- 1Department of Obstetrics, Maternity and Child Health Care of Zaozhuang, Zaozhuang, China
- 2Department of Obstetrics and Gynecology, Maternity and Child Health Care of Zaozhuang, Zaozhuang, China
- 3Department of Cardiology, Heart Center Leipzig at University Leipzig, Leipzig, Germany
Objective: This study presumed that a high or low body mass index (BMI) might increase the risk of infant mortality. Therefore, a meta-analysis was performed to systematically assess the association between maternal BMI and the risk of infant mortality.
Methods: The electronic databases, including Pubmed, Embase database, and Cochrane Library, were systemically searched by two investigators from inception to November 26th, 2020, with no language restriction. In parallel, a dose-response was assessed.
Results: Finally, 22 cohort studies involving 13,532,293 participants were included into this paper, which showed that compared with normal BMI, maternal overweight significantly increased the risks of infant mortality [risk ratio (RR), 1.16; 95% confidence interval (CI), 1.13–1.19], neonatal mortality (RR, 1.23; 95% CI, 1.08–1.39), early neonatal mortality (RR, 1.55; 95% CI, 1.26–1.92) and post-neonatal mortality (RR, 1.18; 95% CI, 1.07–1.29). Similarly, maternal obesity significantly increased the risk of infant mortality (RR, 1.55; 95% CI, 1.41–1.70), neonatal mortality (RR, 1.55; 95% CI, 1.28–1.67), early neonatal mortality (RR, 1.37; 95% CI, 1.13–1.67), and post-neonatal mortality (RR, 1.30; 95% CI, 1.03–1.65), whereas maternal underweight potentially decreased the risk of infant mortality (RR, 0.93; 95% CI, 0.88–0.98). In the dose-response analysis, the risk of infant mortality significantly increased when the maternal BMI was >25 kg/m2.
Conclusions: Maternal overweight or obesity significantly increases the risks of infant mortality, neonatal mortality, early neonatal mortality, and post-neonatal mortality compared with normal BMI in a dose-dependent manner. Besides, maternal underweight will not increase the risk of infant mortality, neonatal mortality, early neonatal mortality, or postneonatal mortality; instead, it tends to decrease the risk of infant mortality. Early weight management may provide potential benefits to infants, and more large-scale prospective studies are needed to verify this finding in the future.
Introduction
Obesity may lead to poor maternal and neonatal health outcomes (1). Obesity is more prevalent in women of reproductive age than in the general population. It has been reported that the prevalence of obesity among women aged 20–39 years increases from 31 to 36% from 2007–2008 to 2015–2016 (2), and such changing demographics represent a new epidemiological trend of particular concern to pregnant women. As recommended by the World Health Organization (WHO), body mass index (BMI) is classified as underweight (BMI<18.5 kg/m2), normal weight (BMI 18.5–24.9 kg/m2), overweight (BMI 25–29.9 kg/m2) or obese (BMI ≥ 30 kg/m2) (3). Obese and pregnant women are more likely to develop intrauterine and fetal complications as well as maternal health problems; meanwhile, obesity during pregnancy may be significantly negatively associated with fetal and maternal health outcomes, including hyperemesis, pre-eclampsia, gestational diabetes, increased incidence of mechanical delivery interventions, and stillbirth (4, 5). Also, compared with normal-weight pregnant women, pregnant women with low pre-pregnancy weight are associated with an increased risk of low birth weight (6, 7), fetal growth restriction (FGR) (8, 9), preterm and premature birth (10) and anemia (11). Therefore, this study presumed that a high or low BMI might increase the risk of infant mortality. To this end, a meta-analysis was carried out in this study to systematically assess the association between maternal BMI and the risk of infant mortality.
Methods
Search Strategy
This study was conducted according to the guidelines for the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) (12). The electronic databases, including Pubmed, Embase database, and Cochrane Library, were systemically searched by two investigators (Huo and Zhang) from inception to November 26th, 2020, with no language restriction. Three sets of medical subject terms (MeSH) were used to search the studies, including “body mass index,” “mortality,” and “infant.” Additionally, a manual library search was also conducted to ensure a comprehensive search. A detailed search strategy is presented in Appendix 1.
Study Selection
The study inclusion criteria were as follows: (1) studies focused on maternal BMI; (2) the study outcomes reported the risk of infant, neonatal, post-neonatal or early neonatal mortality; (3) the study type was restricted to cohort study or randomized controlled trial (RCT); (4) the maximum covariates adjusted hazard ratios (HRs), relative risks (RRs), or odds ratios (ORs) were available, or might be obtained through calculation; (5) if a cohort population was investigated repeatedly, studies containing the longest follow-up period or the largest population were included. At the same time, studies conforming to any one of the following criteria were excluded, including: (1) studies not focusing on maternal BMI or those with unavailable maternal BMI data; (2) the study endpoints did not include risk of infant, neonatal, post-neonatal or early neonatal mortalit; (3) case-control or cross-sectional studies; (4) the related HRs, RRs or ORs were not available; (5) data from one population were used repeatedly; (6) case reports, case series, conference abstracts, reviews or letters were also excluded from this study.
Data Extraction and Quality Assessment
Using a uniform data list, the following data were extracted by two investigators (Huo and Wang), including the first author, published year, country, sample size, study period, parity status, parity, BMI category and outcomes. Any disagreement during the data extraction process was arbitrated by a third investigator (Jia). In addition, the Newcastle-Ottawa Scale (NOS) (13) was also adopted to assess the study quality, with a total score of 9 stars. Studies with a NOS score ≥ 6 stars were considered as high-quality studies, while those with a NOS < 6 stars were considered as low-quality studies.
Statistical Analysis
Infant death was defined as the death of an infant aged < 1 year. Early neonatal death referred to the death of a newborn before 7 days. Neonatal death was defined as the death of an infant within 28 days of birth. Post-neonatal death was defined as the death of an infant older than 28 days but < 1 year of age.
In this study, the primary endpoint was the qualitative analysis on the relationship between maternal BMI and the risk of infant mortality. To be specific, the impacts of maternal underweight, overweight, and obesity on the corresponding risks of infant, neonatal, post-neonatal and early neonatal mortalities were systemically analyzed through comparing the maternal normal to non-normal weights (such as underweight vs. Normal weight, overweight vs. Normal weight, obesity vs. Normal weight). To use more available data, HRs were roughly equal to RRs in cohort studies (14). In addition, due to the low incidence rates of study outcomes in the total population and subgroup populations (<5%), differences between various measures of relative risk were negligible (such as ORs or RRs) (15). All the pooled data from cohort studies were expressed as RRs. Furthermore, the I2 statistic was utilized to evaluate the heterogeneity among studies, and I2 values of 25, 50, and 75% indicated low, moderate, and high inconsistency, respectively. Besides, we performed subgroup and meta-regression analyses to further explore the potential sources of heterogeneity between studies. Moreover, sensitivity analysis, which was performed by excluding one study at a time, was also performed to examine the effect of one study on the pooled results. To more conservatively estimate the pooled RRs, the random-effect model was adopted, since it was able to well-explain the heterogeneity between studies. Besides, Egger's tests were conducted to assess the publication bias (16). If a group of studies contained 2 subgroups (such as obesity 30–39.9 kg/m2, ≥40 kg/m2), they were considered as 2 studies and analyzed separately.
The secondary endpoint of this study was the quantitative analysis on whether maternal BMI level was associated with the risks of infant, neonatal, post-neonatal and early neonatal mortalities. In parallel, a dose-response was assessed. To this end, we performed a dose-response analysis based on the theory put forward by Xu et al. (17). Specifically, in this “one-stage” framework approach, each included study was considered as a cluster across the entire population, which required that the studies should include at least two categories. The method was adopted to the restricted cubic splines to fit the potential non-linear trends at three nodes, and the non-linear p-values were calculated by testing the second spline coefficient to zero. A non-linear model was applied in the case of p ≤ 0.05; conversely, a linear-model was used. Generally speaking, when the reference category included in the analysis was not the lowest, we used the Excel macro file produced by Hamling et al. (18) based on the theory proposed by Greenland and Longnecker (19) to convert risk estimates. When the number of cases in a category was not available, we contacted the original authors. Further, the average of the upper and lower bounds was taken as the midpoint for each BMI category, and then the respective RRs were assigned to each midpoint. Meanwhile, when the study interval was open, the amplitude was assumed to be the same as that of the adjacent category (20).
A total of 1,948 studies were searched from 3 electronic databases, including PubMed, Embase, and Cochrane library, as shown in Figure 1. No additional study was identified by manual search. Of these 1,948 studies, 368 were excluded due to duplication; meanwhile, 1,519 irrelevant studies were also removed after screening the titles and abstracts. The full-texts of the remaining 61 studies were carefully read, among which 39 were excluded for the following reasons: (1) review (n = 2); (2) the exposure was non-maternal BMI (n = 12); (3) non-infant death (n = 22); (4) case-control or cross-sectional study (n = 3). Finally, 22 cohort studies were included for final analysis (21–42). The detailed characteristics of these studies are shown in Table 1. As shown in Supplementary Table 1, 5 of these 22 studies had the NOS scores of 6 stars; 11 of 7 stars; 5 of 8 stars and 1 of 9 stars.
Meta-Analysis
Infant Mortality
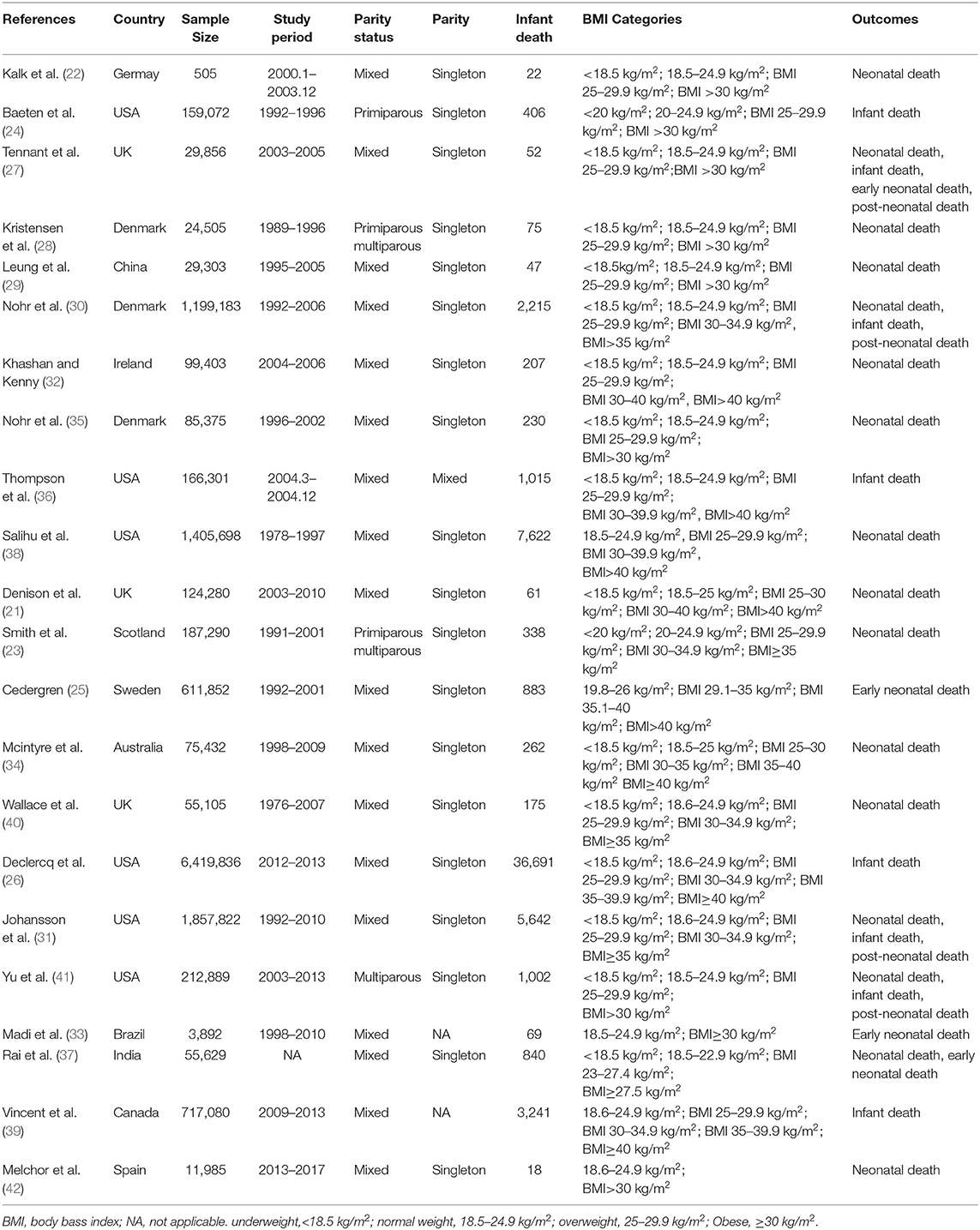
As shown in Figure 2, 7 studies recruiting 10,044,959 participants reported the association between maternal underweight and the risk of infant mortality. Compared with normal-weight pregnant women, underweight pregnant women appeared to have a reduced risk of infant mortality (RR 0.93, 95% CI 0.88–0.98; I2 0%). However, 8 studies including 10,762,039 participants showed that the risk of infant mortality increased by 16% in overweight pregnant women compared with normal-weight pregnant women (RR 1.16, 95% CI 1.13–1.19; I2 0%). Similarly, obese pregnant women had a 55% higher risk of infant mortality than normal weight pregnant women (RR 1.55, 95% CI 1.41–1.70; I2 88.7%). Furthermore, we assessed the publication bias of maternal BMI and the risk of infant mortality by performing funnel plots. Subjectively, the funnel plot appeared to be symmetric, as shown in Supplementary Figure 1A. There was no obvious evidence of publication bias upon Egger's test (p = 0.773).
As displayed in Figure 3A, there was a non-linear relationship between maternal BMI and the risk of infant mortality. Specifically, the dose-response analysis on 8 studies showed that maternal BMI level was non-linearly and positively associated with the risk of infant mortality (pnonlinearity < 0.001). The risk of infant mortality decreased with the increase in BMI level from 16.7 to 25 kg/m2, but with a progressively increasing trend. Typically, the risk of infant mortality increased significantly when maternal BMI was > 25 kg/m2.
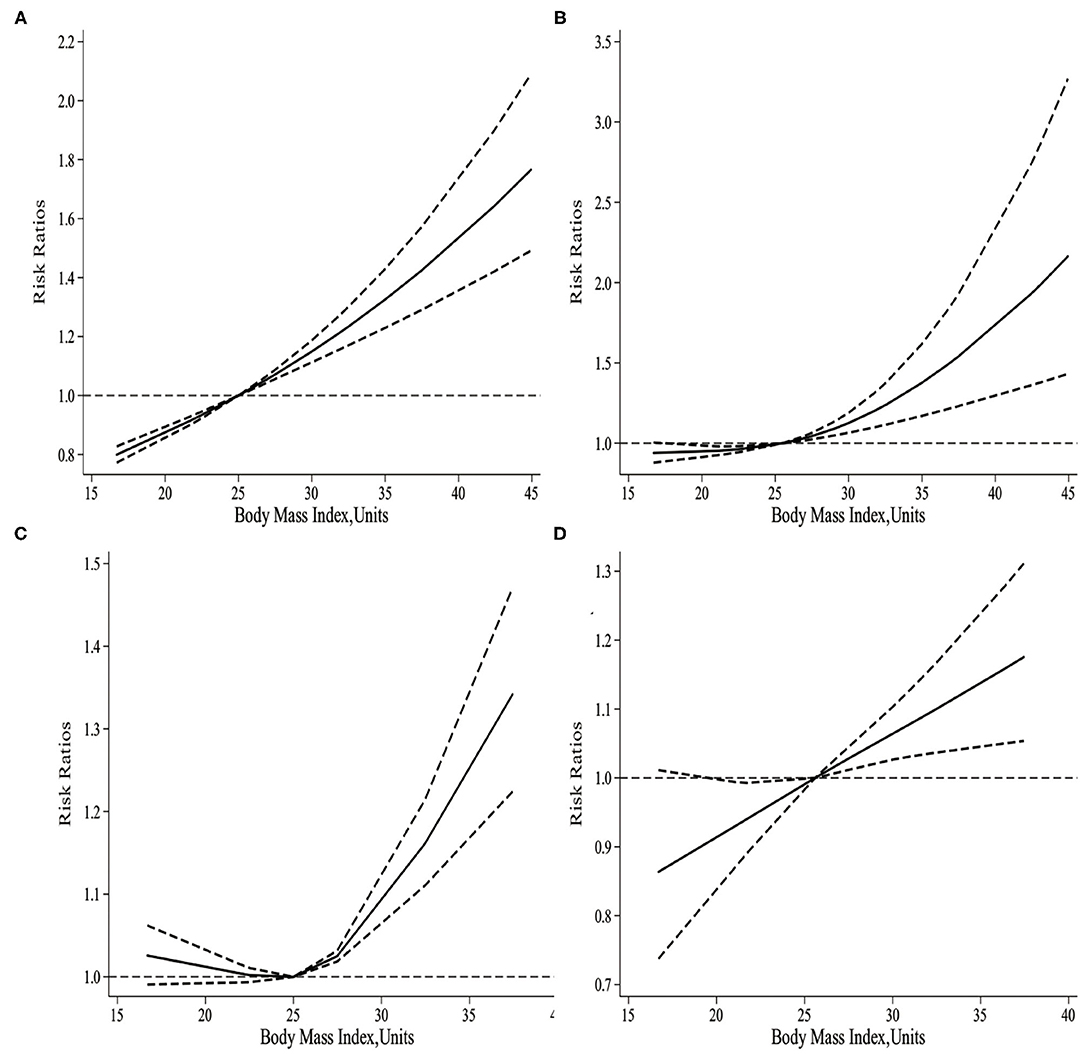
Figure 3. (A) The dose-response of maternal BMI and the risk of infant mortality. (B) The dose-response of maternal BMI and the risk of neonatal mortality. (C) The dose-response of maternal BMI and the risk of post-neonatal mortality. (D) The dose-response of maternal BMI and the risk of early neonatal mortality.
Neonatal Mortality
As presented in Figure 4, 12 studies involving 3,848,782 participants investigated the relationship between maternal underweight and the risk of neonatal mortality. According to the pooled results, underweight pregnant women did not show significantly increased risk of infant mortality compared with normal weight pregnant women (RR 1.08, 95% CI 0.93–1.26; I2 16%). However, the pooled results from 12 studies including 3,848,782 participants indicated that overweight pregnant women contributed to a 23% higher risk of neonatal mortality than normal weight pregnant women (RR 1.23, 95% CI 1.08–1.39; I2 62.4%). Similarly, obese pregnant women showed a 47% increased risk of neonatal mortality compared with normal weight pregnant women (RR 1.55, 95% CI 1.28–1.67; I2 75.6%). Moreover, funnel plot was conducted to assess the publication bias of maternal BMI and the risk of neonatal mortality. Subjectively, the funnel plot was symmetrical, as presented in Supplementary Figure 1B. No obvious evidence of publication bias was found by Egger's test (p = 0.099).
It was illustrated from the dose-response analysis on 15 studies in Figure 3B that, there was a non-linear relationship between maternal BMI and the risk of neonatal mortality. Specifically, maternal BMI level was non-linearly and positively associated with the risk of neonatal mortality (pnonlinearity= 0.002). Besides, the risk of neonatal mortality increased slowly with the maternal BMI level increasing from 16.3 to 25 kg/m2. However, the risk of neonatal mortality significantly increased when the maternal BMI was > 25 kg/m2.
Post-neonatal Mortality
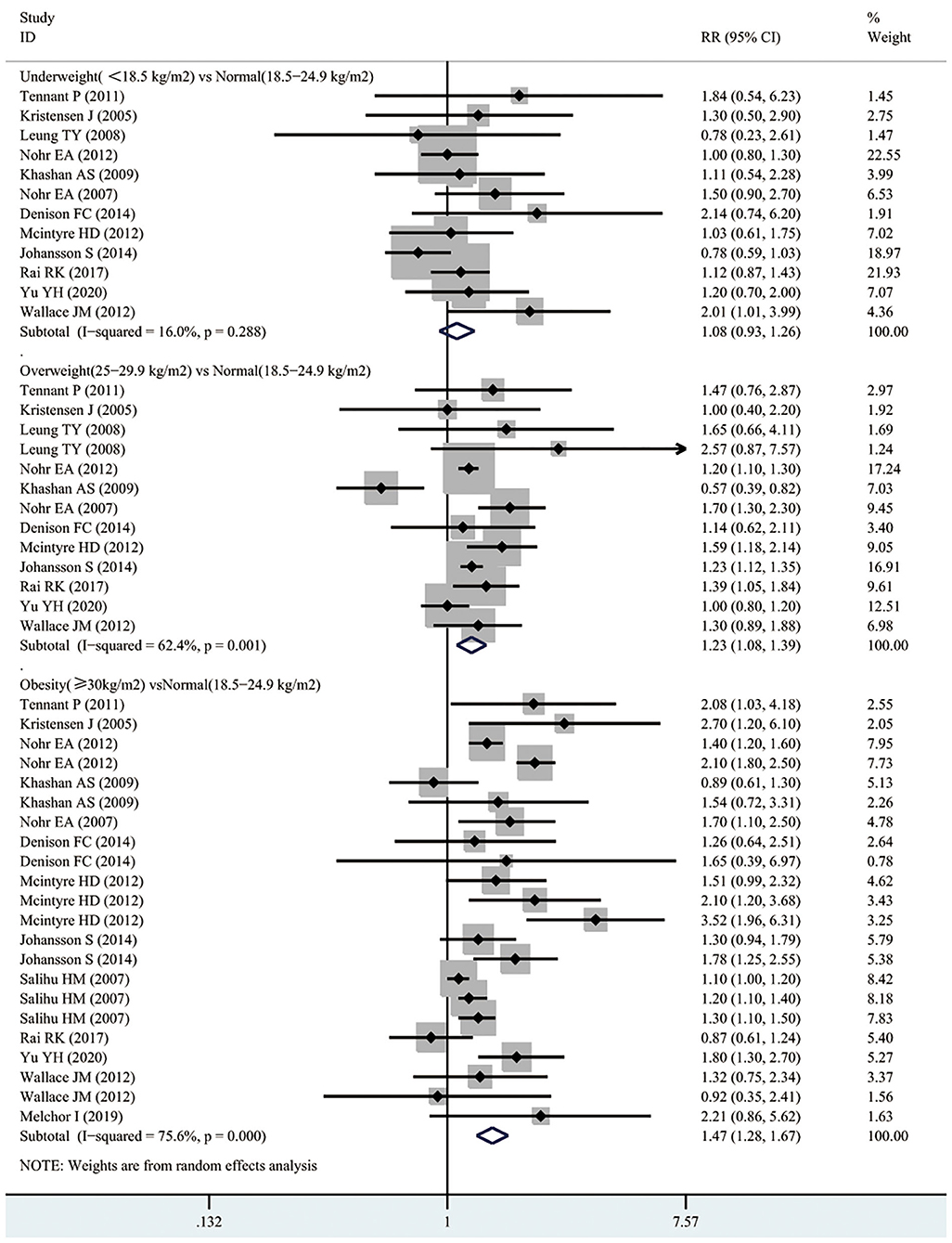
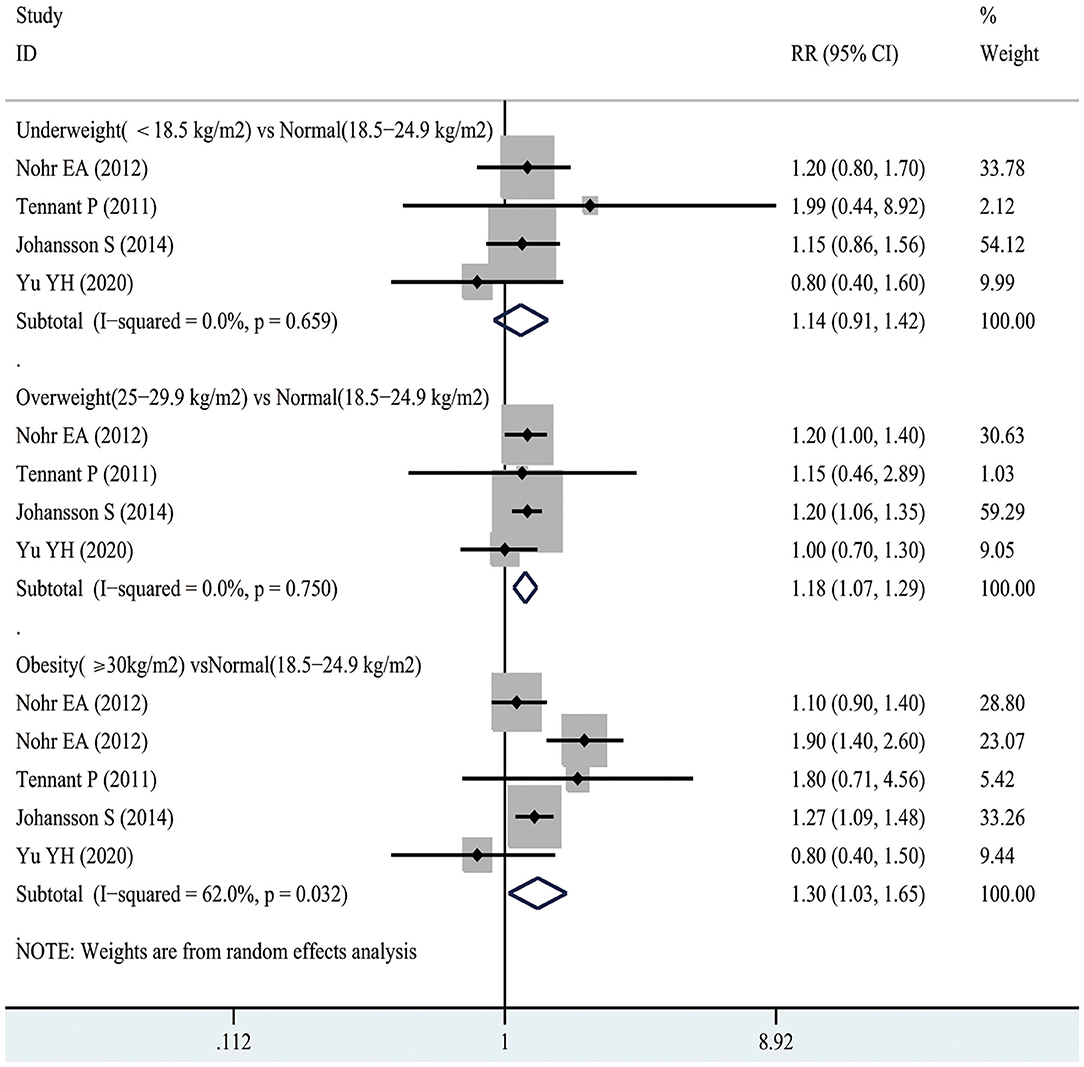
According to Figure 5, 4 studies involving 3,299,750 participants reported the association between maternal underweight and the risk of post-neonatal mortality. As a result, maternal underweight might not lead to an increased risk of infant mortality relative to normal weight pregnant women (RR 1.14, 95% CI 0.91–1.42; I2 0%). However, 4 studies including 3,299,750 participants showed an 18% increased risk of post-neonatal mortality in overweight pregnant women compared with normal-weight pregnant women (RR 1.18, 95% CI 1.07–1.29; I2 0%). Similarly, compared with normal weight pregnant women, the risk of post-neonatal mortality in obese pregnant women increased by 30% (RR 1.30, 95% CI 1.03–1.65; I2 62%). The publication bias of maternal BMI and the risk of post-neonatal mortality was assessed through funnel plot analysis. It was observed from Supplementary Figure 1C that, the funnel plot appeared to be asymmetric, while there was no evidence of publication bias by Egger's test (p = 0.928).
As shown in Figure 3C, there was a non-linear relationship between maternal BMI and the risk of post-neonatal mortality. Specifically, the dose-response analysis on 4 studies revealed a non-linear association between maternal BMI level and the risk of post-neonatal mortality (pnonlinearity= 0.011). With the increase in maternal BMI level from 16.7 to 25 kg/m2, the risk of post-neonatal mortality did not increase, but there was a gradually increasing trend. When the maternal BMI level was > 25 kg/m2, the risk of post-neonatal mortality increased significantly.
Early Neonatal Mortality
According to Figure 6, 2 studies involving 641,708 participants investigated the association between maternal underweight and the risk of early neonatal mortality. As suggested by our results, maternal underweight might not increase the risk of early neonatal mortality compared with normal weight pregnant women (RR 1.01, 95% CI 0.85–1.19; I2 0%). However, 2 studies recruiting 641,708 participants suggested that maternal overweight increased the risk of post-neonatal mortality compared with normal-weight pregnant women (RR 1.55, 95% CI 1.26–1.92; I2 0%). Similarly, maternal obesity also increased the risk of early neonatal mortality (RR 1.37, 95% CI 1.13–1.67; I2 82%). Moreover, funnel plot was conducted to evaluate the publication bias of maternal BMI and the risk of early neonatal mortality, as shown in Supplementary Figure 1D. No obvious evidence of publication bias was observed by Egger's test (p = 0.128).
As observed from Figure 3D, there was a non-linear relationship between maternal BMI and the risk of early neonatal mortality. Specifically, the dose-response analysis on 5 studies showed that maternal BMI level was linearly related to the risk of early neonatal mortality (pnonlinearity = 0.0625). With the increase in maternal BMI level from 16.7 to 25.6 kg/m2, the risk of early neonatal mortality showed an increasing trend; meanwhile, when the maternal BMI level was > 25.6 kg/m2, the risk of early neonatal mortality increased significantly.
Subgroup Analyses and Sensitivity Analyses
Subgroup analyses were performed to explore the potential sources of heterogeneity under these clinical characteristics, including country, NOS scores, infant death cases, and published year. Also, meta-regression was performed to further clarify the potential sources of heterogeneity. In subgroup analysis on infant mortality (obesity vs. normal), the studies reporting infant death cases > 500 and those published after 2000 might partially contribute to the heterogeneity, while meta-regression did not identify any potential source of heterogeneity. In the subgroup analysis on neonatal mortality (overweight vs. normal), studies from Europe and those published before 2000 might partially contribute to the heterogeneity. Similarly, meta-regression did not identify any source of potential heterogeneity. In subgroup analysis on neonatal mortality (obesity vs. normal), no potential source of heterogeneity was identified, and yet meta-regression suggested that the infant death cases (p = 0.06) might partially contribute to heterogeneity. Finally, in the subgroup analysis on post-neonatal mortality (obesity vs. normal) and early neonatal mortality (obesity vs. normal), studies with NOS scores > 8-9 might contribute to the heterogeneity of post-neonatal mortality, whereas no other potential source of heterogeneity was identified. Due to the small number of studies, meta-regression analysis was not performed.
In sensitivity analyses, 1 study was excluded from each analysis at a time, and most results appeared to be robust to the effects of individual studies, as shown in Supplementary Figures 2–4.
Discussion
As shown by the 22 cohort studies including 13,532,293 participants enrolled in this paper, infant mortality increased significantly among overweight or obese pregnant women, compared with normal pregnant women. Simultaneously, maternal underweight might not increase the risk of infant mortality compared with normal-BMI pregnant women, but a trend toward a lower risk of infant mortality was observed.
Two meta-analyses in 2014 systematically analyzed the relationship between maternal BMI level and the risk of infant mortality. For instance, Meehan et al. restricted the study population to obese pregnant women and their results showed that maternal obesity was significantly associated with an increased risk of infant mortality (43). In addition, Aune et al. suggested that even the modest increases in maternal BMI were strongly associated with an increase in infant mortality, while the dose-response analysis showed that low maternal underweight did not significantly increase the risk of infant mortality (44), but only four cohort studies were included. Results obtained from this paper enriched previous studies.
In general, the causes of maternal obesity are complex and multifactorial, and the potential increased risk induced by these causes may be related to obesity itself or the presence of comorbidities such as gestational diabetes and hypertensive disorders (45). A recent meta-analysis shows that maternal overweight or obesity is significantly associated with increased risks of stillbirth, macrosomia, admission to the neonatal intensive care unit (NICU) and large for gestational age (LBW), while maternal underweight is related to the increased risks of preterm birth, small for gestational age (SGA) and LBW (46). Compared with maternal underweight, maternal overweight or obesity appears to be associated with more adverse effects on infants. Besides, it has been shown that even in the absence of clinical disease, obese women have increased inflammatory response (47), vascular and endothelial dysfunction, and lipid metabolic disorders, leading to hyperlipidemia. Hyperlipidemia will cause reduced prostacyclin secretion and increased thromboxane secretion (48), which thus increases the risk of placental thrombosis, reduces placental perfusion (47), elevates the risks of placental infarction and abruption in late pregnancy, leading to preterm birth (49, 50). Also, preterm delivery is found to be closely related to the development of respiratory distress syndrome, which may be the important cause of preterm death (34). On the other hand, most studies have focused on overweight or obese pregnant women, while relatively few studies are conducted on underweight pregnant women.
In the analysis of infant mortality (underweight vs. normal), the risk of infant mortality appeared to be reduced in underweight pregnant women, which seemed to be counter-intuitive to popular convictions. However, in sensitivity analysis, when the study by Declercq et al. was removed, the pooled results were insignificant (RR 1.01, 95% CI 0.88–1.15). Besides, the study by Declercq et al. (26) showed that those underweight pregnant women were linked with a reduced risk of infant mortality compared with normal-weight women, but the primary endpoint of this study was the effect of obesity on infant mortality, and no plausible explanation was given for this results. It has been suggested that women with lower BMI levels can better recognize the reduced fetal movement and therefore take the necessary early interventions, which may partially explain the possible mechanism underlying the risk of infant mortality in underweight pregnant women. In addition, the theory for maternal underweight being protective could also involve mechanisms of cellular hibernation and conservation in the face of stress that may result in earlier maturation of fetal pathways conferring improved survivability. Further research with more prospective studies is needed in the future. Besides, the relationships between maternal BMI level and infant mortality may also be affected by factors such as economic conditions, nutritional status and race. For example, Salihu et al. conducted a restricted analysis on neonatal mortality using data from Missouri in 1978–1997 and found that BMI was associated with the risk of neonatal mortality among white pregnant women, whereas black mothers were related to an overall risk of infant mortality (38).
This study has the following strengths. Firstly, all studies included in this paper were cohort studies with strong levels of evidence, most of them were population-based studies with large samples, and all studies were of high quality. Secondly, this paper systematically conducted qualitative and dose-response analyses to validate the before-and-after results. Findings in this paper also complemented and updated previous studies. Thirdly, sensitivity analysis, subgroup analysis, and meta-regression analysis were simultaneously conducted to maximally search the potential sources of heterogeneity.
Meanwhile, the following limitations should be noted in this work. Firstly, most of the included studies adopted self-reporting for the assessment of BMI, which might produce a certain bias, and for some groups of overweight women, this bias might lead to an underestimation of the measure of effect. Secondly, there were great heterogeneities among some of the studies. Although subgroup and meta-regression analyses were performed, the sources of heterogeneity were not well-explained yet. Thirdly, the maternal BMI level was affected by many factors such as age, economic conditions and nutritional status. Although the extracted RRs were adjusted for maximum covariates, it is still unknown about the potential impacts of other factors on the results. Last but not least, an important limitation was lack of birthweight data that may shed more insight into survival.
Conclusions
Maternal overweight or obesity significantly increases the risks of infant mortality, neonatal mortality, early neonatal mortality, and post-neonatal mortality compared with normal BMI in a dose-dependent manner. Also, maternal underweight does not increase the risk of infant mortality, neonatal mortality, early neonatal mortality, or post-neonatal mortality; instead, it tends to decrease the risk of infant mortality. Early weight management may provide potential benefits to infants, and more prospective studies with large samples are warranted to verify this finding in the future.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
NH participated in the data collection, data review, relevant data extraction, data analysis, statistical analysis, and the writing of the manuscript. KZ, LinW, GJ, and LiW participated in checking data extraction as well as in the data analysis, statistical analysis, and the writing of the manuscript. WL participated in checking data extraction as well as in the statistical analysis. WC participated in performing the risk-of-bias assessment. All authors saw and approved the final version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2021.650413/full#supplementary-material
Supplementary Figure 1. (A) The funnel plot of maternal BMI and the risk of infant mortality. (B) The funnel plot of maternal BMI and the risk of neonatal mortality. (C) The funnel plot of maternal BMI and the risk of post-neonatal mortality. (D) The funnel plot of maternal BMI and the risk of early neonatal mortality.
Supplementary Figure 2. (A) The sensitivity analysis of maternal underweight and the risk of infant mortality. (B) The sensitivity analysis of maternal overweight and the risk of infant mortality. (C) The sensitivity analysis of maternal obesity and the risk of infant mortality.
Supplementary Figure 3. (A) The sensitivity analysis of maternal underweight and the risk of neonatal mortality. (B) The sensitivity analysis of maternal overweight and the risk of neonatal mortality. (C) The sensitivity analysis of maternal obesity and the risk of neonatal mortality.
Supplementary Figure 4. (A) The sensitivity analysis of maternal underweight and the risk of post-neonatal mortality. (B) The sensitivity analysis of maternal overweight and the risk of post-neonatal mortality. (C) The sensitivity analysis of maternal obesity and the risk of post-neonatal mortality. (D) The sensitivity analysis of maternal obesity and the risk of early neonatal mortality.
References
1. McCloud MB. Health behavior change in pregnant women with obesity. Nurs Women Health. (2018) 22:471–80. doi: 10.1016/j.nwh.2018.09.002
2. Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL. Trends in obesity and severe obesity prevalence in US youth and adults by sex and age, 2007-2008 to 2015-2016. JAMA. (2018) 319:1723–5. doi: 10.1001/jama.2018.3060
3. WHO Consultation on Obesity. Obesity: Preventing and Managing the Global Epidemic. Geneva: World Health Organization (2000).
4. Vinayagam D, Chandraharan E. The adverse impact of maternal obesity on intrapartum and perinatal outcomes. ISRN Obstet Gynecol. (2012) 2012:939762. doi: 10.5402/2012/939762
5. Shirazian T, Faris BS, Fox NS, Friedman F Jr, Rebarber A. The lifestyle modification project: limiting pregnancy weight gain in obese women. J Matern Fetal Neonatal Med. (2016) 29:80–4. doi: 10.3109/14767058.2014.987118
6. Haugen M, Brantsæter AL, Winkvist A, Lissner L, Alexander J, Oftedal B, et al. Associations of pre-pregnancy body mass index and gestational weight gain with pregnancy outcome and postpartum weight retention: a prospective observational cohort study. BMC Pregnancy Childbirth. (2014) 14:201. doi: 10.1186/1471-2393-14-201
7. Li N, Liu E, Guo J, Pan L, Li B, Wang P, et al. Maternal prepregnancy body mass index and gestational weight gain on pregnancy outcomes. PLoS ONE. (2013) 8:e82310. doi: 10.1371/journal.pone.0082310
8. Shin D, Song WO. Prepregnancy body mass index is an independent risk factor for gestational hypertension, gestational diabetes, preterm labor, and small- and large-for-gestational-age infants. J Matern Fetal Neonatal Med. (2015) 28:1679–86. doi: 10.3109/14767058.2014.964675
9. Ehrenberg HM, Dierker L, Milluzzi C, Mercer BM. Low maternal weight, failure to thrive in pregnancy, and adverse pregnancy outcomes. Am J Obstet Gynecol. (2003) 189:1726–30. doi: 10.1016/S0002-9378(03)00860-3
10. Schieve LA, Cogswell ME, Scanlon KS, Perry G, Ferre C, Blackmore-Prince C, et al. Prepregnancy body mass index and pregnancy weight gain: associations with preterm delivery. The NMIHS collaborative study group. Obstet Gynecol. (2000) 96:194–200. doi: 10.1097/00006250-200008000-00008
11. Pengpid S, Peltzer K. Lifetime spousal violence victimization and perpetration, physical illness, and health risk behaviours among women in India. Int J Environ Res Public Health. (2018) 15:2737. doi: 10.3390/ijerph15122737
12. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. (2000) 283:2008–12. doi: 10.1001/jama.283.15.2008
13. Zhang J, Yu KF. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. (1998) 280:1690–1. doi: 10.1001/jama.280.19.1690
14. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
15. Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. (1987) 9:1–30. doi: 10.1093/oxfordjournals.epirev.a036298
16. Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol. (2005) 58:882–93. doi: 10.1016/j.jclinepi.2005.01.016
17. Xu C, Doi SAR. The robust error meta-regression method for dose-response meta-analysis. Int J Evidence-Based Healthcare. (2018) 16:138–44. doi: 10.1097/XEB.0000000000000132
18. Hamling J, Lee P, Weitkunat R, Ambuhl M. Facilitating meta-analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category. Stat Med. (2008) 27:954–70. doi: 10.1002/sim.3013
19. Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. (1992) 135:1301–9. doi: 10.1093/oxfordjournals.aje.a116237
20. Cheng W, Zhang Z, Cheng W, Yang C, Diao L, Liu W. Associations of leisure-time physical activity with cardiovascular mortality: a systematic review and meta-analysis of 44 prospective cohort studies. Euro J Prevent Cardiol. (2018) 25:1864–72. doi: 10.1177/2047487318795194
21. Denison FC, Norwood P, Bhattacharya S, Duffy A, Mahmood T, Morris C, et al. Association between maternal body mass index during pregnancy, short-term morbidity, and increased health service costs: a population-based study. BJOG. (2014) 121:72–81. doi: 10.1111/1471-0528.12443
22. Kalk P, Guthmann F, Krause K, Relle K, Godes M, Gossing G, et al. Impact of maternal body mass index on neonatal outcome. Eur J Med Res. (2009) 14:216–22. doi: 10.1186/2047-783X-14-5-216
23. Smith GC, Shah I, Pell JP, Crossley JA, Dobbie R. Maternal obesity in early pregnancy and risk of spontaneous and elective preterm deliveries: a retrospective cohort study. Am J Public Health. (2007) 97:157–62. doi: 10.2105/AJPH.2005.074294
24. Baeten JM, Bukusi EA, Lambe M. Pregnancy complications and outcomes among overweight and obese nulliparous women. Am J Public Health. (2001) 91:436–40. doi: 10.2105/AJPH.91.3.436
25. Cedergren MI. Maternal morbid obesity and the risk of adverse pregnancy outcome. Obstet Gynecol. (2004) 103:219–24. doi: 10.1097/01.AOG.0000107291.46159.00
26. Declercq E, MacDorman M, Cabral H, Stotland N. Prepregnancy Body Mass Index and Infant Mortality in 38 U.S. States, 2012-2013. Obstet Gynecol. (2016) 127:279–87. doi: 10.1097/AOG.0000000000001241
27. Tennant PW, Rankin J, Bell R. Maternal body mass index and the risk of fetal and infant death: a cohort study from the North of England. Hum Reprod. (2011) 26:1501–11. doi: 10.1093/humrep/der052
28. Kristensen J, Vestergaard M, Wisborg K, Kesmodel U, Secher NJ. Pre-pregnancy weight and the risk of stillbirth and neonatal death. BJOG. (2005) 112:403–8. doi: 10.1111/j.1471-0528.2005.00437.x
29. Leung TY, Leung TN, Sahota DS, Chan OK, Chan LW, Fung TY, et al. Trends in maternal obesity and associated risks of adverse pregnancy outcomes in a population of Chinese women. BJOG. (2008) 115:1529–37. doi: 10.1111/j.1471-0528.2008.01931.x
30. Nohr EA, Villamor E, Vaeth M, Olsen J, Cnattingius S. Mortality in infants of obese mothers: is risk modified by mode of delivery? Acta Obstet Gynecol Scand. (2012) 91:363–71. doi: 10.1111/j.1600-0412.2011.01331.x
31. Johansson S, Villamor E, Altman M, Bonamy AK, Granath F, Cnattingius S. Maternal overweight and obesity in early pregnancy and risk of infant mortality: a population based cohort study in Sweden. BMJ. (2014) 349:g6572. doi: 10.1136/bmj.g6572
32. Khashan AS, Kenny LC. The effects of maternal body mass index on pregnancy outcome. Eur J Epidemiol. (2009) 24:697–705. doi: 10.1007/s10654-009-9375-2
33. Madi SRC, Garcia RMR, Souza VC, Rombaldi RL, Araújo BF, Madi JM. Effect of obesity on gestational and perinatal outcomes. Rev Bras Ginecol Obstet. (2017) 39:330–6. doi: 10.1055/s-0037-1603826
34. McIntyre HD, Gibbons KS, Flenady VJ, Callaway LK. Overweight and obesity in Australian mothers: epidemic or endemic? Med J Aust. (2012) 196:184–8. doi: 10.5694/mja11.11120
35. Nohr EA, Vaeth M, Bech BH, Henriksen TB, Cnattingius S, Olsen J. Maternal obesity and neonatal mortality according to subtypes of preterm birth. Obstet Gynecol. (2007) 110:1083–90. doi: 10.1097/01.AOG.0000286760.46679.f8
36. Thompson DR, Clark CL, Wood B, Zeni MB. Maternal obesity and risk of infant death based on Florida birth records for (2004). Public Health Rep. (2008) 123:487–93. doi: 10.1177/003335490812300410
37. Rai RK, Singh L, Singh PK. Is maternal body mass index associated with neonatal mortality? A pooled analysis of nationally representative data from nine Asian countries. Nutrition. (2017) 41:68–72. doi: 10.1016/j.nut.2017.04.002
38. Salihu HM, Alio AP, Wilson RE, Sharma PP, Kirby RS, Alexander GR. Obesity and extreme obesity: new insights into the black-white disparity in neonatal mortality. Obstet Gynecol. (2008) 111:1410–6. doi: 10.1097/AOG.0b013e318173ecd4
39. Vincent S, Czuzoj-Shulman N, Spence AR, Abenhaim HA. Effect of pre-pregnancy body mass index on respiratory-related neonatal outcomes in women undergoing elective cesarean prior to 39 weeks. J Perinat Med. (2018) 46:905–12. doi: 10.1515/jpm-2017-0384
40. Wallace JM, Horgan GW, Bhattacharya S. Placental weight and efficiency in relation to maternal body mass index and the risk of pregnancy complications in women delivering singleton babies. Placenta. (2012) 33:611–8. doi: 10.1016/j.placenta.2012.05.006
41. Yu YH, Bodnar LM, Himes KP, Brooks MM, Naimi AI. Association of Overweight and Obesity Development Between Pregnancies With Stillbirth and Infant Mortality in a Cohort of Multiparous Women. Obstet Gynecol. (2020) 135:634–43. doi: 10.1097/AOG.0000000000003677
42. Melchor I, Burgos J, Del Campo A, Aiartzaguena A, Gutiérrez J, Melchor JC. Effect of maternal obesity on pregnancy outcomes in women delivering singleton babies: a historical cohort study. J Perinat Med. (2019) 47:625–630. doi: 10.1515/jpm-2019-0103
43. Meehan S, Beck CR, Mair-Jenkins J, Leonardi-Bee J, Puleston R. Maternal obesity and infant mortality: a meta-analysis. Pediatrics. (2014) 133:863–71. doi: 10.1542/peds.2013-1480
44. Aune D, Saugstad OD, Henriksen T, Tonstad S. Maternal body mass index and the risk of fetal death, stillbirth, and infant death: a systematic review and meta-analysis. JAMA. (2014) 311:1536–46. doi: 10.1001/jama.2014.2269
45. Kim SS, Zhu Y, Grantz KL, Hinkle SN, Chen Z, Wallace ME, et al. Obstetric and Neonatal Risks Among Obese Women Without Chronic Disease. Obstet Gynecol. (2016) 128:104–12. doi: 10.1097/AOG.0000000000001465
46. Liu P, Xu L, Wang Y, Zhang Y, Du Y, Sun Y, et al. Association between perinatal outcomes and maternal pre-pregnancy body mass index. Obes Rev. (2016) 17:1091–102. doi: 10.1111/obr.12455
47. Ramsay JE, Ferrell WR, Crawford L, Wallace AM, Greer IA, Sattar N. Maternal obesity is associated with dysregulation of metabolic, vascular, and inflammatory pathways. J Clin Endocrinol Metab. (2002) 87:4231–7. doi: 10.1210/jc.2002-020311
48. Eldor A. Thrombophilia and its treatment in pregnancy. J Thromb Thrombolysis. (2001) 12:23–30. doi: 10.1023/A:1012730325902
49. Craven C, Ward K. Stillbirth: tissue findings with environmental and genetic links. Semin Perinatol. (2002) 26:36–41. doi: 10.1053/sper.2002.29836
Keywords: infant, maternal, body mass index, mortality, meta-analysis
Citation: Huo N, Zhang K, Wang L, Wang L, Lv W, Cheng W and Jia G (2021) Association of Maternal Body Mass Index With Risk of Infant Mortality: A Dose-Response Meta-Analysis. Front. Pediatr. 9:650413. doi: 10.3389/fped.2021.650413
Received: 07 January 2021; Accepted: 17 February 2021;
Published: 12 March 2021.
Edited by:
Abhay Gaidhane, Datta Meghe Institute of Medical Sciences, IndiaReviewed by:
Vijay Srinivasan, Children's Hospital of Philadelphia, United StatesJoy D. Doll, Creighton University, United States
Copyright © 2021 Huo, Zhang, Wang, Wang, Lv, Cheng and Jia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: GuangZhu Jia, amlhZ3Vhbmd6aHUyMDE4QDEyNi5jb20=
 Nana Huo1
Nana Huo1 GuangZhu Jia
GuangZhu Jia