ketogenic diet in an animal model of autism induced by prenatal exposure to valproic acid. Nutr Neurosci. (2017) 20:343–50. doi: 10.1080/1028415X.2015.1133029
27. Verpeut JL, DiCicco-Bloom E, Bello NT. Ketogenic diet exposure during the juvenile period increases social behaviors and forebrain neural activation in adult engrailed 2 null mice. Physiol Behav. (2016) 161:90–8. doi: 10.1016/j.physbeh.2016.04.001
28. Wheless JW. History of the ketogenic diet. Epilepsia. (2008) 49(Suppl. 8):3–5. doi: 10.1111/j.1528-1167.2008.01821.x
29. Hirota T, Veenstra-Vanderweele J, Hollander E, Kishi T. Antiepileptic medications in autism spectrum disorder: a systematic review and meta-analysis. J Autism Dev Disord. (2014) 44:948–57. doi: 10.1007/s10803-013-1952-2
30. Schreck KA, Williams K. Food preferences and factors influencing food selectivity for children with autism spectrum disorders. Res Dev Disabil. (2006) 27:353–63. doi: 10.1016/j.ridd.2005.03.005
31. Ułamek-Kozioł M, Pluta R, Bogucka-Kocka A, Czuczwar SJ. To treat or not to treat drug-refractory epilepsy by the ketogenic diet? That is the question. Ann Agric Environ Med. (2016) 23:533–6. doi: 10.5604/12321966.1226841
32. Zhang Y, Zhang S, Marin-Valencia I, Puchowicz MA. Decreased carbon shunting from glucose toward oxidative metabolism in diet-induced ketotic rat brain. J Neurochem. (2015) 132:301–12. doi: 10.1111/jnc.12965
33. Calderón N, Betancourt L, Hernández L, Rada P. A ketogenic diet modifies glutamate, gamma-aminobutyric acid and agmatine levels in the hippocampus of rats: a microdialysis study. Neurosci Lett. (2017) 642:158–62. doi: 10.1016/j.neulet.2017.02.014
34. Paoli A, Mancin L, Bianco A, Thomas E, Mota JF, Piccini F. Ketogenic diet and microbiota: friends or enemies? Genes. (2019) 10:534. doi: 10.3390/genes10070534
35. Ułamek-Kozioł M, Czuczwar SJ, Januszewski S, Pluta R. Ketogenic diet and epilepsy. Nutrients. (2019) 11:2510. doi: 10.3390/nu11102510
36. McDaniel SS, Rensing NR, Thio LL, Yamada KA, Wong M. The ketogenic diet inhibits the mammalian target of rapamycin (mTOR) pathway. Epilepsia. (2011) 52:e7–11. doi: 10.1111/j.1528-1167.2011.02981.x
37. Orozco JS, Hertz-Picciotto I, Abbeduto L, Slupsky CM. Metabolomics analysis of children with autism, idiopathic-developmental delays, and down syndrome. Transl Psychiatry. (2019) 9:243. doi: 10.1038/s41398-019-0578-3
38. Weissman JR, Kelley RI, Bauman ML, Cohen BH, Murray KF, Mitchell RL, et al. Mitochondrial disease in autism spectrum disorder patients: a cohort analysis. PLoS ONE. (2008) 3:e3815. doi: 10.1371/journal.pone.0003815
39. Achanta LB, Rae CD. β-Hydroxybutyrate in the brain: one molecule, multiple mechanisms. Neurochem Res. (2017) 42:35–49. doi: 10.1007/s11064-016-2099-2
40. Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. (2013) 155:1451–63. doi: 10.1016/j.cell.2013.11.024
41. Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. (2005) 57:67–81. doi: 10.1002/ana.20315
42. Perkins ND. NF-kappaB: tumor promoter or suppressor? Trends Cell Biol. (2004) 14:64–9. doi: 10.1016/j.tcb.2003.12.004
43. Young AM, Campbell E, Lynch S, Suckling J, Powis SJ. Aberrant NF-kappaB expression in autism spectrum condition: a mechanism for neuroinflammation. Front Psychiatry. (2011) 2:27. doi: 10.3389/fpsyt.2011.00027
44. Suzuki K, Matsuzaki H, Iwata K, Kameno Y, Shimmura C, Kawai S, et al. Plasma cytokine profiles in subjects with high-functioning autism spectrum disorders. PLoS ONE. (2011) 6:e20470. doi: 10.1371/journal.pone.0020470
45. Manivasagam T, Arunadevi S, Essa MM, SaravanaBabu C, Borah A, Thenmozhi AJ, et al. Role of oxidative stress and antioxidants in autism. Adv Neurobiol. (2020) 24:193–206. doi: 10.1007/978-3-030-30402-7_7
46. Jeong EA, Jeon BT, Shin HJ, Kim N, Lee DH, Kim HJ, et al. Ketogenic diet-induced peroxisome proliferator-activated receptor-γ activation decreases neuroinflammation in the mouse hippocampus after kainic acid-induced seizures. Exp Neurol. (2011) 232:195–202. doi: 10.1016/j.expneurol.2011.09.001
47. Hihi AK, Michalik L, Wahli W. PPARs: transcriptional effectors of fatty acids and their derivatives. Cell Mol Life Sci. (2002) 59:790–8. doi: 10.1007/s00018-002-8467-x
48. Greco T, Glenn TC, Hovda DA, Prins ML. Ketogenic diet decreases oxidative stress and improves mitochondrial respiratory complex activity. J Cereb Blood Flow Metab. (2016) 36:1603–13. doi: 10.1177/0271678X15610584
49. Kim DY, Rho JM. The ketogenic diet and epilepsy. Curr Opin Clin Nutr Metab Care. (2008) 11:113–20. doi: 10.1097/MCO.0b013e3282f44c06
50. Maalouf M, Rho JM, Mattson MP. The neuroprotective properties of calorie restriction, the ketogenic diet, and ketone bodies. Brain Res Rev. (2009) 59:293–315. doi: 10.1016/j.brainresrev.2008.09.002
51. Mirza R, Sharma B. A selective peroxisome proliferator-activated receptor-γ agonist benefited propionic acid induced autism-like behavioral phenotypes in rats by attenuation of neuroinflammation and oxidative stress. Chem Biol Interact. (2019) 311:108758. doi: 10.1016/j.cbi.2019.108758
52. Purcell AE, Jeon OH, Zimmerman AW, Blue ME, Pevsner J. Postmortem brain abnormalities of the glutamate neurotransmitter system in autism. Neurology. (2001) 57:1618–28. doi: 10.1212/WNL.57.9.1618
53. Augustin K, Khabbush A, Williams S, Eaton S, Orford M, Cross JH, et al. Mechanisms of action for the medium-chain triglyceride ketogenic diet in neurological and metabolic disorders. Lancet Neurol. (2018) 17:84–93. doi: 10.1016/S1474-4422(17)30408-8
54. Suzuki Y, Takahashi H, Fukuda M, Hino H, Kobayashi K, Tanaka J, et al. Beta-hydroxybutyrate alters GABA-transaminase activity in cultured astrocytes. Brain Res. (2009) 1268:17–23. doi: 10.1016/j.brainres.2009.02.074
55. Puts NAJ, Wodka EL, Harris AD, Crocetti D, Tommerdahl M, Mostofsky SH, et al. Reduced GABA and altered somatosensory function in children with autism spectrum disorder. Autism Res. (2017) 10:608–19. doi: 10.1002/aur.1691
56. Port RG, Gaetz W, Bloy L, Wang DJ, Blaskey L, Kuschner ES, et al. Exploring the relationship between cortical GABA concentrations, auditory gamma-band responses and development in ASD: evidence for an altered maturational trajectory in ASD. Autism Res. (2017) 10:593–607. doi: 10.1002/aur.1686
57. Li Q, Han Y, Du J, Jin H, Zhang J, Niu M, et al. Recombinant human erythropoietin protects against brain injury through blunting the mTORC1 pathway in the developing brains of rats with seizures. Life Sci. (2018) 194:15–25. doi: 10.1016/j.lfs.2017.12.014
58. Onore C, Yang H, Van de Water J, Ashwood P. Dynamic Akt/mTOR signaling in children with autism spectrum disorder. Front Pediatr. (2017) 5:43. doi: 10.3389/fped.2017.00043
59. Tang G, Gudsnuk K, Kuo SH, Cotrina ML, Rosoklija G, Sosunov A, et al. Loss of mTOR-dependent macroautophagy causes autistic-like synaptic pruning deficits. Neuron. (2014) 83:1131–43. doi: 10.1016/j.neuron.2014.09.001
60. Winden KD, Ebrahimi-Fakhari D, Sahin M. Abnormal mTOR Activation in Autism. Annu Rev Neurosci. (2018) 41:1–23. doi: 10.1146/annurev-neuro-080317-061747
61. Gkogkas CG, Khoutorsky A, Ran I, Rampakakis E, Nevarko T, Weatherill DB, et al. Autism-related deficits via dysregulated eIF4E-dependent translational control. Nature. (2013) 493:371–7. doi: 10.1038/nature11628
62. Gorrindo P, Williams KC, Lee EB, Walker LS, McGrew SG, Levitt P. Gastrointestinal dysfunction in autism: parental report, clinical evaluation, and associated factors. Autism Res. (2012) 5:101–8. doi: 10.1002/aur.237
63. Arentsen T, Raith H, Qian Y, Forssberg H, Diaz Heijtz R. Host microbiota modulates development of social preference in mice. Microb Ecol Health Dis. (2015) 26:29719. doi: 10.3402/mehd.v26.29719
64. Niu M, Li Q, Zhang J, Wen F, Dang W, Duan G, et al. Characterization of intestinal microbiota and probiotics treatment in children with autism spectrum disorders in China. Front Neurol. (2019) 10:1084. doi: 10.3389/fneur.2019.01084
65. Settanni CR, Bibbò S, Ianiro G, Rinninella E, Cintoni M, Mele MC, et al. Gastrointestinal involvement of autism spectrum disorder: focus on gut microbiota. Expert Rev Gastroenterol Hepatol. (2021) 5:1–24. doi: 10.1080/17474124.2021.1869938
66. Newell C, Bomhof MR, Reimer RA, Hittel DS, Rho JM, Shearer J. Ketogenic diet modifies the gut microbiota in a murine model of autism spectrum disorder. Mol Autism. (2016) 7:37. doi: 10.1186/s13229-016-0099-3
67. Ruskin DN, Svedova J, Cote JL, Sandau U, Rho JM, Kawamura M Jr, et al. Ketogenic diet improves core symptoms of autism in BTBR mice. PLoS ONE. (2013) 8:e65021. doi: 10.1371/journal.pone.0065021
68. Tagliabue A, Ferraris C, Uggeri F, Trentani C, Bertoli S, de Giorgis V, et al. Short-term impact of a classical ketogenic diet on gut microbiota in GLUT1 deficiency syndrome: A 3-month prospective observational study. Clin Nutr ESPEN. (2017) 17:33–7. doi: 10.1016/j.clnesp.2016.11.003
69. Xie G, Zhou Q, Qiu CZ, Dai WK, Wang HP, Li YH, et al. Ketogenic diet poses a significant effect on imbalanced gut microbiota in infants with refractory epilepsy. World J Gastroenterol. (2017) 23:6164–71. doi: 10.3748/wjg.v23.i33.6164
70. Olson CA, Vuong HE, Yano JM, Liang QY, Nusbaum DJ, Hsiao EY. The gut microbiota mediates the anti-seizure effects of the ketogenic diet. Cell. (2018) 174:497. doi: 10.1016/j.cell.2018.04.027
71. Kasprowska-Liśkiewicz D, Liśkiewicz AD, Nowacka-Chmielewska MM, Nowicka J, Małecki A, Barski JJ. The ketogenic diet affects the social behavior of young male rats. Physiol Behav. (2017) 179:168–77. doi: 10.1016/j.physbeh.2017.06.007
72. Ruskin DN, Fortin JA, Bisnauth SN, Masino SA. Ketogenic diets improve behaviors associated with autism spectrum disorder in a sex-specific manner in the EL mouse. Physiol Behav. (2017) 168:138–45. doi: 10.1016/j.physbeh.2016.10.023
73. Neal EG, Chaffe H, Schwartz RH, Lawson MS, Edwards N, Fitzsimmons G, et al. The ketogenic diet for the treatment of childhood epilepsy: a randomised controlled trial. Lancet Neurol. (2008) 7:500–6. doi: 10.1016/S1474-4422(08)70092-9
74. Spulber G, Spulber S, Hagenäs L, Amark P, Dahlin M. Growth dependence on insulin-like growth factor-1 during the ketogenic diet. Epilepsia. (2009) 50:297–303. doi: 10.1111/j.1528-1167.2008.01769.x
Keywords: autism spectrum disorder, ketogenic diet, neuroprotection, gut microbiota, blood-brain barrier
Citation: Li Q, Liang J, Fu N, Han Y and Qin J (2021) A Ketogenic Diet and the Treatment of Autism Spectrum Disorder. Front. Pediatr. 9:650624. doi: 10.3389/fped.2021.650624
Received: 07 January 2021; Accepted: 29 March 2021;
Published: 11 May 2021.
Edited by:
Carl E. Stafstrom, Johns Hopkins Medicine, United StatesReviewed by:
David Ruskin, Trinity College, United StatesCaroline Gillis Marleen De Theije, University Medical Center Utrecht, Netherlands
Copyright © 2021 Li, Liang, Fu, Han and Qin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiong Qin, cWluamlvbmdAcGt1cGguZWR1LmNu; Ying Han, aGFueWluZzE1NjhAMTI2LmNvbQ==
 Qinrui Li
Qinrui Li Jingjing Liang1
Jingjing Liang1 Ying Han
Ying Han