- 1Department of Pediatric Cardiovasology, Children's Medical Center, The Second Xiangya Hospital, Central South University, Changsha, China
- 2Jishou University School of Medicine, Jishou, China
Objectives: This study aims to investigate the diurnal variability of heart rate (HR) increment after standing (ΔHR) in pediatric postural tachycardia syndrome (POTS) and explore appropriate cutoff values of ΔHR at different times for the POTS diagnosis.
Materials and Methods: Seventy-eight patients (9–14 years) who presented with orthostatic intolerance symptoms were enrolled. Forty-three patients were diagnosed as POTS (ΔHR ≥40 bpm), and 35 patients were assigned to the non-POTS group (ΔHR <40 bpm). Twenty-six healthy children served as the control group. All subjects completed three standing tests in the morning, afternoon, and evening. Orthostatic HR parameters were analyzed to predict the diagnosis of POTS. Additionally, 41 patients were recruited as an external validation group.
Results: Orthostatic HR increments in both the POTS and non-POTS groups exhibited diurnal variability, which was markedly larger in the morning (P < 0.05), whereas it did not differ with the time of day in the control group. Among the POTS patients, 100% met the diagnostic criteria for POTS in the morning, 44.2% in the afternoon, and 27.9% in the evening. Almost half of the POTS patients (51.2%) displayed a positive result only in the morning standing test. However, in the three standing tests at different times, ΔHR from 1 to 10 min after standing and ΔHRmax were the highest in the POTS group compared with in the non-POTS and control groups (P < 0.05). Furthermore, the maximum ΔHR (ΔHRmax) and ΔHR at 5 and 10 min in the afternoon and evening standing tests yielded moderate predictive values for the POTS diagnosis. The external validation test showed that the afternoon ΔHRmax ≥30 bpm to diagnose POTS yielded sensitivity, specificity, and accuracy of 85, 71.4, and 78%, respectively, and the evening ΔHRmax ≥25 bpm yielded sensitivity, specificity, and accuracy of 85, 76.2, and 80.5%, respectively.
Conclusions: The orthostatic HR increment exhibits diurnal variability in children and adolescents with POTS that may affect the diagnosis of POTS. Supplementary criteria are proposed for the POTS diagnosis based on diurnal variability.
Introduction
Postural tachycardia syndrome (POTS) is a common form of pediatric orthostatic intolerance (OI), characterized by an excessive heart rate (HR) increment after standing and chronic day-to-day OI symptoms, such as dizziness, headache, palpitations, chest discomfort, blurred vision, profuse perspiration, and even syncope (1). Autonomic dysfunction is the principal mechanism for POTS. Many POTS patients have high plasma norepinephrine levels and excessive sympathetic activation upon standing (2, 3). In humans, almost every physiological system has some degree of the circadian rhythm. Many cardiovascular parameters, such as HR, blood pressure (BP), plasma catecholamine concentrations, and sympathetic activity, show distinct circadian rhythm, increasing significantly during the day and decreasing during the night (4). Various cardiovascular events, such as arrhythmias, angina pain, myocardial infarction, stroke, sudden cardiac death, and syncope, exhibit diurnal patterns with morning peaks (5, 6). Similarly, the diurnal variability in POTS adults is observed with orthostatic HR increment shown larger in the morning than in the afternoon or evening (7, 8). Some patients who meet the criteria for POTS in the morning do not always meet the criteria in the afternoon or the evening.
However, there was a lack of studies at present on the diurnal variability of pediatric POTS. Cardiovascular function and the diurnal variability of cardiovascular function are affected by aging (9, 10). We hypothesized that diurnal variability of orthostatic HR increments also existed in children and adolescents with POTS. If so, the existing HR criteria for pediatric POTS diagnosis may not always be appropriate for the standing test or head-up tilt test (HUTT) at different times of the day.
The key diagnostic criterion for POTS in children and adolescents is an HR increment of ≥40 bpm within the initial 10 min of the standing test or HUTT in the absence of orthostatic hypotension (11), but these are not universally accepted. Boris et al. (12) reported that a 40-bpm threshold for children and adolescents on the standing test may be too high because of no significant difference of symptomatic burden between OI pediatric patients with HR increments of 30–39 and ≥40 bpm. We sought to ascertain whether there was a difference in diurnal variability of orthostatic HR increment between OI patients with HR increments of <40 and ≥40 bpm.
Therefore, in the present study, we aimed to investigate the diurnal variability of HR increment after standing in children and adolescents with POTS and explore appropriate cutoff values of HR increment at different times for the POTS diagnosis. We also intended to evaluate the difference in diurnal variability of HR increment between patients with OI symptoms meeting and not meeting the HR criterion of a 40-bpm threshold.
Participants and Methods
Participants
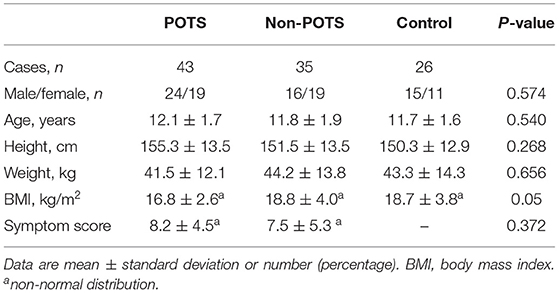
Seventy-eight patients (9–14 years) who presented with OI symptoms were admitted to the Children's Medical Center, The Second Xiangya Hospital, Central South University from 2019 to 2020. The main causes of admission were recurrent dizziness, headache, palpitations, chest discomfort, and syncope. All patients received both HUTT in the morning and standing tests at three different time points within 3 days. Among them, 43 patients who met the HR criterion of a 40-bpm threshold both on the HUTT and on at least one standing test were recruited for the ≥40 bpm group (POTS group), and the remaining 35 patients who did not meet the HR criterion of a 40-bpm threshold were recruited for the <40 bpm group (non-POTS group). Twenty-six healthy children were recruited as the control group who had no history of OI symptoms and normal findings in physical examination, 12-lead electrocardiogram (ECG), and standing test. No one in the control group met the HR criterion of POTS. Cardiovascular, neurological, metabolic, and autoimmune diseases and others (such as infections, arrhythmia, severe anemia, recent long-term bed rest, or deconditioning) that could cause OI symptoms were excluded by a standard basic evaluation that consisted of careful history, physical examination, routine laboratory tests (complete blood count, blood biochemistry, thyroid function, 25-hydroxyvitamin D, autoimmune antibodies), ECG, 24-h Holter ECG, 24-h ambulatory BP monitor, echocardiogram, electroencephalography, and cranium CT/MRI.
Another 41 patients with frequent OI symptoms who were admitted to our hospital in 2020 were recruited for the external validation study. Among them, 20 patients were diagnosed as POTS with an HR increment of ≥40 bpm, and 21 patients were classified as the non-POTS group based on clinical OI symptoms and the morning standing test. The research was approved by the Medical Ethical Committee, The Second Xiangya Hospital, Central South University. All parents or guardians of the subjects signed informed consent.
HUTT and Standing Test
All drugs that might affect the autonomic nervous function were avoided for at least five half-lives before evaluation. The test environment requires a quiet, dim, and suitable temperature. For the HUTT, the tilting device was the SHUT-100 tilt test monitoring software system of Beijing Standley Technology Co., Ltd. (Beijing, China). The subjects were kept supine on the tilt bed over 10 min, and their HR, BP, and ECG were monitored and recorded. After HR and BP stabilized, the bed was tilted upward to a 60° angle within 15 s, and HR, BP, and ECG were continuously monitored over 10 min. HUTT was conducted from 8:00 to 11:00 AM with subjects in a fasting state. On a different day, but within 3 days, the subjects performed standing tests in the morning (between 7:00 and 9:00 AM) in a fasting state, in the afternoon (between 3:00 and 5:00 PM), and in the evening (between 8:00 and 10:00 PM). For the standing test, the subjects laid quietly for 10–30 min, and HR, BP, and ECG were monitored by a multi-lead electrocardiography monitor. A suitable oscillometric BP cuff was used to determine BP. HR was derived automatically from the BP recording. After HR and BP stabilized, subjects were asked to stand upright without support for another 10 min. HR, BP, and ECG were monitored dynamically and recorded at 1, 3, 5, and 10 min after standing (11). The difference between HR at standing 1/3/5/10 min and baseline HR was defined as ΔHR 1 min/ΔHR 3 min/ΔHR 5 min/ΔHR 10 min. The maximum changes in HR, systolic BP (SBP), and diastolic BP (DBP) during the 10-min standing tests compared with those recorded in the supine position were defined as ΔHRmax, ΔSBPmax, and ΔDBPmax, respectively.
Diagnosis of POTS
The criteria for the diagnosis of POTS were ① associated with frequent OI symptoms, such as dizziness, headache, fatigue, blurred vision, chest tightness, palpitations, tremor in the hands, and even syncope during upright posture; ② associated with a positive HUTT or standing test response: supine HR was normal, and during 10 min of the HUTT or standing test, HR increased ≥40 bpm or the maximum HR ≥130 bpm for children 12 years and younger or ≥125 bpm for adolescents 13 years and older, and there was no significant decrease in BP (SBP decreased <20 mmHg and DBP decreased <10 mmHg); and ③ exclusion of other diseases that could cause OI symptoms, such as cardiovascular, metabolic, neurologic, or autoimmune diseases (1, 11, 13).
Symptom Score
Symptom score was based primarily on OI symptoms, including dizziness, headache, blurred vision, chest discomfort, palpitations, vomiting, profuse perspiration, and syncope. The frequency of such symptoms was taken into account. Scoring criteria were based on the number of times (on average) a symptom occurred over a particular period: 0 score, the symptom did not occur; 1 score, occurred once per month; 2 scores, 2–4 times per month; 3 scores, 2–7 times per week; and 4 scores, more than once per day. The resulting score was the sum of individual symptom scores (14).
Statistical Analyses
Data analyses were carried out with the SPSS 25.0 software (IBM Corp, Armonk, NY, USA). Continuous data were expressed as mean ± standard deviation (SD). Data normality was assessed using the Shapiro–Wilk test. Categorical data were expressed as cases (percentage), and the Chi-square test was used for comparisons among groups. The measurement data in multiple groups and hemodynamic parameters of standing tests at different times of the day were compared using one-way ANOVA analysis or the Kruskal–Wallis H test if appropriate. The Bonferroni test was used to adjust for comparisons between the two groups. The receiver operating characteristic (ROC) curve was performed to analyze the sensitivity and specificity of the indicators for predicting the diagnosis. The area under the curve (AUC) represented the predictive values. AUC between 0.5 and 0.7 indicated a relatively low predictive value, between 0.7 and 0.9 had a moderate predictive value, and above 0.9 represented a relatively high predictive value. 95% confidence interval (CI) of the curve area (not including 0.5 or P < 0.05) indicated that the index had a value on the prediction of the research result. The cutoff value was determined by the maximum of the Youden index, which was defined as sensitivity plus specificity minus 1. P < 0.05 was considered statistically significant.
Results
Characteristics of the POTS, Non-POTS, and Control Groups
As shown in Table 1, no statistical difference was found in gender, age, height, weight, and body mass index (BMI) among the POTS, non-POTS, and control groups (P > 0.05). Moreover, the median symptom scores were not significantly different between the POTS and non-POTS groups (P > 0.05).
Diurnal Variability in Orthostatic Hemodynamics of the POTS, Non-POTS, and Control Groups
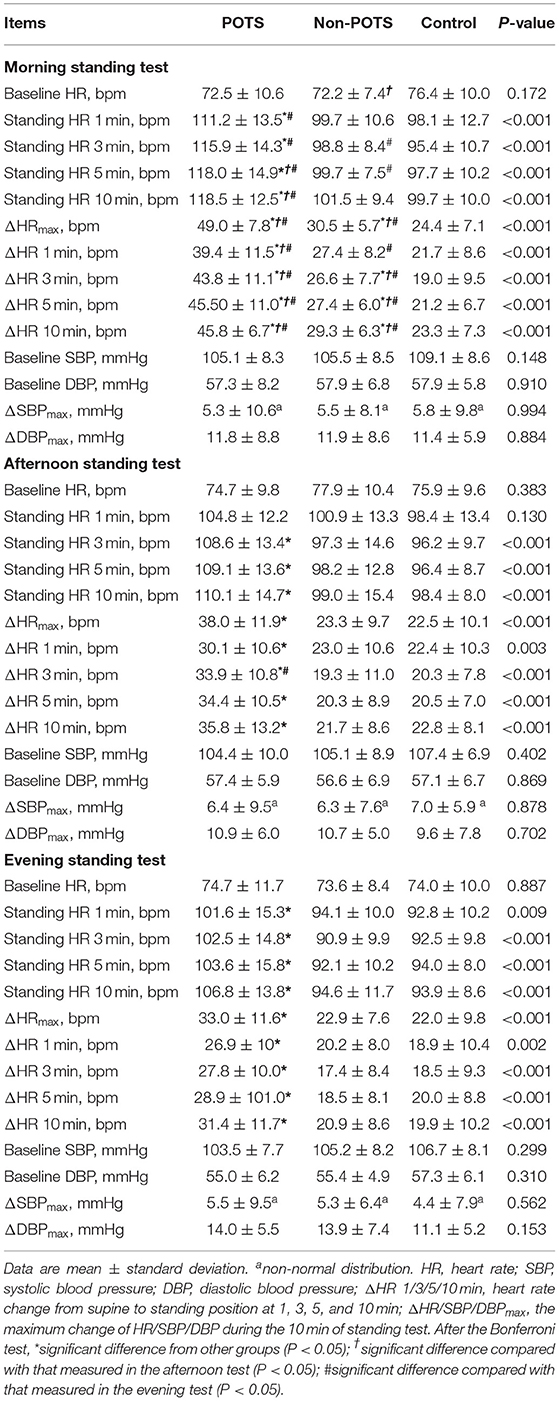
Of the 43 patients with POTS, all 43 patients (100%) met the criterion for POTS hemodynamic response in the morning standing test, 19 patients (44.2%) in the afternoon, and 12 patients (27.9%) in the evening. Among them, only 10 patients (23.3%) met the HR criterion of POTS at all three time points, and another 11 patients (25.6%) met at two time points, including 9 patients in both the morning and afternoon standing tests and 2 patients in both the morning and evening standing tests. Almost half of the POTS patients (51.2%) displayed a positive result only in the morning standing test.
Orthostatic hemodynamic parameters of the three groups were demonstrated in Table 2. At each time point, the baseline HR, SBP, DBP, ΔSBPmax, and ΔDBPmax were not significantly different among the POTS, non-POTS, and control groups (P > 0.05). However, after the Bonferroni test, the orthostatic parameters of HR at the three time points, including ΔHR and absolute standing HR from 1 to 10 min after standing and ΔHRmax, were the highest in the POTS group compared with in the non-POTS and control groups (P < 0.05) except for the afternoon standing HR at 1 min (P > 0.05). The morning ΔHRmax, ΔHR 3 min, ΔHR 5 min, and ΔHR 10 min in the non-POTS group were significantly higher than those in the control group (P < 0.05). Nevertheless, there was no significant difference in orthostatic hemodynamic parameters in the afternoon and evening tests between the non-POTS and control groups (P > 0.05).
Of all subjects, the baseline HR, SBP and DBP, ΔSBPmax, and ΔDBPmax did not differ with the time of day (P > 0.05) except for the baseline HR in the non-POTS group (P < 0.05). In the healthy control group, the orthostatic parameters of HR were not significantly different with the time of day (P > 0.05). In contrast, the orthostatic parameters of HR showed diurnal variability in both the POTS and non-POTS groups. These parameters were significantly greater in the morning than in the afternoon and/or in the evening tests (P < 0.05). However, these parameters were not significantly different between the afternoon and evening tests (P > 0.05) (Figure 1). Additionally, there was no significant difference in ΔHRmax at the three time points between males and females for the POTS group (P > 0.05) (Figure 2).
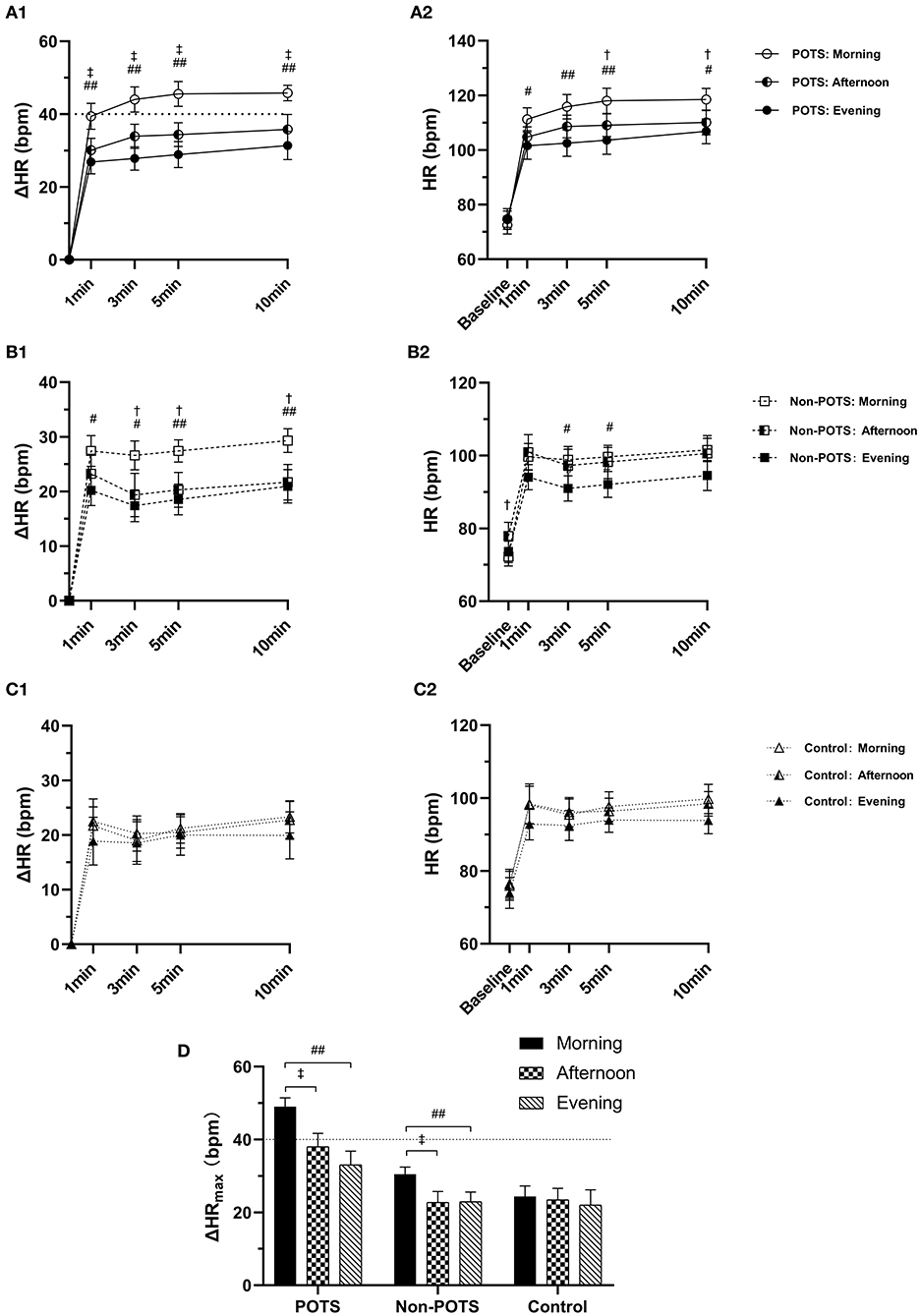
Figure 1. Diurnal variability in orthostatic parameters of HR among the POTS, non-POTS, and control groups. (A1, B1, C1) The heart rate change from supine to standing (ΔHR) at 1, 3, 5, and 10 min in the POTS, non-POTS, and control groups, respectively. (A2, B2, C2) Absolute HR at baseline and 1, 3, 5, and 10 min after standing in the POTS, non-POTS, and control groups, respectively. (D) The maximum HR increment (ΔHRmax) during the 10-min standing test in the POTS, non-POTS, and control groups. Data are mean ± 95% CI. After Bonferroni test, †P < 0.05, ‡ P < 0.001 significant difference compared with that measured in the afternoon test.# P < 0.05, ## P < 0.001 significant difference compared with that measured in the evening test (P < 0.05).
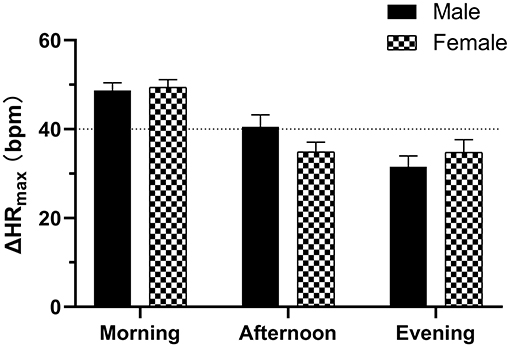
Figure 2. Diurnal variability of the maximum HR increment (ΔHRmax) in the standing tests between males and females with POTS. Data are mean ± 95% CI.
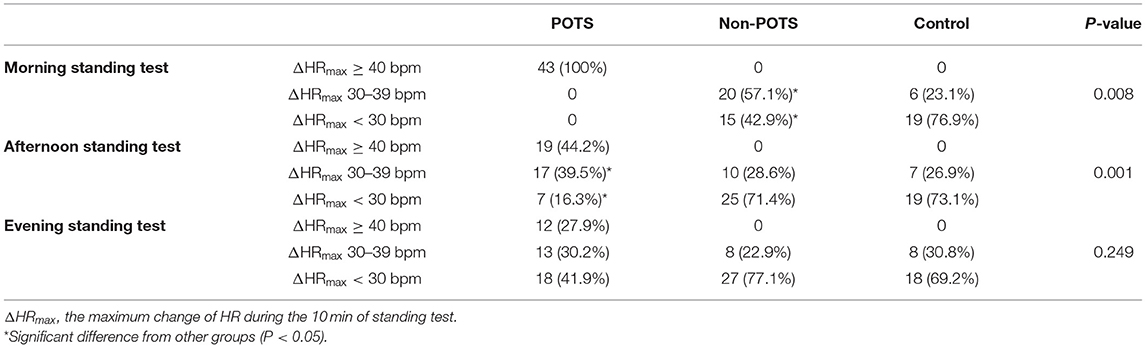
According to ΔHRmax during the 10-min standing test, then, the subjects were divided into three subtypes: those with ΔHRmax <30 bpm, those with ΔHRmax 30–39 bpm, and those with ΔHRmax ≥40 bpm. The distribution in subtypes of ΔHRmax among the POTS, non-POTS, and healthy control groups was seen in Table 3. We used the Chi-square test to compare the percentage of subjects with ΔHRmax <30 bpm and with ΔHRmax 30–39 bpm between the non-POTS and control groups. In the morning test, the percentage of subjects with ΔHRmax 30–39 bpm in the non-POTS group was significantly higher than that in the control group (57.1 vs. 23.1%, P = 0.008). Nevertheless, there was no significant difference between the two groups in the afternoon and evening tests (P > 0.05).
ROC Curve Analysis of the Orthostatic HR Parameters at Different Time Points for the Prediction of the POTS Diagnosis
The ROC curve was used to analyze ΔHR at different time points to predict the POTS diagnosis (Figure 3). The AUC values for ΔHRmax, ΔHR 5 min, and ΔHR 10 min in the afternoon standing test were 0.855 (95% CI 0.776–0.934), 0.856 (95% CI 0.778–0.933), and 0.819 (95% CI 0.727–0.911), respectively, for the prediction of the POTS diagnosis. Cutoff values for ΔHRmax, ΔHR 5 min, and ΔHR 10 min were 32, 32, and 31 bpm, yielding sensitivities of 76.7, 62.8, and 71.4% and specificities of 86, 94.7, and 88.7%, respectively. The AUC values for ΔHRmax, ΔHR 5 min, and ΔHR 10 min in the evening standing test were 0.756 (95% CI 0.657–0.856), 0.756 (95% CI 0.657–0.855), and 0.760 (95% CI 0.622–0.858), respectively, for the prediction of the POTS diagnosis. Cutoff values for ΔHRmax, ΔHR 5 min, and ΔHR 10 min were 25, 23, and 25 bpm, yielding sensitivities of 82.1, 74.4, and 73.7% and specificities of 60.3, 69, and 70.2%, respectively. For easy memory, we suggest that orthostatic HR increment ≥30 bpm in the afternoon standing test and orthostatic HR increment ≥25 bpm in the evening standing test could be considered as POTS, combined with clinical symptoms.
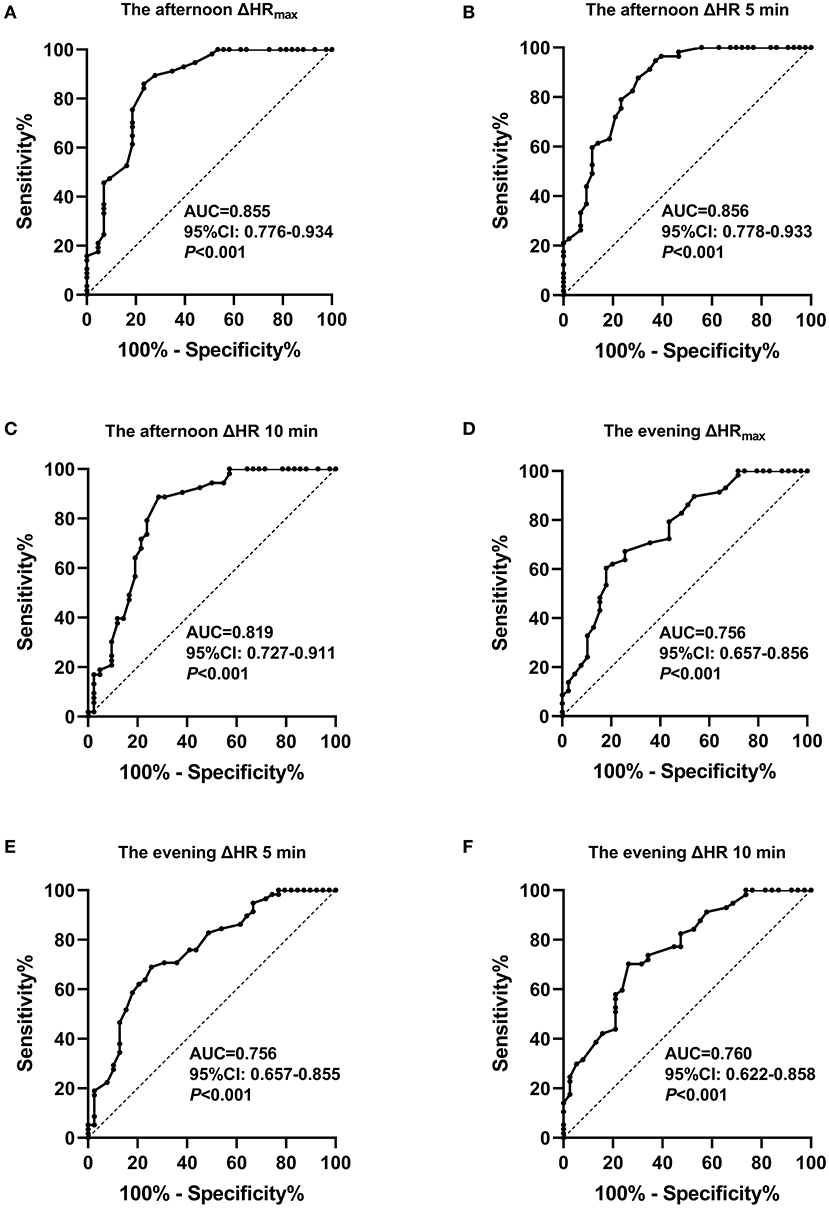
Figure 3. Receiver operating characteristic curve analysis of orthostatic heart rate parameters in the afternoon and evening standing tests to predict the POTS diagnosis. (A) The maximum heart rate increment (ΔHRmax) in the afternoon standing test. (B) ΔHR at 5 min in the afternoon standing test. (C) ΔHR at 10 min in the afternoon standing test. (D) ΔHRmax in the evening standing test. (E) ΔHR at 5 min in the evening standing test. (F) ΔHR at 10 min in the evening standing test.
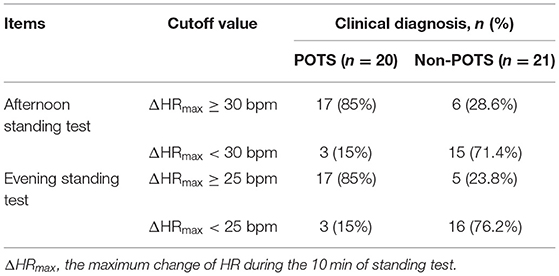
The external validation test in the predictive values of the afternoon and evening ΔHRmax was shown in Table 4. At the afternoon ΔHRmax ≥30 bpm, its sensitivity, specificity, and accuracy in the POTS diagnosis were 85, 71.4, and 78%, respectively. Furthermore, at the evening ΔHRmax ≥25 bpm, its sensitivity, specificity, and accuracy in the POTS diagnosis by the evening ΔHRmax were 85.0, 76.2, and 80.5%, respectively.
Discussion
In this study, we showed that the orthostatic HR increment exhibited diurnal variability in children and adolescents with OI, with a peak in the morning. In contrast, the orthostatic hemodynamics did not change with the time of day in the healthy control group. The orthostatic HR increment in the non-POTS group was higher only in the morning standing test than that in the control group. However, the orthostatic HR increment in the POTS group was markedly the highest in the whole day compared with that in the non-POTS and control groups. Despite the higher HR increment, not all POTS patients can meet the HR criterion of a 40-bpm threshold throughout the day. Only half of the POTS patients (51.2%) displayed a positive result only in the morning standing test. Next, we used the ROC curve analysis to explore the predictive values of orthostatic HR parameters in the afternoon and evening standing tests for the POTS diagnosis. The ΔHRmax and ΔHR at 5 and 10 min in the afternoon and evening tests showed moderate predictive values. On our validation test, with 30 bpm as the cutoff value of the afternoon ΔHRmax, its sensitivity, specificity, and accuracy in the POTS diagnosis were 85, 71.4, and 78%, respectively. Moreover, with 25 bpm as the cutoff value of the evening ΔHRmax, its sensitivity, specificity, and accuracy in the POTS diagnosis were 85.0, 76.2, and 80.5%, respectively.
The diagnosis of POTS is based on the clinical history and the excessive increment in HR after standing without a specific biomarker. The active standing test and the passive HUTT are two main and comparable methods for the assessment of POTS (15). We used the two methods for the POTS diagnosis to exclude transient orthostatic tachycardia. Our study revealed that the morning standing test showed a higher positive rate for the POTS diagnosis than the afternoon and evening standing tests. It implies that the time of day can affect the diagnostic accuracy of the standing test for POTS. Ignoring the diurnal variability of orthostatic HR increment could result in a missed diagnosis of POTS. In our study, although all POTS patients met the HR criterion with ≥40 bpm in the morning standing test, the positive diagnostic rate decreased to 44.2% in the afternoon and 27.9% in the evening. Despite the relatively low positive rate in the afternoon and evening, the orthostatic HR increment in the POTS group was still markedly larger than that in the non-POTS and control groups. We suggest either implementing a standing test in the early morning or redefining the hemodynamic criteria based on diurnal variability. Our findings indicate that a cutoff value of 30 bpm for the afternoon ΔHRmax and a cutoff value of 25 bpm for the evening ΔHRmax to diagnose POTS yielded relatively favorable sensitivity and specificity, which were confirmed by external validation test.
Boris et al. (12) reported that the burden of OI symptoms was unrelated to the degree of tachycardiac response to an upright position. In our study, the POTS (HR increased ≥40 bpm) and non-POTS (HR increased <40 bpm) patients had similar symptom scores. Although the non-POTS patients had significantly larger HR increment than the healthy controls in the morning standing test, the above difference was diminished in the afternoon and evening tests between the non-POTS and control groups.
Another key finding in our study is the diurnal variability of orthostatic HR increment in children and adolescents with POTS. The most probable explanation for the exaggerated orthostatic HR increment observed in the morning is the intrinsic circadian variability. Increasing or decreasing HR is modulated via two opposite pathways of autonomic nervous systems—the catecholaminergic and sympathetic nervous systems and the acetylcholinergic and parasympathetic nervous systems. Plasma catecholamines epinephrine (an indicator of adrenal medullary activity) and norepinephrine (an indicator of sympathetic nervous system activity) levels show significant diurnal variability, with rapid increase after waking and a peak in the late morning, then a gradual decrease throughout the day (16, 17). Plasma norepinephrine levels increase sharply from supine to upright posture, but epinephrine levels are less affected, suggesting that the former is mainly influenced by posture (16, 18). Up to 50% of the POTS patients exhibit a hyperadrenergic state with standing plasma norepinephrine ≥600 pg/ml (13). Salivary cortisol levels can reflect plasma cortisol levels and are positively correlated with norepinephrine levels. Lin et al. (19) reported that salivary cortisol levels were significantly higher in the POTS children than in the control children at all examined time points, with peaking shortly after waking and decreasing during the night. Furthermore, our previous study reported that the rate–pressure product values (multiplying HR and SBP) of the POTS children were significantly higher than those of the controls in the early morning, paralleled with the morning surge of orthostatic HR increment (20).
POTS patients have impaired α1 adrenergic receptor (α1 AR)-mediated vasoconstriction and enhanced β1 adrenergic receptor (β1 AR)-mediated tachycardia (21, 22). Several studies have investigated the presence of autoantibodies in POTS patients, and these autoantibodies induced an allosterically mediated positive modulatory effect on β1 AR and negative modulatory effect on α1 AR activity (23, 24). The cardiac output shows diurnal variability with a steep increase in the early morning, whereas the total peripheral resistance shows an unexpected decrease in the morning (25). Panza et al. (26) showed that the vasodilator effect of phentolamine (an α AR-antagonist drug) was significantly greater in the early morning than in the afternoon and evening. Therefore, when a POTS patient stands from a supine position, α1 AR-mediated vasoconstriction to upright posture could be blunter in the early morning, leading to a further increase of norepinephrine release. Some POTS patients are hypersensitive to β1 AR. The positive chronotropic effect of β1 AR would be enhanced in the morning. Another acknowledged mechanism is central hypovolemia (1, 2). The morning standing test was performed after an overnight fast and overnight bed rest. The hypovolemic state may be exacerbated in the morning resulting from the overnight fast and morning urination.
The exaggerated response to adrenergic receptors, the increased catecholamine levels, and the exacerbated hypovolemic state in the morning may explain the excessive HR increment after standing observed in the morning for POTS children and adolescents.
Despite the promising results, our study still had limitations. This was a single-center study, and the number of cases was not large enough. In addition, all the participants in our study kept their usual routine of daily activities on the day of examination. The diurnal variability of HR is influenced by the physical activities, which needs the participants to avoid strenuous exercise and emotional excitement. More studies with larger sample-sized multicenter studies are needed in the future to establish and validate the hemodynamic criteria for POTS based on diurnal variability.
Conclusions
In summary, we emphasize the significance of diurnal variability for the POTS diagnosis. The diagnostic test performed in the early morning would be the most sensitive for POTS hemodynamic positivity. Nevertheless, new diagnostic criteria for POTS combined with the concept of diurnal variability should be established shortly, which makes the diagnostic test free from the restriction of the time of day.
Data Availability Statement
The data analyzed in this study is subject to the following licenses/restrictions: The datasets generated for this study are available on request to the corresponding author. Requests to access these datasets should be directed to Cheng Wang, wangcheng2nd@csu.edu.cn.
Ethics Statement
The studies involving human participants were reviewed and approved by the Medical Ethical Committee, The Second Xiangya Hospital, Central South University review board approved the study, with waiver of requirement for informed consent. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author Contributions
HC and CW conceived the study. SW, RZ, FL, JZ, YW, and YX collected and reviewed the subjects' data. SW and RZ performed the statistical analysis. HC drafted the manuscript and all authors contributed to its revision. All authors contributed to the article and approved the submitted version.
Funding
This work is supported by grants from Hunan Province Clinical Medical Technology Innovation Guidance Project (2020SK53405).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank the research personnel and study volunteers involved with the project.
References
1. Stewart JM, Boris JR, Chelimsky G, Fischer PR, Fortunato JE, Grubb BP, et al. Pediatric disorders of orthostatic intolerance. Pediatrics. (2018) 141:e20171673. doi: 10.1542/peds.2017-1673
2. Tao CY, Jin HF, Du JB. Management of orthostatic intolerance in children: the state of the art. World J Pediatr. (2020) 16:543–8. doi: 10.1007/s12519-019-00329-0
3. Mar PL, Raj SR. Postural orthostatic tachycardia syndrome: mechanisms and new therapies. Annu Rev Med. (2020) 71:235–48. doi: 10.1146/annurev-med-041818-011630
4. Guo YF, Stein PK. Circadian rhythm in the cardiovascular system: chronocardiology. Am Heart J. (2003) 145:779–86. doi: 10.1016/S0002-8703(02)94797-6
5. Smolensky MH, Portaluppi F, Manfredini R, Hermida RC, Tiseo R, Sackett-Lundeen LL, et al. Diurnal and twenty-four hour patterning of human diseases: cardiac, vascular, and respiratory diseases, conditions, and syndromes. Sleep Med Rev. (2015) 21:3–11. doi: 10.1016/j.smrv.2014.07.001
6. Liao D, Xu Y, Zou R, Wu L, Luo X, Li F, et al. The circadian rhythm of syncopal episodes in patients with neurally mediated syncope. Int J Cardiol. (2016) 215:186–92. doi: 10.1016/j.ijcard.2016.04.086
7. Brewster JA, Garland EM, Biaggioni I, Black BK, Ling JF, Shibao CA, et al. Diurnal variability in orthostatic tachycardia: implications for the postural tachycardia syndrome. Clin Sci(Lond). (2012) 122:25–31. doi: 10.1042/CS20110077
8. Moon J, Lee HS, Byun JI, Sunwoo JS, Shin JW, Lim JA, et al. The complexity of diagnosing postural orthostatic tachycardia syndrome: influence of the diurnal variability. J Am Soc Hypertens. (2016) 10:263–70. doi: 10.1016/j.jash.2016.01.011
9. Massin MM, Maeyns K, Withofs N, Ravet F, Gérard P. Circadian rhythm of heart rate and heart rate variability. Arch Dis Child. (2000) 83:179–82. doi: 10.1136/adc.83.2.179
10. Janz KF, Dawson JD, Mahoney LT. Predicting heart growth during puberty: the muscatine study. Pediatrics. (2000) 105:E63. doi: 10.1542/peds.105.5.e63
11. Wang C, Li Y, Liao Y, Tian H, Huang M, Dong X, et al. 2018 Chinese pediatric cardiology society (CPCS) guideline for diagnosis and treatment of syncope in children and adolescents. Sci Bull. (2018) 63:1558–64. doi: 10.1016/j.scib.2018.09.019
12. Boris JR, Huang J, Bernadzikowski T. Orthostatic heart rate does not predict symptomatic burden in pediatric patients with chronic orthostatic intolerance. Clin Auton Res. (2020) 30:19–28. doi: 10.1007/s10286-019-00622-y
13. Sheldon RS, Grubb BP 2nd, Olshansky B, Shen WK, Calkins H, Brignole M, et al. 2015 heart rhythm society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm. (2015) 12:e41–63. doi: 10.1016/j.hrthm.2015.03.029
14. Cai H, Wang S, Zou R, Liu P, Yang H, Wang Y, et al. Symptom score: a new instrument to assess orthostatic intolerance in children and adolescents. J Child Neurol. (2020) 35:835–43. doi: 10.1177/0883073820936025
15. Kirbiš M, Grad A, Meglič B, Bajrović FF. Comparison of active standing test, head-up tilttest and 24-h ambulatory heart rate and blood pressure monitoring in diagnosing postural tachycardia. Funct Neurol. (2013) 28:39–45.
16. Linsell CR, Lightman SL, Mullen PE, Brown MJ, Causon RC. Circadian rhythms of epinephrine and norepinephrine in man. J Clin Endocrinol Metab. (1985) 60:1210–5. doi: 10.1210/jcem-60-6-1210
17. Monfredi O, Lakatta EG. Complexities in cardiovascular rhythmicity: perspectives on circadian normality, ageing and disease. Cardiovasc Res. (2019) 115:1576–95. doi: 10.1093/cvr/cvz112
18. Dodt C, Breckling U, Derad I, Fehm HL, Born J. Plasma epinephrine and norepinephrine concentrations of healthy humans associated with nighttime sleep and morning arousal. Hypertension. (1997) 30:71–6. doi: 10.1161/01.HYP.30.1.71
19. Lin J, Zhao H, Shen J, Jiao F. Salivary cortisol levels predict therapeutic response to a sleep-promoting method in children with postural tachycardia syndrome. J Pediatr. (2017) 191:91–5.e1. doi: 10.1016/j.jpeds.2017.08.039
20. Cai H, Wang S, Zou R, Wang Y, Wang C. Circadian rhythms of blood pressure and rate pressure product in children with postural tachycardia syndrome. Auton Neurosci. (2020) 228:102715. doi: 10.1016/j.autneu.2020.102715
21. Abe H, Nagatomo T, Kohshi K, Numata T, Kikuchi K, Sonoda S, et al. Heart rate and plasma cyclic AMP responses to isoproterenol infusion and effect of beta-adrenergic blockade in patients with postural orthostatic tachycardia syndrome. J Cardiovasc Pharmacol. (2000) 36(Suppl. 2):S79–82. doi: 10.1097/00005344-200000006-00017
22. Stewart JM, Munoz J, Weldon A. Clinical and physiological effects of an acute alpha-1 adrenergic agonist and a beta-1 adrenergic antagonist in chronic orthostatic intolerance. Circulation. (2002) 106:2946–54. doi: 10.1161/01.CIR.0000040999.00692.F3
23. Li H, Yu X, Liles C, Khan M, Vanderlinde-Wood M, Galloway A, et al. Autoimmune basis for postural tachycardia syndrome. J Am Hear Assoc. (2014) 3:e000755. doi: 10.1161/JAHA.113.000755
24. Fedorowski A, Li H, Yu X, Koelsch KA, Harris VM, Liles C, et al. Antiadrenergic autoimmunity in postural tachycardia syndrome. Europace. (2017) 19:1211–9. doi: 10.1093/europace/euw154
25. Veerman DP, Imholz BP, Wieling W, Wesseling KH, van Montfrans GA. Circadian profile of systemic hemodynamics. Hypertension. (1995) 26:55–9. doi: 10.1161/01.HYP.26.1.55
Keywords: postural tachycardia syndrome, heart rate increment, diurnal variability, diagnosis, children, adolescents
Citation: Cai H, Wang S, Zou R, Li F, Zhang J, Wang Y, Xu Y and Wang C (2021) Diagnostic Value of Diurnal Variability of Orthostatic Heart Rate Increment in Children and Adolescents With POTS. Front. Pediatr. 9:644461. doi: 10.3389/fped.2021.644461
Received: 21 December 2020; Accepted: 22 March 2021;
Published: 13 May 2021.
Edited by:
Oswin Grollmuss, Université Paris-Sud, FranceReviewed by:
Jangsup Moon, Seoul National University Hospital, South KoreaManeesh Kumar, Rajendra Memorial Research Institute of Medical Sciences, India
Copyright © 2021 Cai, Wang, Zou, Li, Zhang, Wang, Xu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cheng Wang, wangcheng2nd@csu.edu.cn
 Hong Cai1
Hong Cai1 Shuo Wang
Shuo Wang Runmei Zou
Runmei Zou Fang Li
Fang Li Juan Zhang
Juan Zhang Yuwen Wang
Yuwen Wang Yi Xu
Yi Xu Cheng Wang
Cheng Wang