- 1Department of Pediatric Nephrology and Rheumatism and Immunology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, China
- 2Department of Pediatric Nephrology and Rheumatism and Immunology, Shandong Provincial Hospital Affiliated to Shandong University, Jinan, China
Objective: The aim of this study was to explore the clinical features, pathological characteristics, and the prognosis of children with microscopic polyangiitis (MPA).
Methods: Ten children with MPA that were hospitalized in our hospital were included in this study. The children's pre-diagnosis status, clinical manifestations, renal pathology, treatment, and prognosis data were analyzed retrospectively.
Results: All 10 cases included female patients with a median age of 8.9 years old at the time of diagnosis. MPO-ANCA antibody was positive in all cases, combined with a positive anti-GBM antibody in two cases. Nine cases had primary AAV and one had antithyroid drug (ATD)-associated MPA (secondary to methimazole). Renal involvement was found in all 10 patients, lung impairment was present in eight cases, and anemia was present in nine patients. Renal biopsies were performed in all 10 patients. Segmental focal or global glomerular necrosis was observed in 70% of the patients (7/10). The treatment mainly included steroid use combined with Cyclophosphamide and Mycophenolate. The follow-up s of the patients revealed normal renal function in eight patients and progression to end-stage renal disease (ESRD) in two patients.
Conclusions: Female predisposition and positive MPO-ANCA antibody were prominent in children with MPA. The patients' kidneys and lungs were the most frequently involved organs. Corticosteroid combined with immunosuppressive therapy was recommended for the treatment of MPA. Early diagnosis, prompt aggressive treatment, and regular follow-ups are also very important factors associated with a good prognosis.
Introduction
Microscopic polyangiitis (MPA) belongs to the antineutrophil cytoplasmic antibody (ANCA) associated vasculitis (AAV). AAV is the necrotizing inflammation of the small and medium vessels and is characterized by anti-neutrophil cytoplasm autoantibody related to a group of diseases. It is also a kind of systemic vasculitis involved with the small vein, mall artery, and blood capillary. MPA typically exhibits multiple organ involvement including the patients' kidneys and lungs, which are the most commonly affected organs. MPA is also considered to occur mostly in adults and rarely in children. Previous reports have shown that AAV disease types are significantly different in different regions and races; GPA was mainly seen in Nordic and British Caucasian AAV patients. However, in southern Europe, Japan, and China, MPA is dominant. This is especially found in China where MPA patients account for the majority (80%) (1). Contrary to adults, female children with AAV have a higher incidence rate than males and their clinical manifestations and treatment programs are similar to adults, but the long-term prognosis is better than in adults (2). Children with MPA are also more likely to be females and the prevalence reaches a peak in early puberty (3, 4). In 2016, it was described in the EMA (the European Medicines Agency) standard of the AR Chi Ve investigation network that among the largest pediatric MPA cohort to date (48 patients), females accounted for 73% of the patients and had an average age of 10.8 years old (5). From 2003 to 2013, the first affiliated hospital of Sun Yat-Sen University diagnosed 20 children with MPA without GPA or EGPA, with a male to female ratio of 4:12 and an average age of 8.9 years old (6).
In this study, the clinical characteristics and pathological features of pediatric patients with MPA were retrospectively analyzed in order to explore the clinical features, pathological characteristics, and the prognosis of children with MPA.
Materials and Methods
The Selection of Patients
Ten female pediatric patients with MPA, aged ≤ 14 years and diagnosed in Shandong Provincial Hospital Affiliated to Shandong First Medical University between January 2000 and December 2018 were enrolled in this study. Their clinical and pathology data were retrospectively analyzed. Patients with MPA who had received the Antithyroid Drug (ATD) were defined as ATD-associated MPA and the other patients were defined as primary MPA (7). The diagnosis of primary MPA was based on the 2012 revised Chapel Hill Conference Nomenclature of Vasculitides (8). All patients were treated according to the same protocol.
The current study was approved by the ethical committee of our hospital and all patients' legal guardians signed written informed consent.
The General Data of the Patients
Renal pathology was performed in all 10 cases and repeated renal biopsy was performed in one case after 2-year treatment.
Disease activity was scored according to the Birmingham Vasculitis Activity Score (BVAS).
The ANCA Analysis
Serum samples of the patients were screened for ANCA by antigen-specific enzyme-linked immunosorbent assay (ELISA) for PR3 or MPO. Serum samples of the patients were screened for PR3-ANCA, MPO-ANCA, anti-GBM and anti-dsDNA by antigen-specific enzyme-linked immunosorbent assay (ELISA). Serum ANA levels were tested by Indirect Immunofluorescence Assay. Serum levels of CRP, C3 and C4 were measured by immune turbidimetry. The changes of erythrocyte sedimentation rate (ESR) were determined by Westergren method.
Renal Pathology
A renal biopsy was performed on all 10 patients. The renal biopsy specimens were examined by both immunofluorescence and light microscopy and electron microscopy. The immune complex deposition was defined as a score of 0 to 4+ in staining for any kind of immunoglobulins (Igs) observed by immunofluorescence microscopy and/or electron-dense deposits observed by electron microscopy. All patients biopsied after the same amount of disease duration.
Treatment Protocols
The induction therapy consisted of Prednisone (1–2 mg/kg per day) plus Cyclophosphamide (CTX, 8–12 mg/kg per day, 2 days, once every 2 weeks, the cumulative dosage <150mg/kg). Patients with severe necrotizing crescentic glomerular nephritis and pulmonary hemorrhage were also treated with Methylprednisone (MP, 7.5–15 mg/kg per day, 3 days) pulse therapy. For maintenance therapy, low doses of Prednisone combined with immunosuppressive drugs such as CTX and Mycophenolate Mofetil (MMF) were administered.
Treatment Response
Complete remission (CR) is defined as (9): (1) The normalization of renal function if renal insufficiency is present; and (2) The disappearance of hematuria, proteinuria, and extra renal manifestations of systemic vasculitis.
Partial remission (PR) is defined as (9): (1) The stabilization or improvement of renal function if renal insufficiency is present; (2) Dialysis independent if renal failure is present; and (3) The resolution of hematuria and proteinuria and/or the resolution of extra renal manifestations of systemic vasculitis.
Treatment failure is defined as (9): (1) The progressive decline in renal function with persistent active urine sediments or; (2) Persistence or the new appearance of any extra-renal manifestations of vasculitis or; (3) Death.
Results
The Clinical and Renal Pathological Features
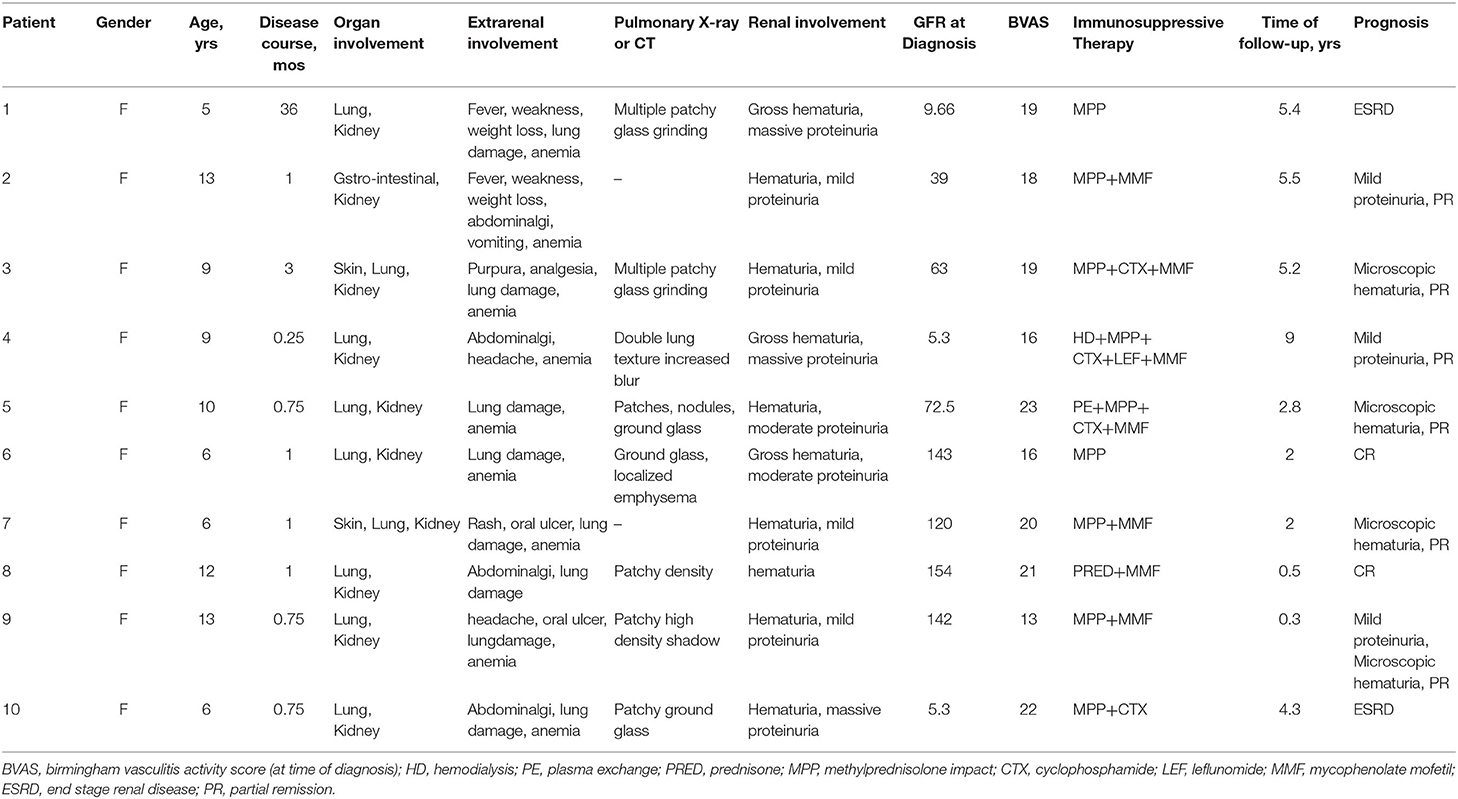
All 10 patients were female and were diagnosed at the age of 5–13 years old, with a median age of 8.9 years old. As shown in Table 1, all 10 patients had extra renal involvement manifestations: 90% of the patients (9/10) had moderate to severe anemia. Eighty percentage of the patients (8/10) had respiratory tract symptoms, such as cough or hemoptysis, abnormal pulmonary X-ray or CT examination, presenting a patchy, nodular grainy image, localized emphysema or increased and blurred lung texture. A rash that was presented as purpura was found in 20% of the patients (2/10). Renal involvement with hematuria and proteinuria was observed in all 10 children. Gross hematuria was seen in 30% of the patients (3/10). Seventy percentage of the patients (7/10) showed mild to moderate proteinuria and 30% of the patients (3/10) showed nephrotic proteinuria. Impaired renal function was seen in 60% of the patients (6/10). Only one patient had Hashimoto's thyroiditis. Other patients have no other diseases. None of them have a family history of autoimmune diseases. Nine of the 10 cases were diagnosed as primary AAV. The other 1 case included a history of hyperthyroidism and received a Methiomidazole administration and was diagnosed as ATD-associated MPA (case 9). The time from onset (time provided by the chief complaint) to diagnosis ranged from 1 week to 3 years, with an average of 4.5 months (Table 1).
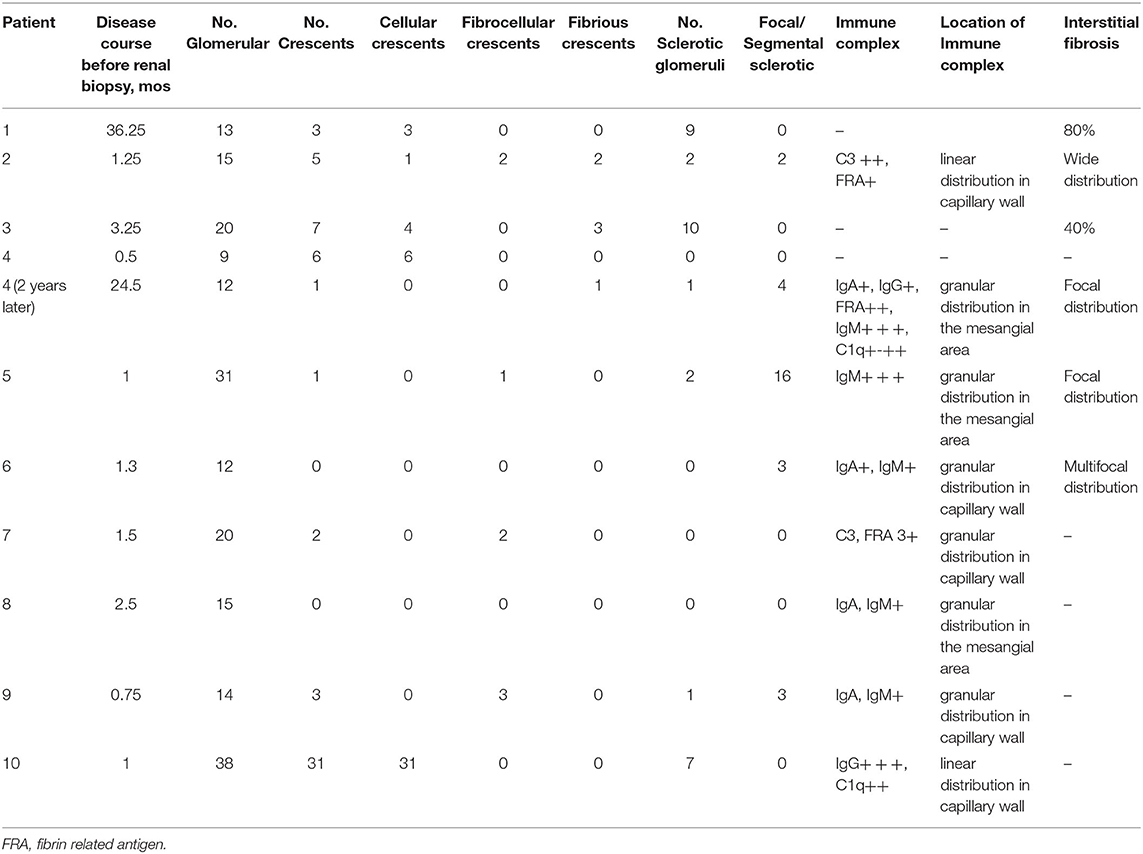
As shown in Table 2, renal biopsies were obtained in all 10 patients. Case 4 underwent a second renal biopsy after 2 years of the drug administration. The cellular or fibrous crescents were shown in the renal specimens of 80% of the patients (8/10). Segmental focal or global glomerular necrosis was observed in 70% of the patients (7/10). Extensive or focal renal interstitial fibrosis was found in 60% of the patients (6/10). Immunofluorescence staining of Igg, IgA, IGM, C3, C1q, and FRA was performed in all 10 children with renal biopsy; no IC deposits were observed in 30% of the patients (3/10), a small amount of deposition was seen in 40% of the patients (4/10), and granular or linear distribution in the mesangial area or capillary wall was observed in 70% of the patients (7/10).
ANCA and Other Laboratory Results
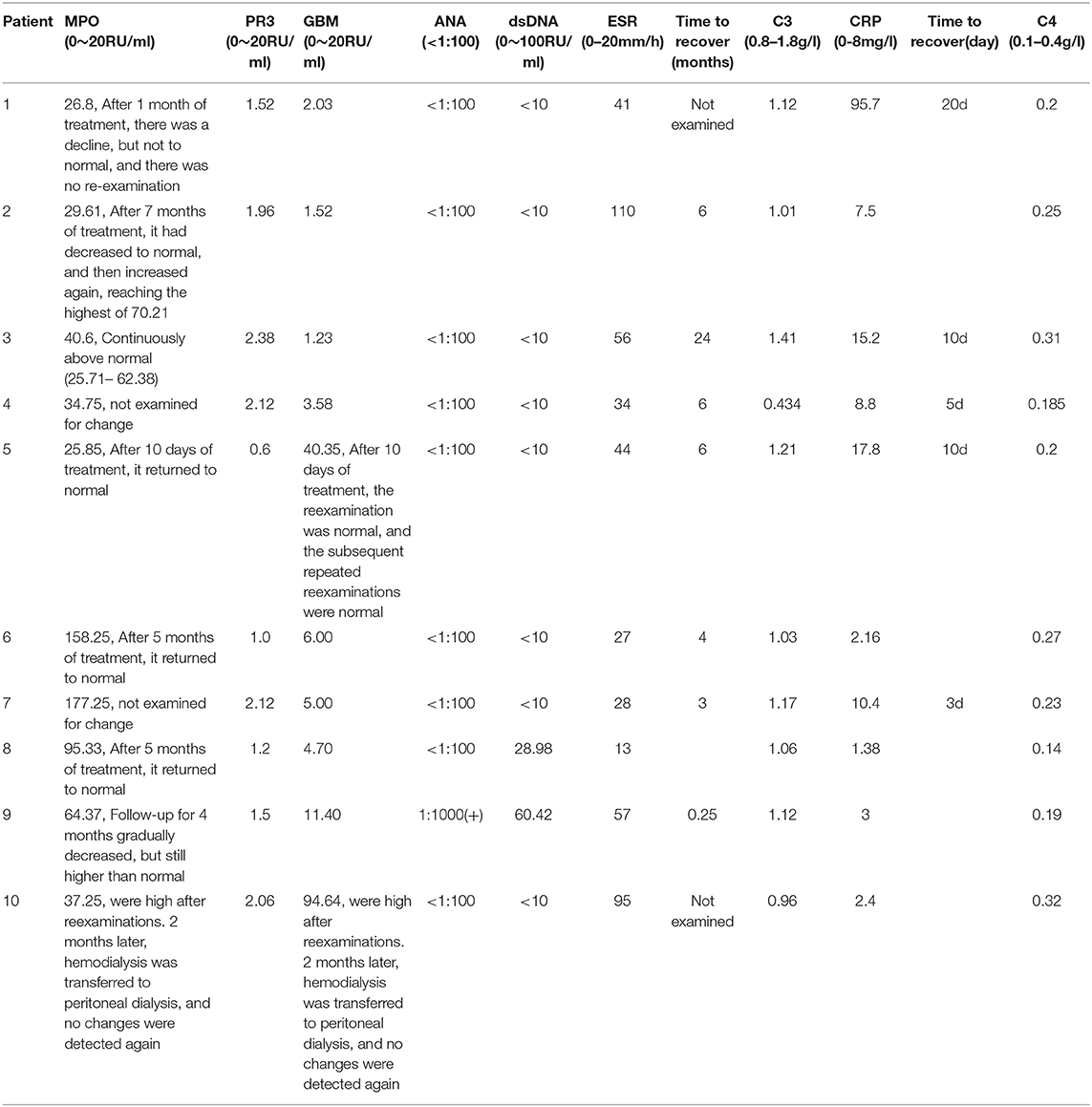
As shown in Table 3, positive MPO-ANCA and negative PR3-ANCA were observed in 100% of the patients (10/10). Twenty percentage of the patients (2/10) had a positive GBM antibody. ANA was tested positive and DSDNA was negative in 1 child with secondary MPA. Fifty percentage of the patients showed increased CRP and 90% showed elevated ESR levels. Decreased C3 levels were found in 10% of the cases and no abnormal serum C4 levels were seen in all 10 patients. Serum CRP levels were elevated in 5 children with AAV, which were reduced to normal level within 20 days after steroid therapy. ESR values were increased in 9 children with AAV, which recover to normal level within 0.25–24 months after steroid combined with immunosuppressive drugs therapy in seven patients. Decreased C3 was found in patient 4. She was given the combination therapy of hemodialysis, methylprednisone pulse and CTX therapy. Serum C3 level returned to normal 6 months later.
Treatment and Prognosis
All of the 10 children were given immunosuppression and symptomatic supportive treatment. 90% of the patients (9/10) received Methylprednisolone pulse therapy, 40% of the patients (4/10) received Cyclophosphamide pulse treatment, 70% (7/10) received MMF treatment, and 10% (1/10) received leflunomide treatment. Hemodialysis and plasmapheresis were performed on the basis of immunosuppressive therapy (Table 1). The follow-up times of the 10 patients ranged from 4 months to 9 years. Two children presented as treatment failure and progressed to ESRD. Eighty percentage of the patients (8/10) maintained their clinical remission. Among them, two patients had complete remission and 6 had partial remission. At present, 1 of the 8 children with clinical remission had stopped taking corticosteroids and immunosuppressive therapy, three had stopped taking corticosteroids therapy and only take oral mycophenolate ester therapy, and three had oral corticosteroids and mycophenolate ester maintenance therapy. The patients' urine test results were normal in two patients with microscopic hematuria in three patients, mild proteinuria in two patients, and microscopic hematuria and mild proteinuria in two patient (Table 1).
Discussion
MPA is rare in childhood and its pathogenesis is not fully understood. ANCA can be detected in the serum of most MPA patients, most of which are positive for MPO/PANCA, but a few patients can be positive for PR3/CANCA (10, 11). The pathogenesis of AAVs involves a complex interplay network of helper T (TH; TH-17) cells, antigen-presenting cells (APCs), and various cytokines like IL-23, IL-1β, TNFα, myeloperoxidase (MPO), and proteinase 3 (PR3), as well as the complement system (12, 13). A variety of environmental factors such as infection and drugs are also believed to be related to ANCA production and vasculitis, among which the reported anti-thyroid drugs mainly include Tabazole, Propylthiazine, and Methimazole (14). In this study, 10 cases of children with MPA tested positive for the serum MPO antibody and 2 cases tested positive for the anti-GBM antibody. Nine patients were diagnosed as primary AAV and 1 13-year-old patient was diagnosed with Hashimoto thyroiditis with hyperthyroidism for 4 years. The long-term oral administration of Methimazole was considered as secondary MPA. ANA was negative in all patients with primary MPA but positive in the girl with ATD-associated MPA. One patient had a low C3 serum level,9 patients had an increased ESR, and elevated CRP was found in 5 patients. However, the relationships between disease activity and serum C3, ESR, CRP levels were not observed in this study.
MPA usually affects multiple organs. The lungs are the most frequently involved extra renal organ. MPA is the most common cause of pneumohemorrhagic nephritis syndrome. The common manifestations of lung damage include diffuse alveolar hemorrhage, dyspnea, hemoptysis, cough, chest pain and imaging features are mainly ground glass and patchy shadows (15). In this study, 8 of the 10 MPA patients (80%) had respiratory symptoms, such as cough or hemoptysis and abnormal lung X-ray or CT examination, which manifested as a patchy, nodular grind glass shadow, localized emphysema or increased and blurred lung texture. The kidneys are also most commonly affected in MPA, and may also be the only affected organ. In the AR Chi Ve survey network study, according to the EMA criteria, renal involvement, including proteinuria, microscopic hematuria, and renal insufficiency were present in 3/4 of the patients and serum creatinine levels were moderately to severely elevated in 48% of the children with renal involvement. 1/3 of the children developed hypertension and 1/4 of the children needed renal replacement therapy treatment at the onset (16). In this study, all 10 children had renal impairment, hematuria, and proteinuria, including three cases of gross hematuria, three cases of nephrotic proteinuria, six cases with renal impairment at diagnosis, and two cases of hemodialysis or plasmapheresis.
The pathological features of the kidneys in MPA patients were glomerular capillary segmental fibrinoid necrosis with a crescent formation. Different stages of the glomerular disease were also found in one patient (16). Currently, there are no guidelines for the treatment of MPA or AAV in children and the clinical treatment scheme mainly refers to the recommendations of adult AAV. The 2016 AAV treatment recommendation issued by the European Federation of Rheumatology (EULAR) and the European Dialysis and Transplantation Association (EDTA) stated that glucocorticoid (GC) (including Methylprednisolone pulse) combined with Cyclophosphamide or Rituximab is recommended for AAV-induced remission therapy that threatens organ function and life. For AAV induction and remission therapy without organ function involvement, GC combined with Methotrexate or Mycophenate is recommended (17). In 2017, the expert consensus of the children's blood purification committee of the pediatrician branch of the Chinese Medical Doctor Association stated that plasma exchange can be used in AAV children (18). Early diagnosis and early treatment are key factors for patients with MPA. Although most of the patients responded to induction therapy, some patients progressed to CKD stages.
In our study, the follow-up time of the 10 cases was 4 months to 9 years; 8 cases had a normal renal function and two cases progressed to ESRD.As long as AAV was diagnosed, early and intensive immunosuppressive therapy was used. Our remission rate was 80% and was similar to previous studies (3). At present, one of the 8 children with normal renal function had stopped taking prednisone and immunosuppressive therapy; three patients had stopped taking corticosteroids therapy and received Mycophenolate, and; three patients received oral corticosteroids therapy and Mycophenolate in the maintenance phase. The patients' urine test results were normal in two patients, microscopic hematuria was found in three patients, mild proteinuria in two patients, and microscopic hematuria and mild proteinuria in one patient. Therefore, regular and long-term follow-ups are needed for evaluating the prognosis of children with MPA. However, our limitation is that 4 months is still too short to assess the effect of therapy and disease prognosis. In addition, retrospective nature of the research and the wide range of follow up also limited our study because it is difficult to draw firm conclusions if one patient is followed for 4 months and the other for 9 years.
Conclusions
Female predisposition and positive MPO-ANCA antibody were prominent in children with MPA. The kidneys and lungs were the most frequently involved organs in MPA. Corticosteroid combined with immunosuppressive therapy was recommended for the treatment of the disease. Take-home messages are early diagnosis, prompt aggressive treatment, and regular follow-ups are very important for improving the patients' long-term outcomes.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by Shandong Provincial Hospital Affiliated to Shandong First Medical University. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author Contributions
QL and L-CY conceptualized and designed the study, drafted the initial manuscript, and reviewed and revised the manuscript. F-XL, JW, and YC designed the data collection instruments, collected data, carried out the initial analyses, and reviewed and revised the manuscript. S-ZS coordinated and supervised data collection, and critically reviewed the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
MPA, microscopic polyangiitis; ATD, antithyroid drug; ESRD, end-stage renal disease; ANCA, antineutrophil cytoplasmic antibody; AAV, antineutrophil cytoplasmic antibody associated vasculitis; EMA, European Medicines Agency; ELISA, enzyme-linked immunosorbent assay; CTX, Cyclophosphamide; MP, Methyl prednisone; MMF, Mycophenolate Mofetil; CR, Complete remission; PR, Partial remission; MPO, myeloperoxidase.
References
1. Arulkumaran N, Jawad S, Smith SW, Harper L, Brogan P, Pusey CD, et al. Long- term outcome of paediatric patients with ANCA vasculitis. Pediatr Rheumatol J. (2011) 9:12. doi: 10.1186/1546-0096-9-12
2. Calatroni M, Oliva E, Gianfreda D, Gregorini G, Allinovi M4, Ramirez GA5, et al. ANCA-associated vasculitis in childhood: recent advances. Ital J Pediatr. (2017) 43:46. doi: 10.1186/s13052-017-0364-x
3. Özçelik G, Sönmez HE, Sahin S, Özagari A, Bayram MT, Çiçek RY, et al. Clinical and histopathological prognostic factors affecting the renal outcomes in childhood ANCA-associated vasculitis. Pediatr Nephrol. (2019) 34:847–54. doi: 10.1007/s00467-018-4162-5
4. Plumb LA, Oni L, Marks SD, Tullus K. Paediatric anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis: an update on renal management. Pediatr Nephrol. (2018) 33:25–39. doi: 10.1007/s00467-016-3559-2
5. Cabral DA, Canter DL, Muscal E, Nanda K, Wahezi DM, Spalding SJ, et al. Comparing presenting clinical features in 48 children with microscopic polyangiitis to 183 children who have granulomatosis with polyangiitis (Wegener's): an AR Chi Ve cohort study. Arthritis Rheumatol. (2016) 68:2514–26. doi: 10.1002/art.39729
6. Barut K, Sahin S, Kasapcopur O. Pediatric vasculitis. Curr Opin Rheumatol. (2016) 28:29–38. doi: 10.1097/BOR.0000000000000236
7. Hasegawa J, Hoshino J, Sekine A, Hayami N, Suwabe T, Sumida K, et al. Clinical and histological features of antineutrophil cytoplasmic antibody-associated vasculitis related to antithyroid drugs. Clin Nephrol. (2018) 89:438–44. doi: 10.5414/CN109364
8. Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 revised international chapel hill consensus conference nomenclature of vasculitides. Arthritis Rheum. (2013) 65:1–11. doi: 10.1002/art.37715
9. Yu F, Huang JP, Zou WZ, Zhao MH. The clinical features of anti-neutrophil cytoplasmic antibody-associated systemic vasculitis in chinese children. Pediatr Nephrol. (2006) 21:497–502. doi: 10.1007/s00467-006-0028-3
10. Li ZY, Ma TT, Chen M, Zhao MH. The prevalence and management of antineutrophil cytoplasmic antibody-associated vasculitis in China. J Kidney Dis. (2016) 1:216–23. doi: 10.1159/000441912
11. Noone D, Hebert D, Licht C. Pathogenesis and treatment of ANCA-associated vasculitis: a role for complement. Pediatr Nephrol. (2018) 33:1–11. doi: 10.1007/s00467-016-3475-5
12. Lionaki S, BoIetis JN. The prevalence and management of pauci-immune glomerulonephritis and vasculitis in western countries. Kidney Dis. (2016) 1:224–34. doi: 10.1159/000442062
13. Sun LZ, Wang HY. Progress in diagnosis and treatment of pediatric microscopic polyangiitis. Clin J Appl Clin Pediatr. (2017) 32:333–7. doi: 10.3760/cma.j.issn.2095-428X.2017.05.004
14. Gao Y, Zhao MH. Drug-induced anti-neutrophil cytoplasmic antibody-associated vasculitis. Nephrology. (2009) 14:33–41. doi: 10.1111/j.1440-1797.2009.01100.x
15. Wang H, Sun L, Tan W. Clinical features of children with pulmonary microscopic polyangiitis: report of 9 cases. PLoS ONE. (2014) 10:e0124352. doi: 10.1371/journal.pone.0124352
16. Peco-Antic A, Bonaci-Nikolic B, Basta-Jovanovic G, Kostic M, Markovic-Lipkovski J, Nikolic M, et al. Childhood microscopic polyangiitis associated with MPO-ANCA. Pediatr Nephrol. (2006) 21:46–53. doi: 10.1007/s00467-005-2063-x
17. Yates M, Watts RA, Bajema IM, Cid MC, Crestani B, Hauser T, et al. EULAR/ERA-EDTA recommendations for the management of ANCA-associated vasculitis. Ann Rheum Dis. (2016) 75:1583–94. doi: 10.1136/annrheumdis-2016-209133
Keywords: children, microscopic polyangiitis, clinical pathology, female, prognosis
Citation: Li Q, Yu L-C, Li F-X, Wang J, Chen Y and Sun S-Z (2021) The Clinical and Pathological Features of Children With Microscopic Polyangiitis. Front. Pediatr. 9:645785. doi: 10.3389/fped.2021.645785
Received: 26 January 2021; Accepted: 16 March 2021;
Published: 15 April 2021.
Edited by:
Jordi Anton, Hospital Sant Joan de Déu Barcelona, SpainReviewed by:
Marija Jelusic, University of Zagreb, CroatiaOzgur Kasapcopur, Istanbul University-Cerrahpasa, Turkey
Copyright © 2021 Li, Yu, Li, Wang, Chen and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shu-Zhen Sun, c2h1emhlbnN1bjI0QDE2My5jb20=
 Qian Li1,2
Qian Li1,2 Shu-Zhen Sun
Shu-Zhen Sun