- 1Faculty of Medicine & Dentistry, University of Alberta, Edmonton, AB, Canada
- 2Department of Pediatric Nephrology, Evelina London Children's Hospital, London, United Kingdom
- 3Division of Pediatric Critical Care, Department of Pediatrics, University of Alberta and Stollery Children's Hospital, Edmonton, AB, Canada
- 4Department of Pediatric Nephrology, Stollery Children's Hospital, Edmonton, AB, Canada
- 5Department of Critical Care Medicine, Faculty of Medicine and Dentistry and Alberta Health Services—Edmonton Zone, University of Alberta, Edmonton, AB, Canada
- 6Alberta Critical Care Strategic Clinical Network, Alberta Health Services, Edmonton, AB, Canada
- 7King's College London, London, United Kingdom
Acute kidney injury (AKI) in the pediatric intensive care unit (PICU) is an important risk factor for increased morbidity and mortality during hospitalization. Over the past decade, accumulated data on children and young people indicates that acute episodes of kidney dysfunction can have lasting consequences on multiple organ systems and health outcomes. To date, there are no guidelines for follow-up of surviving children that may be at risk of long-term sequelae following AKI in the PICU. This narrative review aims to describe literature from the last 5 years on the risk of medium and long-term kidney and non-kidney outcomes after AKI in the PICU. More specifically, we will focus on outcomes in children and young people following AKI in the general PICU population and children undergoing cardiac surgery. These outcomes include mortality, hypertension, proteinuria, chronic kidney disease, and healthcare utilization. We also aim to highlight current gaps in knowledge in medium and long-term outcomes in this pediatric population. We suggest a framework for future research to develop evidence-based guidelines for follow-up of children surviving an episode of critical illness and AKI.
Introduction
Acute kidney injury (AKI) is defined as an abrupt onset of kidney dysfunction. AKI occurs in 20–30% of the general pediatric intensive care (PICU) population and up to 50% in children following cardiac surgery (1–6). Compared to children who do not develop AKI in the PICU, children with AKI are at higher risk of poor early outcomes, including mortality, receipt and longer duration of mechanical ventilation, and prolonged length of PICU and subsequent hospital stay (7–10).
More recently, research has focused on evaluating the medium and long-term sequelae of AKI sustained in the PICU. One of the largest early follow-up studies found that 10% of children with AKI in the PICU developed chronic kidney disease (CKD) [defined as glomerular filtration rate (GFR) <60 ml/min/1.73 m2 or albuminuria] 1–3 years later (11). Additionally, 47% of children with AKI were considered to be at risk of CKD [defined as GFR 60–90 ml/min/1.73 m2, hypertension, and/or hyperfiltration (GFR ≥ 150 ml/min/1.73m2)]. In 2014, Greenberg et al. performed a systematic review and meta-analysis to examine the long-term kidney outcomes after AKI (12). These early studies found that children with AKI had higher rates of hypertension, proteinuria, CKD, and mortality relative to the general pediatric population. However, the authors highlighted that there was significant heterogeneity between studies with an absence of contemporaneous patients with no AKI, and variable definitions of AKI, which together made it difficult to evaluate the association between AKI and outcomes. These issues have been addressed systematically in recent studies standardizing AKI definitions, with large study cohorts that include non-AKI comparison groups, and an overall increase in the quality of research and rigor of analyses. This has allowed for a better understanding of the association between AKI and long-term outcomes.
The objectives of this narrative review are to: (i) summarize literature over the past 5 years for relevant medium and long-term outcomes following AKI in the general PICU population and children undergoing cardiac surgery; (ii) highlight risk associations for reported adverse outcomes; and (iii) discuss future directions for clinical guidelines and research to mitigate adverse outcomes in this high-risk population.
Mortality
Previous studies evaluating short- and long-term mortality following AKI lacked non-AKI comparison groups, however, they uniformly highlight that mortality rates in patients with AKI were higher than in the general population (12, 13). Since 2015, we identified eight studies that examined the association of AKI with mortality and compared this to children who did not develop AKI, with follow-up ranging from 28-days to 5–7 years (Table 1) (3, 6, 14–19). As highlighted in Table 1, despite differences in study populations and duration of follow-up, all studies reported higher mortality in those with AKI when compared with children who did not experience AKI whilst in the PICU, independent of illness severity and other important confounders.
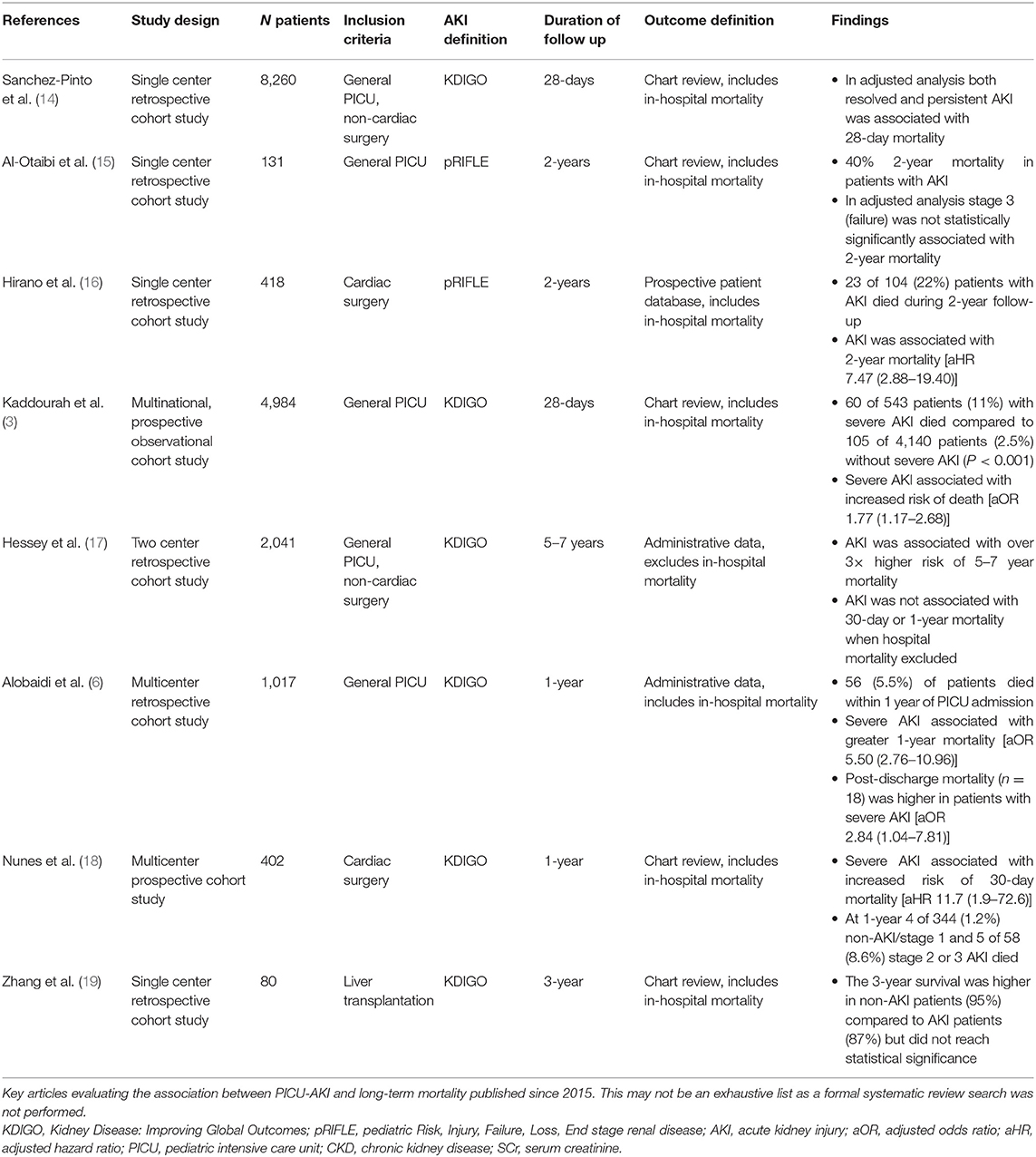
Table 1. Summary of studies evaluating the association between AKI and mortality published over the past 5-years.
General PICU Population
In a multinational study of 4,984 general PICU patients, Kaddourah et al. showed that severe AKI [defined throughout this review as stage 2 or 3 AKI by Kidney Disease: Improving Global Outcomes (KDIGO) criteria unless specified] was associated with 77% greater odds of 28-day mortality following adjustments for risk factors that differed between survivors and non-survivors including admission diagnoses, comorbidities, illness severity, and PICU interventions (3). Similarly, when compared to those with no AKI, a large multicenter Canadian study observed that severe AKI was associated with over 5 times greater risk of mortality 1-year after PICU admission following adjustment for illness severity and other important confounders (6). It is important to highlight that both these studies included in-hospital mortality from initial admission in their outcomes. In the Canadian study 52 (5.5%) patients died, 18 of whom died after discharge. Severe AKI was still associated with post-discharge mortality [odds ratio [OR] (95% confidence interval [CI]): 2.84 (10.4–7.81)] (6).
The longest follow-up study looked at mortality 5–7 years after hospital discharge in a non-cardiac surgery PICU population and found that AKI was associated with over 3 times higher risk of mortality (17). Interestingly, this paper demonstrated that the association between AKI and 30-day and 1-year mortality was conditional on the inclusion of hospital mortality in the outcome. When hospital mortality was included, AKI was associated with 30-day and 1-year mortality with a similar magnitude of association as reported in previous studies (3, 6, 17).
Cardiac Surgery Population
Two studies specifically focused on mortality in children with AKI following cardiac surgery. In a multicenter study of over 400 patients, Nune et al. found that children with severe AKI post-cardiac surgery had over 11 times higher risk of mortality 30-day after surgery, following adjustment for age, surgical risk score, cardiopulmonary bypass time, and cyanotic heart disease (18). In a similar-sized population, Hirano et al. reported a higher risk of 2-year mortality in children with AKI [defined by Pediatric Risk, Injury, Failure, Loss, End Stage Renal Disease (pRIFLE) criteria] post-cardiac surgery [adjusted hazard ratio (95% CI): 7.47 (2.88–19.40)] (16). Both of these studies included hospital mortality from the initial cardiac surgery admission.
What's New?
Since the publication of the systematic review by Greenberg et al. we now have comparable groups (with and without AKI during hospitalization) that allow us to evaluate the association between AKI and mortality (12). All studies found that children who develop AKI in the PICU had higher mortality, independent of illness severity measures, compared to patients admitted to the PICU who did not develop AKI. However, it is important to highlight that many of these studies included in-hospital mortality from the index admission.
Hypertension, Proteinuria, and Chronic Kidney Disease
In adult studies, AKI in the ICU is independently associated with significantly worse long-term kidney outcomes including hypertension, proteinuria, and CKD (20–22). The potential mechanism for the progression of AKI to CKD includes loss of kidney mass during the acute event resulting in progressive hyperfiltration damage, glomerulosclerosis, and fibrosis (23). This relationship has been challenging to study in the pediatric population as a result of the known physiologic maturation in glomerular filtration rate following birth, the current lack of consensus guidelines on how best to monitor these children over time resulting in varying follow-up practices, and inconsistent definitions of long-term kidney outcomes in the literature. Initial findings showed a higher prevalence of adverse kidney outcomes following AKI compared to the general population; however, in these studies subject numbers were small, follow-up duration was limited, and many lacked non-AKI comparison groups (12). Since 2015 we found eleven studies examining the association between PICU-AKI and hypertension, proteinuria, and CKD (Table 2) (2, 4, 15, 24–32).
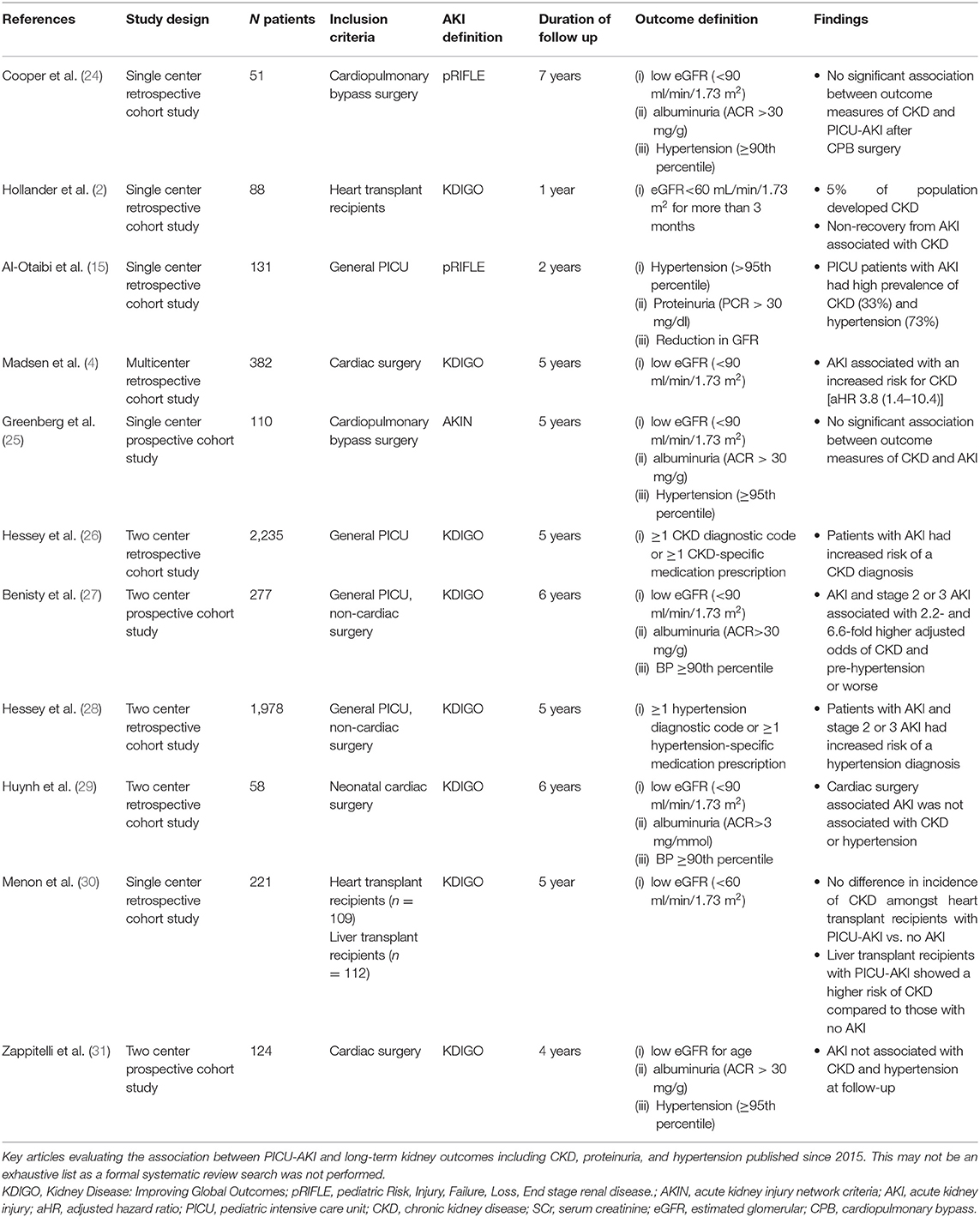
Table 2. Summary of studies evaluating the association between AKI and long-term kidney outcomes published over the past 5-years.
General PICU Population
There have been four studies examining the association of CKD following AKI in general PICU cohorts. The follow-up time across these studies varied from 2 to 6 years, as did the methods of defining outcomes. However, consistently across all studies patients with mixed etiology, PICU-AKI were at higher risk of developing hypertension, proteinuria, and/or CKD over the long-term (15, 26–28). Two studies in cohorts of general PICU-AKI patients measured outcomes using laboratory defined CKD (albumin/creatinine >30 mg/g or GFR <90 ml/min/1.73 m2) or measured office blood pressure abnormalities (15, 27). The prevalence of CKD and hypertension was high in the AKI population in both studies. One of these studies by Benisty et al. found that 6 years after PICU admission any AKI (i.e., stage 1, 2, or 3 by KDIGO criteria) and severe AKI (stage 2 or 3) were associated with a 2.2 [95% CI: 1.1–4.4] and 6.6 [95% CI: 1.5–28.3] higher adjusted odds ratio for CKD and pre-hypertension or worse, respectively (27).
Two studies from the same cohort in Quebec alternatively used administrative data (diagnostic and medication codes) to define CKD and hypertension outcomes (26, 28). In this large two-center retrospective cohort of 2,245 subjects, even following mild (stage 1) AKI, children had a significantly increased risk of CKD diagnosis after a 5-year follow-up period (26). At follow-up, 2% had a diagnosis of CKD. Those with mild AKI (stage 1) had an increased risk of CKD with an adjusted hazard ratio of 2.2 [95% CI:1.1–4.5]. Those with more severe AKI (stage 2 or 3) had a higher risk of CKD with an adjusted hazard ratio of 2.5 [95% CI: 1.1–5.7] (26). Looking exclusively at hypertension diagnosis based on administrative data in the same large two-center cohort, Hessey et al. found that patients with PICU-AKI had over twice the risk of a hypertension diagnosis with an adjusted hazard ratio of 2.19 [95% CI: 1.47–3.26] (28).
Cardiac Surgery Population
Six studies reported long-term kidney outcomes following PICU-AKI exclusively in subjects who underwent cardiac surgery (2, 4, 24, 25, 29, 31). Unlike the general PICU population, there is conflicting evidence on whether AKI after cardiac surgery is associated with negative long-term kidney outcomes. A two-center prospective cohort study of 124 children undergoing cardiac surgery found no association between AKI and CKD or hypertension after 2 years (31). The overall prevalence of CKD and hypertension was high at 20 and 30%, respectively, and young age at surgery was the only factor associated with CKD development. Contradicting these findings, in a single-center retrospective cohort study of 382 subjects who had undergone congenital cardiac surgery, after a median 3-year follow-up period, 11% of patients with AKI demonstrated CKD (4). The hazard ratio for CKD development amongst those with AKI compared with the non-AKI group was notably high, at 3.8 [95% CI: 1.4–10.4] following adjustment for sex, age, and surgical complexity (4). Similarly, two studies looking at long-term kidney outcomes exclusively in subjects who had undergone cardiopulmonary bypass surgery did not find an association between AKI and CKD (GFR <90 ml/min/1.73 m2 or albuminuria) or hypertension (blood pressure >95th percentile) (24, 25).
In a cardiac sub-group of 88 heart transplant recipients, advanced CKD (GFR <60 mL/min/1.73 m2) developed in 5% of the population after 1-year follow-up and was more common in those who did not fully recover kidney function and in those with more severe AKI (stage 2 or 3 by KDIGO) (2). Lastly, in an exclusively neonatal cohort of 58 subjects who underwent cardiac surgery, with a median follow-up of 6 years, AKI was not associated with CKD or hypertension despite a high prevalence overall (17 and 30%, respectively) (29). Post-operative cyanosis was the only independent predictor of CKD.
Biomarkers have been explored as a way to detect kidney injury earlier than rising serum creatinine and to predict hospital outcomes in both adult and pediatric settings (33–37). These biomarkers are also being explored as possible markers of CKD and its progression (38). Two pediatric studies looked at the long-term changes in biomarkers, both in cardiac surgery populations. The Translational Research Investigating Biomarker End Points in AKI (TRIBE-AKI) consortium found that urinary neutrophil gelatinase-associated lipocalin (NGAL) and urinary interleukin-18 (IL-18) concentrations rose acutely postoperatively in children with AKI but then decreased by the 5-year follow-up (25). They did not find a difference in median urine biomarker levels in children with severe AKI at 5-year follow-up compared to non-AKI patients. Unlike the TRIBE-AKI study, the Follow-Up Renal Assessment of Injury Long-Term After Acute Kidney Injury (FRAIL-AKI) study demonstrated that 7–8 years after cardiac surgery, children with AKI had higher urinary IL-18, kidney injury molecule-1 (KIM-1), and liver-type fatty acid-binding protein (L-FABP) concentrations (24). Despite these changes in biomarker profiles, these studies have not reported higher risk associations for adverse kidney outcomes in children with AKI post-cardiac surgery.
What Is New?
One of the major improvements in published studies since 2015 has been the standardization of AKI definitions, the inclusion of non-AKI comparison groups, and standardized definitions of hypertension, proteinuria, and CKD. The previous meta-analysis found an overall high prevalence of adverse kidney outcomes in AKI populations, however, more recent studies with non-AKI comparison groups and larger cohorts allow us to better understand this population's long-term risk (12). In the general PICU population, AKI is associated with an increased risk of CKD and hypertension compared to the non-AKI population after controlling for important confounders using both measured outcome data and administrative data. In the cardiac surgery population, intriguingly this risk association remains less clear, however as previously highlighted, this population has a high prevalence of kidney outcomes in long-term follow-up (12). Early research on the use of biomarkers for evaluating kidney recovery or CKD development has been published but validation of these biomarkers against gold standard measurements of kidney function are needed before they can be widely used.
Healthcare Utilization
In adults, AKI is associated with an increased risk of rehospitalization. In this population, the KDIGO guidelines suggest follow-up care 3 months after an AKI episode to assess new onset or worsening CKD (i.e., assess serum creatinine, urine for protein, blood pressure) (39–41). Limited data have been published on the impact of AKI on long-term healthcare utilization in children following their initial admission to the PICU. Two studies reporting on healthcare utilization, one in a non-cardiac surgery population and the other in those following cardiac surgery, have been published (Table 3).
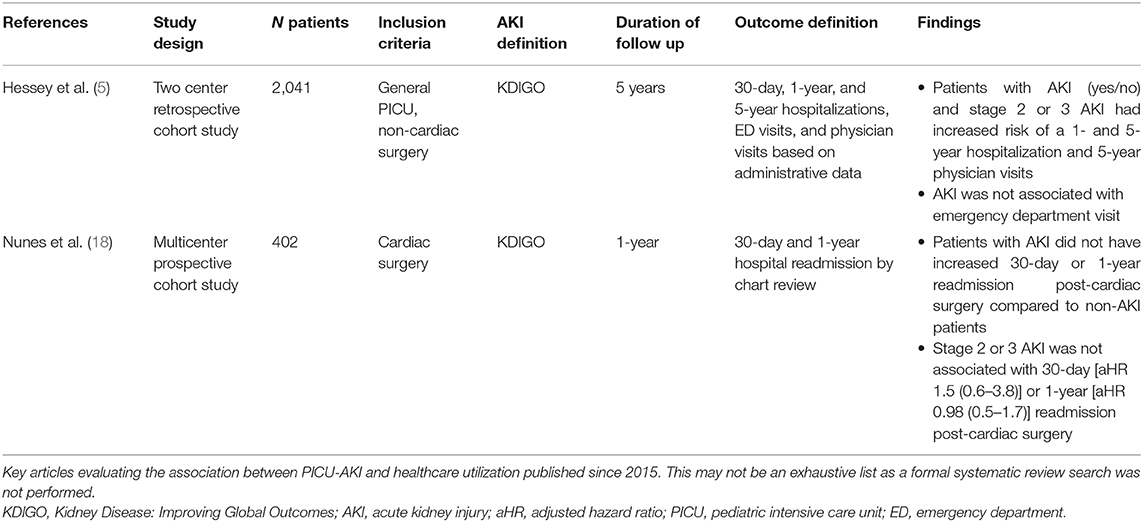
Table 3. Summary of studies reporting AKI and long-term healthcare utilization published over the past 5-years.
General PICU Population
In the non-cardiac surgery PICU population, although there was no association with increased emergency department visits in those who developed AKI, children with AKI had a 35% higher risk of 1-year hospitalization, and 59% higher risk of 5-year hospitalizations compared to children without AKI (5). AKI was also associated with increased physician visits over 5 years [relative risk (95% CI) 1.12 (1.07–1.18)]. Importantly, <25% of the children with AKI saw a nephrologist in a 5-year follow-up (5). The reason for nephrology follow-up was not reported nor was a diagnosis of CKD or hypertension, however, this still highlights that even children with severe AKI in the PICU do not have regular follow-up with kidney specialists.
Cardiac Surgery Population
In the cardiac surgery population, neither any AKI (i.e., stage 1, 2, or 3) nor severe AKI (stage 2 or 3 by KDIGO criteria) were associated with 30-day or 1-year hospital readmission (18). Unlike the aforementioned study in the non-cardiac population which evaluated the number of events per person-time (count data), this study focused on readmissions as a binary outcome. Therefore, further research evaluating cumulative use healthcare is required to better understand if there are differences in healthcare utilization in this population.
What's New?
Evaluating the impact of AKI on long-term healthcare utilization is a new area of research that has emerged in the pediatric literature over the past 5 years. At this time, it remains unclear if AKI itself contributes to increased healthcare utilization in the general PICU population either by long-term kidney and non-kidney sequelae that may progress following AKI or if perhaps, AKI is a marker of a patient's medical complexity. Due to the limited data on healthcare utilization following AKI in the PICU, we are not able to draw strong conclusions at this time. Research comparing healthcare utilization prior to critical illness and after critical illness (i.e., a difference-in-difference approach), specifically evaluating AKI as a risk factor, would help to clarify this. Although there are currently no guidelines for post-discharge monitoring after pediatric AKI, non-cardiac surgery children who experience AKI whilst in PICU may require closer follow-up by primary care providers, hence leading to increased physician visits. In those following cardiac surgery, this is “in built” to their current care pathways with sub-specialist cardiac reviews and follow-up. Research on provider practice is needed to further evaluate this.
Discussion
With the development of a standardized definition for AKI and increased awareness of long-term sequelae of AKI described in the adult literature, more research evaluating pediatric AKI and long-term outcomes has been reported (21, 39). In the general PICU population, AKI is associated with an increased risk of long-term mortality, healthcare utilization, CKD, and hypertension. In the cardiac surgery population, the association between AKI and long-term outcomes is less clear; however, these children remain at high risk of kidney sequelae compared to the general population. Since the meta-analysis by Greenberg et al. published in 2014, non-AKI comparison cohorts have been included in research studies allowing for the evaluation of AKI with long-term outcomes rather than simply making comparisons with the general pediatric population (12). This has not only allowed us to identify AKI as an important risk factor for poor long-term outcomes, but it has also allowed us to quantify the magnitude of risk of developing these outcomes.
Over the past 5 years, more data has been published beyond early mortality (within 28-days) describing both medium (1–2 year) (6, 15, 16, 18) and long-term mortality risk (>3 years) (17, 19). Importantly, only two studies evaluated post-discharge mortality (i.e., excluded hospital mortality from the index admission). While the focus of this review was on long-term outcomes, we included key studies evaluating 28-day mortality as we felt this data frames the population and demonstrates the differences in risk of mortality during the acute illness and the long-term. This data highlights potential future research directions and the need to focus on evaluating in-hospital vs. post-discharge mortality to improve our understanding of the timelines and etiologies of mortality risk in this heterogeneous population of children with hospital-acquired AKI.
There remains a divergence of reported long-term kidney outcomes in children who developed PICU-AKI. Larger cohorts with general PICU populations show a consistent association between PICU-AKI and hypertension, proteinuria, and CKD. In exclusively cardiac surgery populations this association is less clear and requires further investigation. It remains to be shown if this is a result of study cohort size and shorter follow-up duration, if it is due to differences in the patient population, or the management of the patients in the peri-operative period or subsequently in the immediate post-operative period. It is well-known that there is a high prevalence of AKI after cardiac surgery and this is associated with poor short-term outcomes. There may be more strategies in place to identify and mitigate post-operative declines in kidney function. This population of patients needs further evaluation in larger multi-center cohorts with longer follow-up duration to better determine the long-term risk of kidney sequelae.
Biomarkers are an important emerging area of research and may allow for earlier detection of both acute and chronic kidney problems. However, standardized methods for measuring biomarkers and validated CKD definitions showing biomarker level associations with gold standard GFR measurements in children are needed before they can be widely used. At this time, proteinuria is the urinary marker used to identify early kidney disease. Disappointingly, rates of proteinuria are not reported uniformly in studies evaluating long-term CKD. However, this is important information as monitoring albumin-to-creatinine ratio or protein-to-creatinine ratio in a child with previous AKI is a simple investigation, less invasive than serial serum creatinine measurements, and may detect early kidney disease in this population.
All the studies that evaluated the long-term risk of kidney outcomes report composite measures, which include some combination of hypertension, proteinuria, albuminuria, and reduced GFR. Although composite measures have been used in the literature, moving forward it is important to report kidney outcomes more systematically, categorizing by presence (yes/no) and where applicable, by level for proteinuria, albuminuria, hypertension, CKD, and reduced eGFR. Detailed reporting is likely to improve our understanding of kidney outcomes, as often CKD is used as a “blanket term.” Before these recent publications, there was little data on the prevalence of kidney outcomes in the pediatric AKI population, and these diagnoses are relatively uncommon in the general pediatric population. Therefore, early studies likely used composite measures to first assess if there was an association between AKI and long-term kidney outcomes. With the data showing increased prevalence of kidney outcomes in this population, future studies should aim to report these data with improved granularity using consensus definitions.
With many medical systems transitioning to electronic medical records, we are beginning to see larger population-based studies emerge in the pediatric AKI literature (6). While prospective follow-up studies provide exceptional information, these are labor-intensive and costly, and the retention of patients can be a challenge. Using administrative data is a cost-effective method for examining long-term outcomes in a large population. A systematic review in adults has demonstrated that AKI is identified by administrative health data with low sensitivity, but high specificity; similar results were also obtained from a validation study for identifying AKI in the PICU (42, 43). Adult studies have used databases to evaluate the association between AKI and long-term CKD development (22). In the pediatric literature, studies have started exploring the use of administrative data; however, the algorithms used to define CKD and hypertension have not yet been validated (26, 28). A validation study of long-term kidney outcome definitions with administrative data would allow for much larger population based studies with longer follow-up times. Validation studies in adults have demonstrated low sensitivity of these diagnostic code algorithms which is a major limitation (44, 45). These administrative data definitions seem to identify the more severe disease and therefore are likely to underestimate the prevalence of outcomes. It is important to identify the population of patients with early CKD or mild proteinuria, as early intervention can slow the progression of the disease. In the future, as more electronic medical record databases become available, combining what we have learned from administrative database studies with patient measurements will allow for larger population studies with more sensitive outcome measurements.
Recovery from AKI has also been an important variable to monitor in this population and has been associated with long-term CKD development in children (46). Unfortunately, in retrospective studies, this can be difficult to assess as many patients with AKI do not have repeat creatinine measurements (47). At this time there is no standardized definition for kidney function recovery and therefore studies evaluating AKI recovery with outcomes can be difficult to compare and interpret. Further research is needed to examine the association between incomplete recovery after AKI and long-term outcomes. Various definitions should be evaluated in these studies to better determine what level of incomplete recovery puts children at higher risk. This information would be invaluable for long-term follow-up guideline development and risk stratification.
As our detection and treatment of various illnesses in the PICU improves, so does PICU survival; however, this may also be associated with greater long-term morbidity (48). At this time, it remains unclear whether the association between AKI and long-term mortality and healthcare utilization is related to the direct effect of kidney damage or if AKI is a marker of medical complexity. This also means further exploring the impact AKI has on long-term health-related quality of life (HRQoL). One study in children with sepsis in the PICU showed that children with severe AKI (stage 2 or 3 by KDIGO) had poorer HRQoL at 3-months than children with no AKI or stage 1 AKI (49). Future research is needed to further assess the relationship between AKI and long-term markers of morbidity. These studies should also evaluate other clinical factors that may contribute, to allow for more targeted risk stratification models and follow-up guidelines.
There are many important outcomes that have been explored in the adult AKI literature that remain as gaps in knowledge in the pediatric population. Specifically, there are validated administrative data definitions for kidney outcomes, more evidence on recovery after AKI as well as recurrent AKI events, and measures of quality of life (44, 45, 50, 51). Adult studies have also shown that AKI increases direct and indirect healthcare costs and resource utilization (50). These outcomes remain as gaps in our current pediatric AKI research and warrant further attention. The use of standardized AKI definitions and non-AKI comparison groups should also be continued in all future pediatric AKI research.
Overall, it is clear from the literature that children with PICU associated AKI have an increased risk of adverse medium and long-term outcomes including mortality. Future challenges include standardizing our approach to the recognition and management of these patients beyond the critical care and nephrology healthcare professionals. It is important therefore to have the diagnosis of AKI documented when the patient is discharged from the PICU and subsequently from the hospital so that both non-critical care professionals who are hospital-based and those outside the hospital know to monitor these children more closely for resolution of AKI and for long-term sequelae. This would also allow providers to make more educated decisions about future treatments, including avoiding nephrotoxic medications where possible and providing lifestyle modification advice. To date, outlines of possible follow-up guidelines have been suggested but no formal guidelines exist (52, 53). Adult AKI guidelines suggest assessing the resolution of AKI 3 months after the initial insult (39). As highlighted in this review, children who develop severe AKI in the PICU are at increased risk of adverse kidney and non-kidney outcomes, so similar follow-up recommendations are required.
The 22nd Acute Disease Quality Initiative (ADQI) conference recently published quality improvement recommendations for pediatric AKI (46). This consensus statement recommends kidney health assessment every 1–2 years, which includes an AKI history, blood pressure measurement, serum creatinine measurement, and drug list review, in high-risk populations which include patients admitted to the PICU. They also recommended kidney health assessment following an unplanned acute exposure such as PICU admission (46). These recommendations need evaluation for implementation in different health settings but are a welcome expert consensus statement that is likely to mitigate adverse outcomes in this high-risk population. One of the challenges of developing AKI guidelines in this population remains that all children with AKI cannot be followed by nephrologists alone both because of large numbers of patients (6) but also because of the increasing burden of healthcare visits on children and families. Therefore, a coordinated effort must be made between general pediatricians and practitioners that follow these children in the community and the discharging hospitalist or specialists. While future research will continue to help us better understand additional risk factors for long-term outcomes and develop better risk stratification models, it is clear that children with PICU-AKI require closer follow-up. Early CKD and hypertension are treatable and timely detection and intervention will improve outcomes in these children.
Author Contributions
EH conceptualized the review, performed the literature review, and drafted and edited the manuscript. NM conceptualized the review, assisted with the literature review, drafted and edited the manuscript, and provided expert knowledge. MS conceptualized the review, assisted with the literature review, edited the manuscript, and provided expert knowledge. RA assisted with the literature review, edited the manuscript, and provided expert knowledge. EU, CM, and SB provided expert knowledge and edited the manuscript. All authors agreed to the final submitted manuscript.
Funding
MS acknowledges financial support from the Department of Health via the National Institute for Health Research (NIHR) comprehensive Biomedical Research Center and Clinical Research Facilities awards to Guy's and St Thomas' NHS Foundation Trust in partnership with King's College London and King's College Hospital NHS Foundation Trust.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Greenberg JH, Zappitelli M, Devarajan P, Thiessen-Philbrook HR, Krawczeski C, Li S, et al. Kidney outcomes 5 years after pediatric cardiac surgery: the TRIBE-AKI study. JAMA Pediatr. (2016) 170:1071–8. doi: 10.1001/jamapediatrics.2016.1532
2. Hollander SA, Montez-Rath ME, Axelrod DM, Krawczeski CD, May LJ, Maeda K, et al. Recovery from acute kidney injury and CKD following heart transplantation in children, adolescents, and young adults: a retrospective cohort study. Am J Kidney Dis. (2016) 68:212–8. doi: 10.1053/j.ajkd.2016.01.024
3. Kaddourah A, Basu RK, Bagshaw SM, Goldstein SL. Epidemiology of acute kidney injury in critically ill children and young adults. N Engl J Med. (2017) 376:11–20. doi: 10.1056/NEJMoa1611391
4. Madsen NL, Goldstein SL, Froslev T, Christiansen CF, Olsen M. Cardiac surgery in patients with congenital heart disease is associated with acute kidney injury and the risk of chronic kidney disease. Kidney Int. (2017) 92:751–6. doi: 10.1016/j.kint.2017.02.021
5. Hessey E, Morissette G, Lacroix J, Perreault S, Samuel S, Dorais M, et al. Healthcare utilization after acute kidney injury in the pediatric intensive care unit. Clin J Am Soc Nephrol. (2018) 13:685–92. doi: 10.2215/CJN.09350817
6. Alobaidi R, Morgan C, Goldstein SL, Bagshaw SM. Population-based epidemiology and outcomes of acute kidney injury in critically ill children. PediatrCrit Car Med. (2020) 21:82–91. doi: 10.1097/PCC.0000000000002128
7. Schneider J, Khemani R, Grushkin C, Bart R. Serum creatinine as stratified in the RIFLE score for acute kidney injury is associated with mortality and length of stay for children in the pediatric intensive care unit. Crit Care Med. (2010) 38:933–9. doi: 10.1097/CCM.0b013e3181cd12e1
8. Alkandari O, Eddington KA, Hyder A, Gauvin F, Ducruet T, Gottesman R, et al. Acute kidney injury is an independent risk factor for pediatric intensive care unit mortality, longer length of stay and prolonged mechanical ventilation in critically ill children: a two-center retrospective cohort study. Crit Care. (2011) 15:R146. doi: 10.1186/cc10269
9. Li S, Krawczeski CD, Zappitelli M, Devarajan P, Thiessen-Philbrook H, Coca SG, et al. Incidence, risk factors, and outcomes of acute kidney injury after pediatric cardiac surgery: a prospective multicenter study. Crit Care Med. (2011) 39:1493–9. doi: 10.1097/CCM.0b013e31821201d3
10. Sutherland SM, Byrnes JJ, Kothari M, Longhurst CA, Dutta S, Garcia P, et al. AKI in hospitalized children: comparing the pRIFLE, AKIN, and KDIGO definitions. Clin J Am Soc Nephrol. (2015) 10:554–61. doi: 10.2215/CJN.01900214
11. Mammen C, Al Abbas A, Skippen P, Nadel H, Levine D, Collet JP, et al. Long-term risk of CKD in children surviving episodes of acute kidney injury in the intensive care unit: a prospective cohort study. Am J Kidney Dis. (2012) 59:523–30. doi: 10.1053/j.ajkd.2011.10.048
12. Greenberg JH, Coca S, Parikh CR. Long-term risk of chronic kidney disease and mortality in children after acute kidney injury: a systematic review. BMC Nephrol. (2014) 15:184. doi: 10.1186/1471-2369-15-184
13. Askenazi DJ, Feig DI, Graham NM, Hui-Stickle S, Goldstein SL. 3-5 year longitudinal follow-up of pediatric patients after acute renal failure. Kidney Int. (2006) 69:184–9. doi: 10.1038/sj.ki.5000032
14. Sanchez-Pinto LN, Goldstein SL, Schneider JB, Khemani RG. Association between progression and improvement of acute kidney injury and mortality in critically ill children. Pediatr Crit Care Med. (2015) 16:703–10. doi: 10.1097/PCC.0000000000000461
15. Al-Otaibi NG, Zeinelabdin M, Shalaby MA, Khathlan N, Mashat GD, Zahrani AA, et al. Impact of acute kidney injury on long-term mortality and progression to chronic kidney disease among critically ill children. Saudi Med J. (2017) 38:138–42. doi: 10.15537/smj.2017.2.16012
16. Hirano D, Ito A, Yamada A, Kakegawa D, Miwa S, Umeda C, et al. Independent risk factors and 2-year outcomes of acute kidney injury after surgery for congenital heart disease. Am J Nephrol. (2017) 46:204–9. doi: 10.1159/000480358
17. Hessey E, Morissette G, Lacroix J, Perreault S, Samuel S, Dorais M, et al. Long-term mortality after acute kidney injury in the pediatric ICU. Hosp Pediatr. (2018) 8:260–8. doi: 10.1542/hpeds.2017-0215
18. Nunes S, Brown J, Parikh CR, Greenberg JH, Devarajan P, Philbrook HT, et al. The association of acute kidney injury with hospital readmission and death after pediatric cardiac surgery. JTCVS Open. (2020) 4:70–85. doi: 10.1016/j.xjon.2020.07.006
19. Zhang Y, Xiang B, Wu Y, Xie X, Wang J, Jin S. Risk factors and associated outcomes of early acute kidney injury in pediatric liver transplant recipients: a retrospective study. J Pediatr Surg. (2020) 55:446–50. doi: 10.1016/j.jpedsurg.2019.07.019
20. Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. (2007) 11:R31. doi: 10.1186/cc5713
21. Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. (2012) 81:442–8. doi: 10.1038/ki.2011.379
22. Heung M, Steffick DE, Zivin K, Gillespie BW, Banerjee T, Hsu CY, et al. Acute kidney injury recovery pattern and subsequent risk of CKD: an analysis of veterans health administration data. Am J Kidney Dis. (2016) 67:742–52. doi: 10.1053/j.ajkd.2015.10.019
23. Ferenbach DA, Bonventre JV. Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat Rev Nephrol. (2015) 11:264–76. doi: 10.1038/nrneph.2015.3
24. Cooper DS, Claes D, Goldstein SL, Bennett MR, Ma Q, Devarajan P, et al. Follow-up renal assessment of injury long-term after acute kidney injury (FRAIL-AKI). Clin J Am Soc Nephrol. (2016) 11:21–9. doi: 10.2215/CJN.04240415
25. Greenberg JH, Devarajan P, Thiessen-Philbrook HR, Krawczeski C, Parikh CR, Zappitelli M. Kidney injury biomarkers 5 years after AKI due to pediatric cardiac surgery. Pediatr Nephrol. (2018) 33:1069–77. doi: 10.1007/s00467-018-3888-4
26. Hessey E, Perreault S, Dorais M, Roy L, Zappitelli M. Acute kidney injury in critically ill children and subsequent chronic kidney disease. Can J Kidney Health Dis. (2019) 6:2054358119880188. doi: 10.1177/2054358119880188
27. Benisty K, Morgan C, Hessey E, Huynh L, Joffe AR, Garros D, et al. Kidney and blood pressure abnormalities 6 years after acute kidney injury in critically ill children: a prospective cohort study. Pediatr Res. (2020) 88:271–8. doi: 10.1038/s41390-019-0737-5
28. Hessey E, Perreault S, Roy L, Dorais M, Samuel S, Phan V, et al. Acute kidney injury in critically ill children and 5-year hypertension. Pediatr Nephrol. (2020) 35:1097–107. doi: 10.1007/s00467-020-04488-5
29. Huynh L, Rodriguez-Lopez S, Benisty K, Dancea A, Garros D, Hessey E, et al. Follow-up after neonatal heart disease repair: watch out for chronic kidney disease and hypertension! Pediatr Nephrol. (2020) 35:2137–45. doi: 10.1007/s00467-020-04621-4
30. Menon S, Pollack AH, Sullivan E, Murphy T, Smith J. Acute kidney injury and chronic kidney disease after non-kidney solid organ transplantation. Pediatr Transplant. (2020) 24:e13753. doi: 10.1111/petr.13753
31. Zappitelli M, Parikh CR, Kaufman JS, Go AS, Kimmel PL, Hsu CY, et al. Acute Kidney Injury and Risk of CKD and Hypertension after Pediatric Cardiac Surgery. Clin J Am Soc Nephrol. (2020) 18:18. doi: 10.2215/CJN.00150120
32. Monet-Didailler C, Godron-Dubrasquet A, Madden I, Delmas Y, Llanas B, Harambat J. Long-term outcome of diarrhea-associated hemolytic uremic syndrome is poorly related to markers of kidney injury at 1-year follow-up in a population-based cohort. Pediatr Nephrol. (2019) 34:657–62. doi: 10.1007/s00467-018-4131-z
33. Zappitelli M, Greenberg JH, Coca SG, Krawczeski CD, Li S, Thiessen-Philbrook HR, et al. Association of definition of acute kidney injury by cystatin C rise with biomarkers and clinical outcomes in children undergoing cardiac surgery. JAMA Pediatr. (2015) 169:583–91. doi: 10.1001/jamapediatrics.2015.54
34. Palermo J, Dart AB, De Mello A, Devarajan P, Gottesman R, Garcia Guerra G, et al. Biomarkers for early acute kidney injury diagnosis and severity prediction: a pilot multicenter canadian study of children admitted to the ICU. Pediatr Crit Care Med. (2017) 18:e235–44. doi: 10.1097/PCC.0000000000001183
35. Hoste E, Bihorac A, Al-Khafaji A, Ortega LM, Ostermann M, Haase M, et al. Identification and validation of biomarkers of persistent acute kidney injury: the RUBY study. Intensive Care Med. (2020) 46:943–53. doi: 10.1007/s00134-019-05919-0
36. Stanski NL, Stenson EK, Cvijanovich NZ, Weiss SL, Fitzgerald JC, Bigham MT, et al. PERSEVERE biomarkers predict severe acute kidney injury and renal recovery in pediatric septic shock. Am J Respir Crit Care Med. (2020) 201:848–55. doi: 10.1164/rccm.201911-2187OC
37. Yoneyama F, Okamura T, Takigiku K, Yasukouchi S. Novel urinary biomarkers for acute kidney injury and prediction of clinical outcomes after pediatric cardiac surgery. Pediatr Cardiol. (2020) 41:695–702. doi: 10.1007/s00246-019-02280-3
38. Greenberg JH, Abraham AG, Xu Y, Schelling JR, Feldman HI, Sabbisetti VS, et al. Plasma biomarkers of tubular injury and inflammation are associated with ckd progression in children. J Am Soc Nephrol. (2020) 31:1067–77. doi: 10.1681/ASN.2019070723
39. Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. (2012) 120:c179–84. doi: 10.1159/000339789
40. Koulouridis I, Price LL, Madias NE, Jaber BL. Hospital-acquired acute kidney injury and hospital readmission: a cohort study. Am J Kidney Dis. (2014) 65:275–82. doi: 10.1053/j.ajkd.2014.08.024
41. Brown JR, Hisey WM, Marshall EJ, Likosky DS, Nichols EL, Everett AD, et al. Acute kidney injury severity and long-term readmission and mortality after cardiac surgery. Ann Thorac Surg. (2016) 102:1482–9. doi: 10.1016/j.athoracsur.2016.04.020
42. Vlasschaert ME, Bejaimal SA, Hackam DG, Quinn R, Cuerden MS, Oliver MJ, et al. Validity of administrative database coding for kidney disease: a systematic review. Am J Kidney Dis. (2011) 57:29–43. doi: 10.1053/j.ajkd.2010.08.031
43. D'arienzo D, Hessey E, Ali R, Perreault S, Samuel S, Roy L, et al. A validation study of administrative health care data to detect acute kidney injury in the pediatric intensive care unit. Can J Kidney Health Dis. (2019) 6:2054358119827525. doi: 10.1177/2054358119827525
44. Ronksley PE, Tonelli M, Quan H, Manns BJ, James MT, Clement FM, et al. Validating a case definition for chronic kidney disease using administrative data. Nephrol Dial Transplant. (2012) 27:1826–31. doi: 10.1093/ndt/gfr598
45. Fleet JL, Dixon SN, Shariff SZ, Quinn RR, Nash DM, Harel Z, et al. Detecting chronic kidney disease in population-based administrative databases using an algorithm of hospital encounter and physician claim codes. BMC Nephrol. (2013) 14:81. doi: 10.1186/1471-2369-14-81
46. Selewski DT, Askenazi DJ, Kashani K, Basu RK, Gist KM, Harer MW, et al. Quality improvement goals for pediatric acute kidney injury: pediatric applications of the 22nd acute disease quality initiative (ADQI) conference. Pediatr Nephrol. (2021) 36:733–46. doi: 10.1007/s00467-020-04828-5
47. Hessey E, Ali R, Dorais M, Morissette G, Pizzi M, Rink N, et al. Renal function follow-up and renal recovery after acute kidney injury in critically ill children. Pediatr Crit Care Med. (2017) 18:733–40. doi: 10.1097/PCC.0000000000001166
48. Heneghan JA, Pollack MM. Morbidity: changing the outcome paradigm for pediatric critical care. Pediatr Clin North Am. (2017) 64:1147–65. doi: 10.1016/j.pcl.2017.06.011
49. Starr MC, Banks R, Reeder RW, Fitzgerald JC, Pollack MM, Meert KL, et al. Severe acute kidney injury is associated with increased risk of death and new morbidity after pediatric septic shock. Pediatr Crit Care Med. (2020) 21:e686–95. doi: 10.1097/PCC.0000000000002418
50. Rewa O, Bagshaw SM. Acute kidney injury-epidemiology, outcomes and economics. Nat Rev Nephrol. (2014) 10:193–207. doi: 10.1038/nrneph.2013.282
51. James MT, Bhatt M, Pannu N, Tonelli M. Long-term outcomes of acute kidney injury and strategies for improved care. Nat Rev Nephrol. (2020) 16:193–205. doi: 10.1038/s41581-019-0247-z
52. Sigurjonsdottir VK, Chaturvedi S, Mammen C, Sutherland SM. Pediatric acute kidney injury and the subsequent risk for chronic kidney disease: is there cause for alarm? Pediatr Nephrol. (2018) 33:2047–55. doi: 10.1007/s00467-017-3870-6
Keywords: acute kideny injury, chronic kidney disease, long-term follow up, hypertension, healthcare utilization, mortality, critical care
Citation: Hessey E, Melhem N, Alobaidi R, Ulrich E, Morgan C, Bagshaw SM and Sinha MD (2021) Acute Kidney Injury in Critically Ill Children Is Not all Acute: Lessons Over the Last 5 Years. Front. Pediatr. 9:648587. doi: 10.3389/fped.2021.648587
Received: 31 December 2020; Accepted: 22 February 2021;
Published: 15 March 2021.
Edited by:
Katja Michelle Gist, Children's Hospital Colorado, United StatesReviewed by:
Gerard Cortina, Innsbruck Medical University, AustriaAftab S. Chishti, University of Kentucky, United States
Scott Sutherland, Lucile Packard Children's Hospital, United States
Copyright © 2021 Hessey, Melhem, Alobaidi, Ulrich, Morgan, Bagshaw and Sinha. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Erin Hessey, ZXJpbi5oZXNzZXlAZ21haWwuY29t
 Erin Hessey
Erin Hessey Nabil Melhem2
Nabil Melhem2 Manish D. Sinha
Manish D. Sinha