- 1Department of Obstetrics and Gynecology, University of Toyama, Toyama, Japan
- 2Clinical Laboratory Center, Toyama University Hospital, Toyama, Japan
- 3Division of Neonatology, Maternal and Perinatal Center, Toyama University Hospital, Toyama, Japan
- 4University of Toyama, Toyama, Japan
Objective: The purpose of this study was to investigate perinatal factors associated with a poor neurodevelopmental outcome in preterm infants.
Methods: A retrospective study was conducted by searching our clinical database between January 2006 and December 2016. A total of 165 singleton children who were born between 23 and 33 weeks of gestation were included. We defined poor neurological development outcomes as follows: cerebral palsy; intellectual disability; developmental disorder including autism and attention-deficit/hyperactivity disorder; low score (<85 points) on Bayley Scales of Infant and Toddler Development, Third Edition (Bayley-III); or low score of Kyoto Scale of Psychological Development corrected at 3 years old. We diagnosed maternal renal dysfunction according to the Clinical Practice Guideline for chronic kidney disease 2018 and the Best Practice Guide 2015 for Care and Treatment of Hypertension in Pregnancy.
Results: The rate of poor neurological development was 25/165 (15.2%): cerebral palsy (n = 1), intellectual disability (n = 1), developmental disorder (n = 2), low score of Bayley-III (n = 20), and low score of Kyoto Scale of Psychological Development (n = 1). Preeclampsia complicated with maternal renal dysfunction (P = 0.045) and delivery at <30 weeks of gestation (P = 0.007) were independent risk factors for poor neurological development.
Conclusions: In addition to previous risk factors such as delivery at <30 weeks of gestation, preeclampsia complicated with renal dysfunction was also associated with poor neurodevelopmental outcomes corrected at 3 years old.
Introduction
Poor neurological development of preterm infants is related to delivery weeks (1, 2), fetal growth restriction (3, 4), chorioamnionitis, maternal infection (5), maternal increased body mass index (BMI) (6), socioeconomic disadvantage (7, 8), not receiving breast milk at discharge (7), neonatal intensive care unit (NICU) noise (9), longer intensive care unit stay, more complex forms of congenital heart disease (8), and maternal preeclampsia (PE) (10, 11).
Children born to mothers with PE show developmental disorders (10, 11) that contain cognitive, behavioral, and mood deficits compared with offspring from mothers with non-complicated pregnancies (12). Severe PE is a disease complicated with hypertension and proteinuria with or without kidney and liver damage, oliguria, PE, cerebral edema, and cerebral hemorrhage. This will lead to perinatal and neonatal problems, including preterm birth, fetal growth restriction (FGR), reduced birth weight, and NICU stays.
There is also the Baker hypothesis that undernutrition during pregnancy increases the risk of adult diseases (13). Barker (13) demonstrated that many human fetuses have to adapt to a limited supply of nutrients, and so they change their metabolism and physiology. These changes may be the origins of many diseases in later life, including diabetes, hypertension, and coronary heart disease (13).
In Japan, the examination of neurological development is usually assessed by the Tsumori-Inage Infant's developmental test (14) or Kyoto Scale of Psychological Development (15). However, these tests are unique to Japan, and so studies cannot be compared internationally.
Bayley Scales of Infant and Toddler Development, Third Edition (Bayley-III), is an independently examined test designed to assess developmental functioning of infants and toddlers. Bayley-III checks development in five areas: cognitive, language, motor, social–emotional, and adaptive behavior (16). Bayley-III is globally used as a method for evaluating development from 1 month to the age of 3.5 years, and there are many reports (17–20).
The objective of this study was to examine the association of perinatal factors and neurological developmental outcomes evaluated by Bayley-III in preterm infants. We examined just one case by Kyoto Scale of Psychological Development.
Materials and Methods
This was a retrospective study, conducted by examining hospitalized patients in Toyama University Hospital, Department of Obstetrics and Gynecology, between January 2006 and December 2016. Figure 1 shows a flow diagram of the study population. The study group consisted of 243 infants delivered between 23 and 33 weeks of gestation. We excluded cases with children born to mothers with abruptio placenta (n = 4), with congenital malformation (n = 15), intrauterine fetal death (IUFD) (n = 15), who died as neonates (n = 7), those transferred to another hospital (n = 36), and cases of sudden infant death syndrome (SIDS) (n = 1). A total of 165 infants were included in this study; 92 cases were followed up to corrected age of 3 years, and 73 cases were followed up to completion before 3 years old (Figure 1). Informed consent for clinical data, purpose of the study, etc. were signed by all patients. The research protocols were accepted by the institutional review board of Toyama University Hospital.
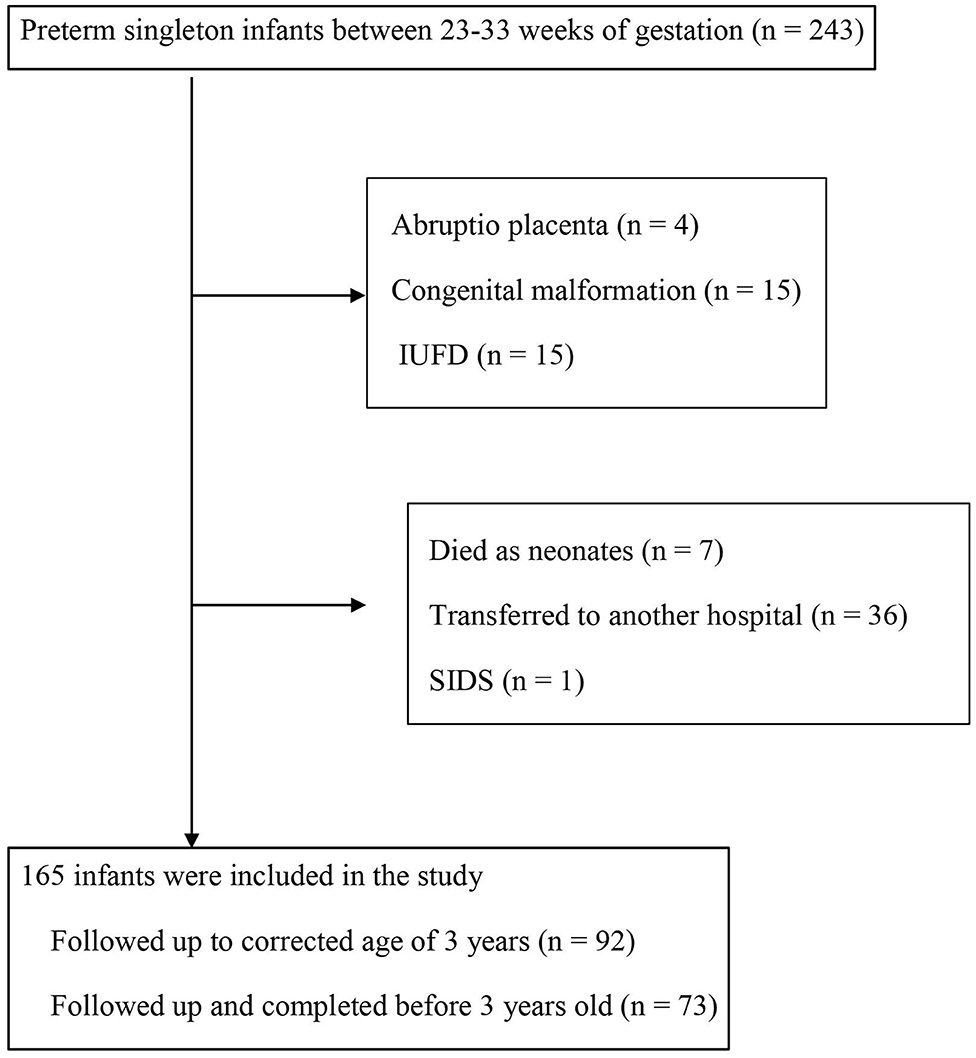
Figure 1. Flow diagram of the study population. IUFD, intrauterine fetal death; SIDS, sudden infant death syndrome.
Histological Examination of the Placenta
We defined chorioamnionitis according to acute inflammatory cell infiltration in the chorion-decidua and amnion by Blanc's classification: deciduitis (stage I), chorionitis (stage II), and amnionitis (stage III) (21).
Maternal Factors
PE was diagnosed by the new onset of hypertension and proteinuria or fetal growth restriction or end-organ dysfunction after 20 weeks' gestation. Gestational hypertension (GH) was defined by only hypertension. Hypertension during pregnancy was diagnosed as systolic blood pressure over 140 mmHg and/or diastolic blood pressure over 90 mmHg. Proteinuria was defined by over 300 mg/day. Superimposed PE was diagnosed as chronic hypertension before 20 weeks' gestation and the new onset of proteinuria and/or end-organ dysfunction after 20 weeks' gestation. Preterm premature rupture of membranes (PROM) was diagnosed by the rupture of membranes before 37 weeks' gestation. Preterm PROM was diagnosed to verify the pooling of amniotic fluid in the vagina or the presence of insulin-like growth factor binding protein-1 (IGFBP-1) (Check PROM test; MedixBiochemica, Finland). Clinical chorioamnionitis was diagnosed according to Lencki's criteria (22).
Neonatal Factors
Respiratory distress syndrome (RDS) was defined by radiographic findings and the administration of surfactant. Chronic lung disease (CLD) was diagnosed by over 28 days' age supplemental oxygen and/or pneumonia; intraventricular hemorrhage (IVH) was diagnosed by cranial ultrasound. Periventricular leukomalacia (PVL) was defined by magnetic resonance imaging and head ultrasound according to injury of deep cerebral white matter and focal necrosis. Necrotizing enterocolitis (NEC) was diagnosed by portal venous gas and pneumatosis. Retinopathy of prematurity (ROP) was identified by retinoscopy, and all patients were treated with laser photocoagulation or anti-vascular endothelial growth factor (VEGF) drugs. Mechanical ventilation was administered for managing preterm neonates with RDS. Non-invasive positive pressure ventilation (NPPV) was used to help remove invasive mechanical ventilation or positive airway pressure and/or inspiratory pressure support. Circulation problem was defined by the need for surgical ligation of patent ductus arteriosus (PDA) and/or late-onset glucocorticoid-responsive circulatory collapse. Postnatal infection was defined by the presence of bacteria in blood culture. Visual impairment and hearing impairment were identified at 3 years old. Visual impairment included amblyopia and squint. Hearing impairment was diagnosed by hearing test.
Developmental Follow-Up
Neurodevelopmental outcomes were examined by Bayley-III. Composite scores were obtained for motor, cognitive, and emotion domains, with a normal mean of 100 and SD of 15. We defined poor neurodevelopmental outcomes as follows: cerebral palsy, intellectual disability, developmental disorder including autism and attention-deficit/hyperactivity disorder (ADHD), and low score (<85 points) of Bayley-III. One patient was examined by Kyoto Scale of Psychological Development. Kyoto Scale of Psychological Development is one of the most generally used developmental examinations in Japan. This test is an individualized person-to-person test examined by expert psychologists to check a child's development in three areas: Cognitive-Adaptive, Language-Social, and Postural-Motor (15). A total of 73 cases were followed up to completion before 3 years old. Of these 73 cases, all cases were diagnosed as normal development. Clinical examination by the neonatologist and parental questionnaires were inspected at follow-up. Infants who were followed up and completed before 3 years old were instructed by the neonatologists to go and see a neonatologist/pediatrician in our hospital if they had developmental problems at health checkup at 3 years old.
Diagnosis of Maternal Renal Dysfunction
We diagnosed maternal renal dysfunction as follows: at least one of creatinine clearance under 70 ml/min, oliguria ≤ 400 ml/day, urine protein ≥5 g/day, and pleural effusion and ascites according to the Clinical Practice Guideline for chronic kidney disease (CKD) 2018 (23) and the Best Practice Guide 2015 for Care and Treatment of Hypertension in Pregnancy (24).
Statistical Analysis
To compare clinical variables between both outcome groups, we used the Fisher's exact test or chi-square test. We used the Mann–Whitney U test and Kruskal–Wallis test to compare the differences in continuous variables. We checked the association between perinatal factors and poor neurological outcomes by multiple logistic regression analysis. We adjusted odds ratios (ORs) and 95% confidence intervals (95% CIs). All analyses were operated by statistical analysis software JMP 11.2.0 (SAS Institute Inc., Tokyo, Japan). Significant difference was described as P < 0.05.
Results
Poor neurological development was noted in 25 cases (15.2%) of 165 infants as follows: cerebral palsy (n = 1), intellectual disability (n = 1), developmental disorder including autism and ADHD (n = 2), low score (<85 points) of Bayley-III (n = 20), and low score of Kyoto Scale of Psychological Development (n = 1) (Figure 2). A total of 91 cases were examined by Bayley-III, and one case was examined by Kyoto Scale of Psychological Development. High grades of developmental delay were: ID (n = 1), CP (n = 1), developmental disorder (n = 2), Bayley-III score <70 points (n = 4), and 50 points of Kyoto Scale of Psychological Development (n = 1) at a corrected age of 3 years. Mild developmental delay was noted in 16 cases with Bayley-III scores of 70–84 points.
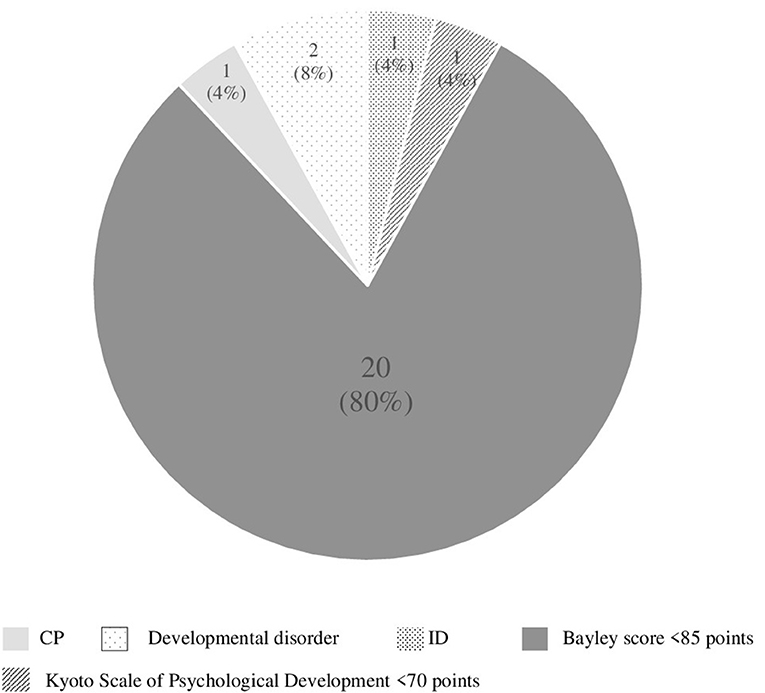
Figure 2. Details of poor neurological development cases (n = 25). CP, cerebral palsy; ID, intellectual disability.
Risk Factors for Poor Neurological Development in Preterm Infants
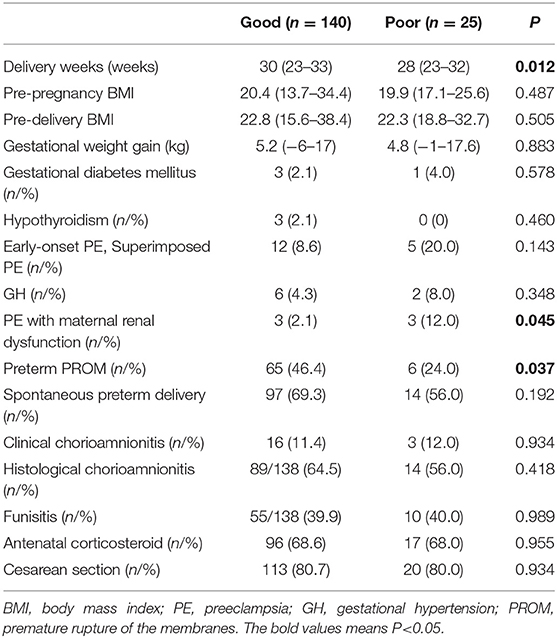
Table 1 demonstrates perinatal factors according to neurological development. The delivery week was significantly earlier in the poor outcome group (n = 25) than that in the good outcome group (n = 140) (28 vs. 30, respectively; P = 0.012). No significant differences were observed between the good outcome group and the poor outcome group in clinical characteristics including pre-pregnancy BMI (20.4 vs. 19.9, respectively, P = 0.487), pre-delivery BMI (22.8 vs. 22.3, respectively; P = 0.505), gestational weight gain (kg) (5.2 vs. 4.8, respectively; P = 0.883), rates of gestational diabetes mellitus (%) (2.1 vs. 4.0, respectively; P = 0.578), hypothyroidism (%) (2.1 vs. 0, respectively; P = 0.460), spontaneous preterm delivery (%) (69.3 vs. 56, respectively; P = 0.192), clinical chorioamnionitis (%) (11.4 vs. 12.0, respectively; P = 0.934), histological chorioamnionitis (%) (64.5 vs. 56, respectively; P = 0.418), funisitis (%) (39.9 vs. 40, respectively; P = 0.989), antenatal corticosteroid use (%) (68.6 vs. 68, respectively; P = 0.955), and cesarean section (%) (80.7 vs. 80, respectively; P = 0.934). The rates of preterm PROM (%) were higher in the good outcome group than those in the poor outcome group (46.4 vs. 24, respectively; P = 0.037).
The rates of early-onset PE including superimposed PE (%) (8.6 vs. 20, respectively; P = 0.143) and GH (%) (4.3 vs. 8, respectively; P = 0.348) were not related to poor neurological development. On the other hand, rates of PE with maternal renal dysfunction (%) was significantly higher in the poor outcome group than those in the good outcome group (12 vs. 2.1, respectively; P = 0.045). Univariable analyses revealed that preterm infants born early to PE mothers with renal dysfunction were more likely to show poor neurological development (Table 1).
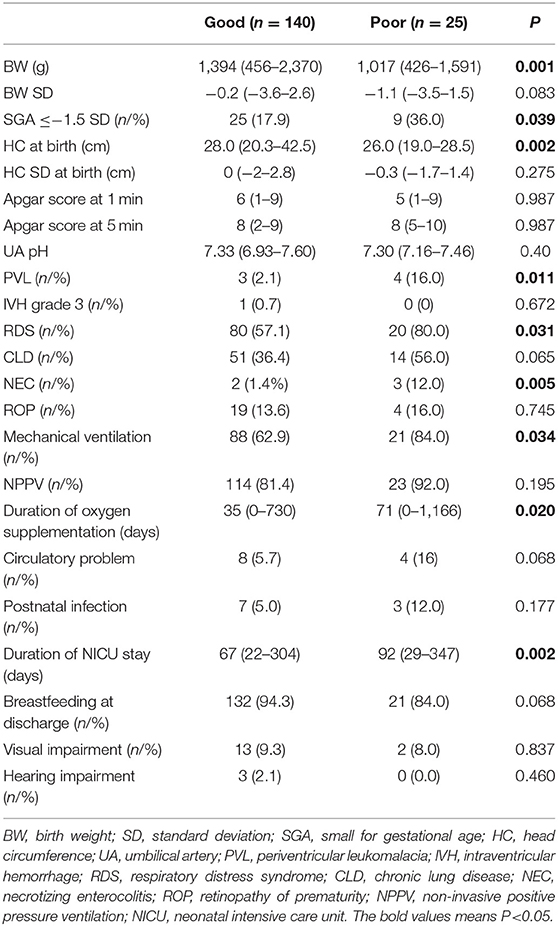
Of the neonatal factors examined, low birth weight (g) (1,017 vs. 1,394, respectively; P = 0.001), rates of small for gestational age (SGA) (%) (36 vs. 17.9, respectively; P = 0.039), head circumference (HC) at birth (cm) (26.0 vs. 28.0, respectively; P = 0.002), rates of PVL (%) (16 vs. 3, respectively; P = 0.011), RDS (%) (80.0 vs. 57.1, respectively; P = 0.031), NEC (%) (12.0 vs. 1.4, respectively; P = 0.005), mechanical ventilation (%) (84.0 vs. 62.9, respectively; P = 0.034), duration of oxygen supplementation (days) (71 vs. 35, respectively; P = 0.020), and duration of NICU stay (days) (92 vs. 67, respectively; P = 0.002) were significantly correlated with poor neurological development (poor outcome group vs. good outcome group) (Table 2). Other neonatal factors such as birth weight SD, Apgar score, and umbilical cord blood pH, rates of CLD, ROP, circulation problem, postnatal infection, breastfeeding at discharge, and visual and hearing impairment were not related to a poor neurological outcome. The rates of IVH grade 3 (0 vs. 0.7%, respectively; P = 0.672) were not different between the poor outcome group and the good outcome group. In the current study, there were no cases of IVH grade 4.
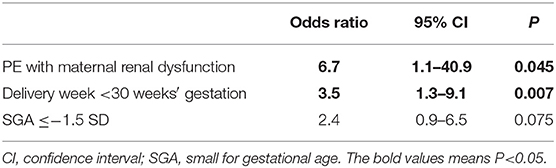
Table 3 shows risk factors for a poor neurological outcome. PE complicated with maternal renal dysfunction was an independent risk factor for a poor neurological outcome (OR: 6.7, 95% CI: 1.1–40.9, P = 0.045). Delivery at <30 weeks of gestation was also an independent risk factor for a poor neurological outcome (OR: 3.5, 95% CI: 1.3–9.1, P = 0.007).
Discussion
To study risk factors of a poor neurodevelopmental outcome in preterm singleton children delivered at <34 weeks of gestation, we investigated 92 preterm children at a corrected age of 3 years old using Bayley-III (n = 91) and Kyoto Scale of Psychological Development (n = 1). We included follow-up children before 3 years old (n = 73) in the good outcome group. The rate of a poor neurological outcome was 15.2% (25/165). A total of 80% (20/25) Bayley-III scores under 85 points. Also, 80% (16/20) of those with a poor neurodevelopmental outcome had shown mild neurodevelopmental delay with Bayley-III scores of 70–84 points, and 20% (4/20) showed <70 points. Therefore, this study included those with relatively mild developmental delay.
In addition, as in previous studies, our study showed that the delivery week (28 vs. 30 weeks, respectively; P = 0.012), a low birth weight (1,017 vs. 1,394 g, respectively; P = 0.001), and SGA (36 vs. 17.9%, respectively; P = 0.039) were associated with a poor neurodevelopmental outcome in preterm children. However, there was no association between the onset of PE alone and a poor neurodevelopmental outcome (8.6 vs. 20%, respectively; P = 0.143), and it was significantly correlated with PE complicated with maternal renal dysfunction (12 vs. 2.1%, respectively; P = 0.045).
Also, there was no difference between IVH grade 3, low Apgar score, and poor neurological development. These results differ from those of previous reports (25–27). There were only one IVH grade 3 case and small population in our study, so a false negative could be possible. Duration of oxygen supplementation and duration of NICU stay, rates of RDS, NEC, and mechanical ventilation were significantly higher in the poor outcome group than the good outcome group. These results were consistent with those of previous reports (8, 28, 29) and considered to be strongly associated with early delivery weeks of gestation and low birth weight.
Table 3 shows that delivery at <30 weeks of gestation and PE complicated with maternal renal dysfunction were independent risk factors for poor neurological development. However, the association between the delivery week and a poor neurological outcome was previously reported (1, 2). The most meaningful and new detection of our study is that PE complicated with maternal renal dysfunction was associated with poor neurological development in preterm birth infants.
Table 4 shows the findings related to neurological impairment assessed in 1.5–11.5-year-olds, antecedently reported in offspring born to mothers with hypertensive disorder in pregnancy (HDP). Offspring whose mothers develop PE (PE-F1s) exhibit decreased cognitive function. PE was identified as a risk factor and has been associated with cognitive impairment in some studies (Nos. 1, 2, 4–14, and 16) (30, 31, 33–42, 44, 45).
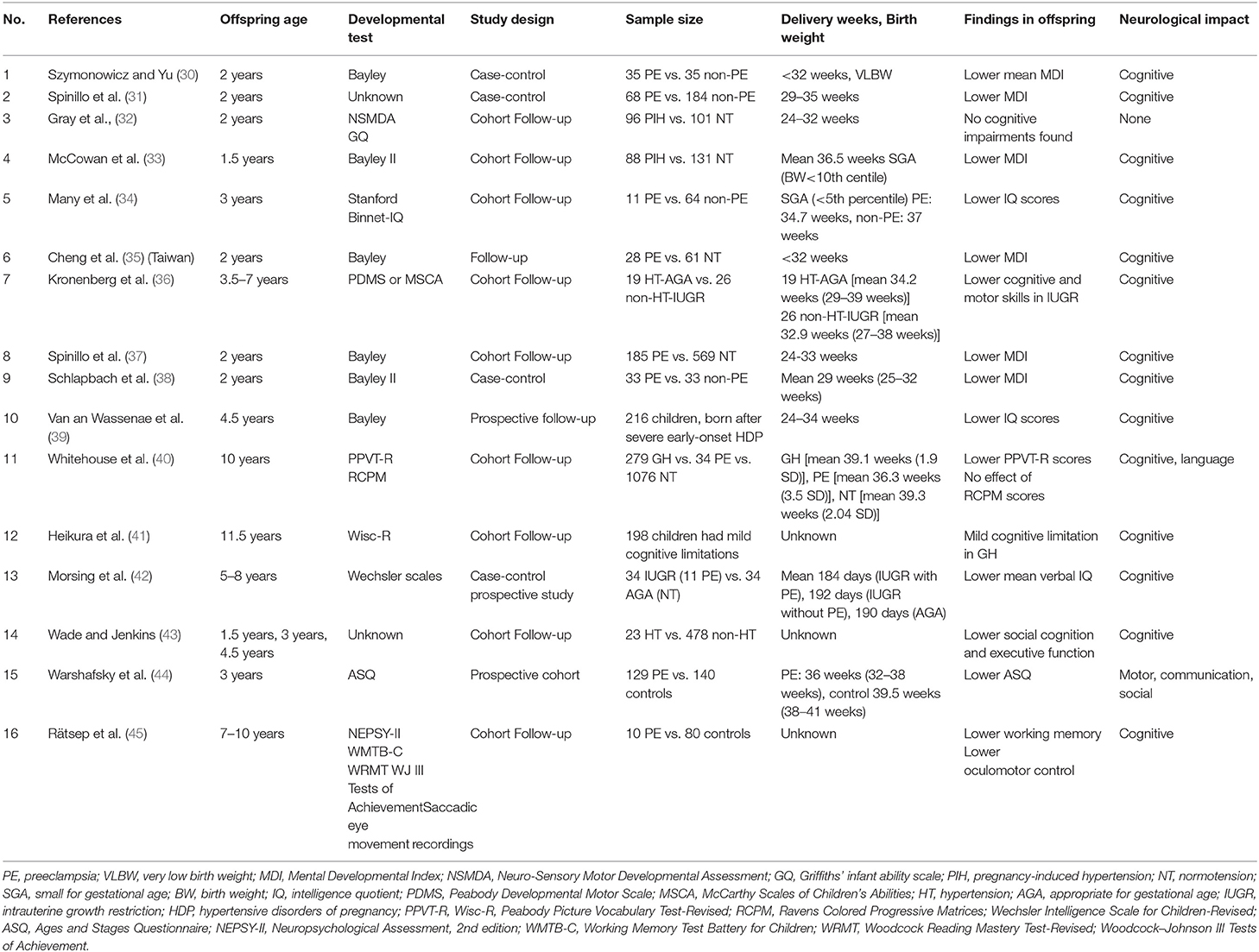
Table 4. Findings related to neurological impairment previously reported in offspring born to mothers with hypertensive disorder in pregnancy.
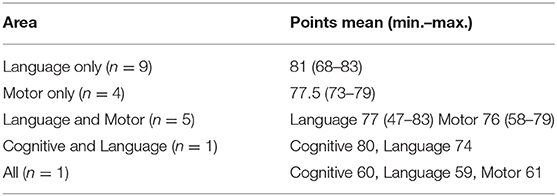
In our study, cognitive and language functions decreased in one case. Cognitive, language, and motor functions decreased in one case. Only language function decreased in nine cases, and only motor function decreased in four cases (Table 5). In our study, there were only two cases of cognitive decline, with the highest rate of decrease in language function. In cases of a poor neurological outcome complicated with maternal renal dysfunction (n = 3), one case showed a decrease only in the language function by Bayley-III, one case showed decreased language and motor functions by Bayley-III, and one case showed decreases in all areas by Kyoto Scale of Psychological Development.
Three published reports (Nos. 5, 14, and 15) involved patients at the same age of 3 years old as in our study; two (Nos. 5 and 14) involved a cognitive decline; and one (No. 15) involved a motor, communication, or social decrease (34, 43, 44). Other reports examined a wide age range, from 1.5 to 11.5 years old, and the neurodevelopment test methods differ, making it difficult to compare with our study.
Figueiró-Filho et al. (46) reviewed 27 out of 464 studies reporting on the neurological development of offspring born to HDP mothers. The current studies support the behavioral and cognitive deviations in offspring whose mothers developed PE. Figueiró-Filho et al. (46) demonstrated the relation between mechanisms of PE and physiology of placenta and neurological development. From first to third trimesters of pregnancy, PE causes disorders along with cardiovascular, immune, metabolic, etc. and dysfunction of placenta such as placentation, immune tolerance, and then lead to neurological developmental disorders. PE may lead to breakdown such as hypoxia, inflammation, etc. and influence physiology and neurological development. Some knockout models of angiogenesis such as placental growth factor (PlGF) show dysfunction of angiogenesis. These knockout models indicate neurodevelopmental disorders of children of PE mothers.
The most meaningful and new detection of our study is that PE complicated with maternal renal dysfunction is related to poor neurological development in children at 3 years.
However, there has been no study on infants' neurological development focused on PE with maternal renal dysfunction. PE is related to renal dysfunction owing to deficiency in podocyte-specific VEGF (47). Placental hypoxic condition in PE cases causes elevated levels of fms-like tyrosine kinase 1 (sFlt-1) and a soluble receptor of VEGF. An increased level of sFlt-1 binds to VEGF and PlGF, which are important angiogenesis and vasculogenesis factors, resulting in endothelial dysfunction in various organs, including the kidney (48). A recent study demonstrated the effects of PlGF deficiency on the behavior, neuroanatomy, and cerebrovasculature of mice (49).
The specific character of sFlt-1 in adapting neuroanatomical and vascular construction and circulatory characteristics at the central nervous system level are unclear (50). sFlt-1 high levels are related to adult psychiatric and neurological alterations (51, 52). Vogtmann et al. (53) demonstrated that human sFlt-1 arrests placental differentiation, exclusively arresting fetal capillary branching by decreasing VEGF signaling and inducing apoptosis in fetal embryonic stem cells. This arrest could lead to a stagnation of maternal blood, improving dilatation of the maternal sinusoids according to damage of the fetal vessel system. The changed morphology of placenta finally results in insufficiency of utero and placenta, containing decreased nutrient transport (chiefly influencing the fatty acid supply) and induction of FGR (53).
High sFlt-1 levels are considered to cause glomerular damage, proteinuria, and renal damage. In cases of PE complicated with renal dysfunction, hyperproteinemia can lead to malnutrition of the infant and neurological adverse effects as a result of systemic vascular endothelial dysfunction.
Barker et al. (13) showed the differentiation of risk factors for adult diseases after adulthood depending on the stage of pregnancy when the mother was exposed to starvation and those born to mothers who are underweight at birth are at higher risk of developing cardiovascular disease, diabetes, and hypertension. A mechanism exists in the fetus to adapt (e.g., expression of a thrifty gene) to environmental stimuli (insufficient supply of nutrients from the placenta to the fetus due to malnutrition of the mother) in each organ and tissue, and this program also exists in adulthood. It is hypothesized that it increases the risk of developing the disease (54, 55).
Fetal growth in the uterus is by cell division and proliferation in all tissues, and undernutrition during pregnancy leads to fetal organs and placenta showing low cell numbers (56). From this experiment, fetal undernutrition, low protein nutrition, and early postnatal malnutrition may cause brain damage.
Offspring of mothers with PE complicated with maternal renal dysfunction, hyperproteinemia, and undernutrition may show a poor neurodevelopmental outcome as preterm infants.
Strengths and Limitations
This study is the first report to show the relationship between PE with maternal renal dysfunction and infants' poor neurological development.
The strengths of the present study include neurological development being evaluated by Bayley-III in Japan. In Japan, there have been only seven studies about neurodevelopment of infants assessed by Bayley-II or III (57–63). However, there has been only one study on preterm infants among these reports (63). A previous study compared 23 low-birth weight infants diagnosed with PVL (median gestational age: 30 weeks) with 209 normal-weight infants (gestational age range: 25–39 weeks). In this study, we reported the neurological development of Japanese preterm infants evaluated by Bayley Scales of Infants and Toddler Development for the first time.
Major limitations of our study are retrospective study design and small sample size in a single institution. Also, this study did not involve cases of severe developmental delay, only relatively mild developmental delay. Only 91 cases (55.2%) out of 165 included infants were evaluated with the Bayley-III method as the selected tool to assess the neurodevelopmental outcome. There is attrition bias. Ideally, all cases should have Bayley-III assessment at 3 years old. However, rest of all cases (73 cases, 44.2% out of 165 cases) were followed up until the neonatologists diagnose they are normally developed and instructed to see a neonatologist/pediatrician in our hospital if they had developmental problems at health checkup at 3 years old. Our data did not include toxic exposure such as NICU noise and social deprivation such as poor socioeconomic status, so there is a relevant bias. Bayley-III was originally developed by American norms, so there could be a difference between Japan norms and American norms.
Conclusion
Offspring of mothers with PE complicated with maternal renal dysfunction required long-term neurodevelopmental follow-up.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by The Institutional Review Board of Toyama University Hospital. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author Contributions
NY: conception of the work, analysis, interpretation, and writing this paper. SY, ST, MI, AS, HN, TY, and AN: contribution to the design of the work, revising it critically for important intellectual content, agreement to be acccountable for all aspects of the work. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by JSPS KAKENHI [a Grant-in-Aid for Scientific Research (C), 20K09663 to NY and HN, Grand-in-Aid for Clinical and Scientific Research to NY] from the University of Toyama.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We appreciate the funding support, a grant for Clinical and Scientific Research from the University of Toyama. The authors are grateful to Tae Kusabiraki, Jun Inasaka, Mutsumi Kobayashi, Ippei Yasuda, and Sho Soeda for data collection.
References
1. Boyle EM, Poulsen G, Field DJ, Kurinczuk JJ, Wolke D, Alfirevic Z, et al. Effects of gestational age at birth on health outcomes at 3 and 5 years of age: population based cohort study. BMJ. (2012) 344:e896. doi: 10.1136/bmj.e896
2. Poulsen G, Wolke D, Kurinczuk JJ, Boyle EM, Field D, Alfirevic Z, et al. Gestational age and cognitive ability in early childhood: a population-based cohort study. Paediatr Perinat Epidemiol. (2013) 27:371–9. doi: 10.1111/ppe.12058
3. El Ayoubi M, Patkai J, Bordarier C, Desfrere L, Moriette G, Jarreau PH, et al. Impact of fetal growth restriction on neurodevelopmental outcome at 2 years for extremely preterm infants: a single institution study. Dev Med Child Neurol. (2016) 58:1249–56. doi: 10.1111/dmcn.13218
4. Guellec I, Lapillonne A, Marret S, Picaud J-C, Mitanchez D, Charkaluk M-L, et al. Effect of intra-and extrauterine growth on long-term neurologic outcomes of very preterm infants. J Pediatr. (2016) 175:93–9. e1. doi: 10.1016/j.jpeds.2016.05.027
5. Harry GJ, Lawler C, Brunssen SH. Maternal infection and white matter toxicity. Neurotoxicology. (2006) 27:658–70. doi: 10.1016/j.neuro.2006.05.004
6. Brodowski L, Büter W, Kohls F, Hillemanns P, von Kaisenberg C, Dammann O. Maternal overweight, inflammation and neurological consequences for the preterm child: results of the ELGAN study. Geburtshilfe Frauenheilkd. (2019) 79:1176. doi: 10.1055/a-0960-0939
7. Johnson S, Evans TA, Draper ES, Field DJ, Manktelow BN, Marlow N, et al. Neurodevelopmental outcomes following late and moderate prematurity: a population-based cohort study. Arch Dis Child Fetal Neonatal Ed. (2015) 100:F301–8. doi: 10.1136/archdischild-2014-307684
8. Latal B. Neurodevelopmental outcomes of the child with congenital heart disease. Clin Perinatol. (2016) 43:173–85. doi: 10.1016/j.clp.2015.11.012
9. Brown G. NICU noise and the preterm infant. Neonat Netw. (2009) 28:165–73. doi: 10.1891/0730-0832.28.3.165
10. Hakim J, Senterman MK, Hakim AM. Preeclampsia is a biomarker for vascular disease in both mother and child: the need for a medical alert system. Int J Pediatr. (2013) 2013:1–8. doi: 10.1155/2013/953150
11. Davis EF, Newton L, Lewandowski AJ, Lazdam M, Kelly BA, Kyriakou T, et al. Pre-eclampsia and offspring cardiovascular health: mechanistic insights from experimental studies. Clin Sci. (2012) 123:53–72. doi: 10.1042/CS20110627
12. Tuovinen S, Eriksson JG, Kajantie E, Räikkönen K. Maternal hypertensive pregnancy disorders and cognitive functioning of the offspring: a systematic review. J Am Soc Hypertens. (2014) 8:832–47.e1. doi: 10.1016/j.jash.2014.09.005
13. Barker DJ. Maternal nutrition, fetal nutrition, and disease in later life. Nutrition. (1997) 13:807–13. doi: 10.1016/S0899-9007(97)00193-7
14. Tsumori M, Inage N, Isobe K. Assessment of Infant and Child Mental Development. Tokyo: Dainihonn-tosho-kabusikigaisya (1961).
15. Koyama T, Osada H, Tsujii H, Kurita H. Utility of the Kyoto Scale of Psychological Development in cognitive assessment of children with pervasive developmental disorders. Psychiatry Clin Neurosci. (2009) 63:241–3. doi: 10.1111/j.1440-1819.2009.01931.x
16. Bayley N. Bayley Scales of Infant and Toddler Development®. 3rd ed. (Bayley-III®). San Antonio, TX: The Psychological Corporation (2006).
17. Johnson S, Moore T, Marlow N. Using The Bayley-III to assess neurodevelopmental delay: which cut-off should be used? Pediatr Res. (2014) 75:670–4. doi: 10.1038/pr.2014.10
18. Chalak LF, Dupont TL, Sánchez PJ, Lucke A, Heyne RJ, Morriss MC, et al. Neurodevelopmental outcomes after hypothermia therapy in the era of Bayley-III. J Perinatol. (2014) 34:629–33. doi: 10.1038/jp.2014.67
19. O'connor DL, Gibbins S, Kiss A, Bando N, Brennan-Donnan J, Ng E, et al. Effect of supplemental donor human milk compared with preterm formula on neurodevelopment of very low-birth-weight infants at 18 months: a randomized clinical trial. JAMA. (2016) 316:1897–1905. doi: 10.1001/jama.2016.16144
20. Acton BV, Biggs WS, Creighton DE, Penner KA, Switzer HN, Thomas JHP, et al. Overestimating neurodevelopment using the Bayley-III after early complex cardiac surgery. Pediatrics. (2011) 128:e794–e800. doi: 10.1542/peds.2011-0331
21. Blanc W. Pathology of the placenta, membranes and umbilical cord in bacterial infections in man. Perinat Dis. (1981) 22:167–132.
22. Lencki SG, Maciulla MB, Eglinton GS. Maternal and umbilical cord serum interleukin levels in preterm labor with clinical chorioamnionitis. Am J Obstetr Gynecol. (1994) 170:1345–51. doi: 10.1016/S0002-9378(94)70154-7
24. Takagi K, Yamasak M, Nakamoto O, Saito S, Suzuki H, Seki H, et al. A review of best practice guide 2015 for care and treatment of hypertension in pregnancy. Hypertens Res Pregnancy. (2015) 3:65–103. doi: 10.14390/jsshp.3.105
25. Calisici E, Eras Z, Oncel MY, Oguz SS, Gokce IK, Dilmen U. Neurodevelopmental outcomes of premature infants with severe intraventricular hemorrhage. J Matern Fetal Neonat Med. (2015) 28:2115–20. doi: 10.3109/14767058.2014.979783
26. Brouwer A, Groenendaal F, Van Haastert I-L, Rademaker K, Hanlo P, De Vries L. Neurodevelopmental outcome of preterm infants with severe intraventricular hemorrhage and therapy for post-hemorrhagic ventricular dilatation. J Pediatr. (2008) 152:648–54. doi: 10.1016/j.jpeds.2007.10.005
27. Badr LK, Bookheimer S, Purdy I, Deeb M. Predictors of neurodevelopmental outcome for preterm infants with brain injury: MRI, medical and environmental factors. Early Hum Dev. (2009) 85:279–84. doi: 10.1016/j.earlhumdev.2008.11.005
28. Shah DK, Doyle LW, Anderson PJ, Bear M, Daley AJ, Hunt RW, et al. Adverse neurodevelopment in preterm infants with postnatal sepsis or necrotizing enterocolitis is mediated by white matter abnormalities on magnetic resonance imaging at term. J Pediatr. (2008) 153:170–5. e1. doi: 10.1016/j.jpeds.2008.02.033
29. Coletti MF, Caravale B, Gasparini C, Franco F, Campi F, Dotta A. One-year neurodevelopmental outcome of very and late preterm infants: risk factors and correlation with maternal stress. Infant Behav Dev. (2015) 39:11–20. doi: 10.1016/j.infbeh.2015.01.003
30. Szymonowicz W, Yu V. Severe pre-eclampsia and infants of very low birth weight. Arch Dis Child. (1987) 62:712–6. doi: 10.1136/adc.62.7.712
31. Spinillo A, Iasci A, Capuzzo E, Egbe TO, Colonna L, Fazzi E. Two-year infant neurodevelopmental outcome after expectant management and indicated preterm delivery in hypertensive pregnancies. Acta Obstet Gynecol Scand. (1994) 73:625–9. doi: 10.3109/00016349409013455
32. Gray PH, O'Callaghan MJ, Mohay HA, Burns YR, King JF. Maternal hypertension and neurodevelopmental outcome in very preterm infants. Arch Dis Child Fetal Neonatal Ed. (1998) 79:F88–93. doi: 10.1136/fn.79.2.f88
33. Mccowan LM, Pryor J, Harding JE. Perinatal predictors of neurodevelopmental outcome in small-for-gestational-age children at 18 months of age. Am J Obstetr Gynecol. (2002) 186:1069–75. doi: 10.1067/mob.2002.122292
34. Many A, Fattal A, Leitner Y, Kupferminc MJ, Harel S, Jaffa A. Neurodevelopmental and cognitive assessment of children born growth restricted to mothers with and without preeclampsia. Hypertens Pregnancy. (2003) 22:25–9. doi: 10.1081/PRG-120016791
35. Cheng S-W, Chou H-C, Tsou K-I, Fang L-J, Tsao P-N. Delivery before 32 weeks of gestation for maternal pre-eclampsia: neonatal outcome and 2-year developmental outcome. Early Hum Dev. (2004) 76:39–46. doi: 10.1016/j.earlhumdev.2003.10.004
36. Kronenberg ME, Raz S, Sander CJ. Neurodevelopmental outcome in children born to mothers wit hypertension in pregnancy: the significance of suboptimal intrauterine growth. Dev Med Child Neurol. (2006) 48:200–6. doi: 10.1017/S0012162206000430
37. Spinillo A, Montanari L, Gardella B, Roccio M, Stronati M, Fazzi E. Infant sex, obstetric risk factors, and 2-year neurodevelopmental outcome among preterm infants. Dev Med Child Neurol. (2009) 51:518–25. doi: 10.1111/j.1469-8749.2009.03273.x
38. Schlapbach LJ, Ersch J, Adams M, Bernet V, Bucher HU, Latal B. Impact of chorioamnionitis and preeclampsia on neurodevelopmental outcome in preterm infants below 32 weeks gestational age. Acta Paediatr. (2010) 99:1504–9. doi: 10.1111/j.1651-2227.2010.01861.x
39. Van Wassenaer AG, Westera J, Van Schie PE, Houtzager BA, Cranendonk A, De Groot L, et al. Outcome at 4.5 years of children born after expectant management of early-onset hypertensive disorders of pregnancy. Am J Obstet Gynecol. (2011) 204:510.e1–510.e9. doi: 10.1016/j.ajog.2011.02.032
40. Whitehouse AJ, Robinson M, Newnham JP, Pennell CE. Do hypertensive diseases of pregnancy disrupt neurocognitive development in offspring? Paediatr Perinat Epidemiol. (2012) 26:101–8. doi: 10.1111/j.1365-3016.2011.01257.x
41. Heikura U, Hartikainen A-L, Nordström T, Pouta A, Taanila A, Marjo-Riitta J. Maternal hypertensive disorders during pregnancy and mild cognitive limitations in the offspring. Paediatr Perinat Epidemiol. (2013) 27:188–98. doi: 10.1111/ppe.12028
42. Morsing E, Maršál K. Pre-eclampsia—an additional risk factor for cognitive impairment at school age after intrauterine growth restriction and very preterm birth. Early Hum Dev. (2014) 90:99–101. doi: 10.1016/j.earlhumdev.2013.12.002
43. Wade M, Jenkins JM. Pregnancy hypertension and the risk for neuropsychological difficulties across early development: a brief report. Child Neuropsychol. (2016) 22:247–54. doi: 10.1080/09297049.2014.958070
44. Warshafsky C, Pudwell J, Walker M, Wen S-W, Smith GN. Prospective assessment of neurodevelopment in children following a pregnancy complicated by severe pre-eclampsia. BMJ Open. (2016) 6:1–7. doi: 10.1136/bmjopen-2015-010884
45. Rätsep MT, Hickman AF, Maser B, Pudwell J, Smith GN, Brien D, et al. Impact of preeclampsia on cognitive function in the offspring. Behav Brain Res. (2016) 302:175–81. doi: 10.1016/j.bbr.2016.01.030
46. Figueiró-Filho EA, Croy BA, Reynolds JN, Dang F, Piro D, Rätsep M, et al. Diffusion tensor imaging of white matter in children born from preeclamptic gestations. Am J Neuroradiol. (2017) 38:801–6. doi: 10.3174/ajnr.A5064
47. Sani HM, Vahed SZ, Ardalan M. Preeclampsia: a close look at renal dysfunction. Biomed Pharmacother. (2019) 109:408–16. doi: 10.1016/j.biopha.2018.10.082
48. Jardim LL, Rios DRA, Perucci LO, De Sousa LP, Gomes KB, Dusse LMS. Is the imbalance between pro-angiogenic and anti-angiogenic factors associated with preeclampsia? Clin Chim Acta. (2015) 447:34–8. doi: 10.1016/j.cca.2015.05.004
49. Kay VR, Rätsep MT, Cahill LS, Hickman AF, Zavan B, Newport ME, et al. Effects of placental growth factor deficiency on behavior, neuroanatomy, and cerebrovasculature of mice. Physiol Genomics. (2018) 50:862–75. doi: 10.1152/physiolgenomics.00076.2018
50. Lara E, Acurio J, Leon J, Penny J, Torres-Vergara P, Escudero C. Are the cognitive alterations present in children born from preeclamptic pregnancies the result of impaired angiogenesis? Focus on the potential role of the VEGF family. Front Physiol. (2018) 9:1591. doi: 10.3389/fphys.2018.01591
51. Emanuele E, Orsi P, Barale F, Di Nemi SU, Bertona M, Politi P. Serum levels of vascular endothelial growth factor and its receptors in patients with severe autism. Clin Biochem. (2010) 43:317–9. doi: 10.1016/j.clinbiochem.2009.10.005
52. Pillai A, Howell K, Ahmed A, Weinberg D, Allen K, Bruggemann J, et al. Association of serum VEGF levels with prefrontal cortex volume in schizophrenia. Mol Psychiatry. (2016) 21:686–92. doi: 10.1038/mp.2015.96
53. Vogtmann R, Kuhnel E, Dicke N, Verkaik-Schakel RN, Plösch T, Schorle H, et al. Human sFLT1 leads to severe changes in placental differentiation and vascularization in a transgenic hsFLT1/rtTA FGR mouse model. Front Endocrinol. (2019) 10:165. doi: 10.3389/fendo.2019.00165
54. Barker DJ, Osmond C, Winter P, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet. (1989) 334:577–80. doi: 10.1016/S0140-6736(89)90710-1
55. Godfrey KM, Barker DJ. Fetal nutrition and adult disease. Am J Clin Nutr. (2000) 71:1344S−52S. doi: 10.1093/ajcn/71.5.1344s
57. Nakajima S, Saijo Y, Miyashita C, Ikeno T, Sasaki S, Kajiwara J, et al. Sex-specific differences in effect of prenatal exposure to dioxin-like compounds on neurodevelopment in Japanese children: sapporo cohort study. Environ Res. (2017) 159:222–31. doi: 10.1016/j.envres.2017.08.006
58. Minatoya M, Sasaki S, Araki A, Miyashita C, Ikeno T, Nakajima T, et al. Effects of prenatal phthalate exposure on thyroid hormone levels, mental and psychomotor development of infants: the Hokkaido Study on Environment and Children's Health. Sci Total Environ. (2016) 565:1037–43. doi: 10.1016/j.scitotenv.2016.05.098
59. Goudarzi H, Nakajima S, Ikeno T, Sasaki S, Kobayashi S, Miyashita C, et al. Prenatal exposure to perfluorinated chemicals and neurodevelopment in early infancy: The Hokkaido Study. Sci Total Environ. (2016) 541:1002–10. doi: 10.1016/j.scitotenv.2015.10.017
60. Shoji H, Ikeda N, Hosozawa M, Ohkawa N, Matsunaga N, Suganuma H, et al. Oxidative stress early in infancy and neurodevelopmental outcome in very low-birthweight infants. Pediatr Int. (2014) 56:709–13. doi: 10.1111/ped.12332
61. Kon N, Tanaka K, Sekigawa M, Negishi Y, Yoshikawa N, Hisata K, et al. Association between iron status and neurodevelopmental outcomes among VLBW infants. Brain Dev. (2010) 32:849–54. doi: 10.1016/j.braindev.2009.12.003
62. Matsuzaki T, Matsui M, Ichida F, Nakazawa J, Hattori A, Yoshikosi K, et al. Neurodevelopment in 1-year-old Japanese infants after congenital heart surgery. Pediatr Int. (2010) 52:420–7. doi: 10.1111/j.1442-200X.2009.02974.x
Keywords: maternal renal dysfunction, neurological development, pre-eclampsia, preterm delivery, small for gestational age
Citation: Yoneda N, Yoneda S, Tsuda S, Ito M, Shiozaki A, Niimi H, Yoshida T, Nakashima A and Saito S (2021) Pre-eclampsia Complicated With Maternal Renal Dysfunction Is Associated With Poor Neurological Development at 3 Years Old in Children Born Before 34 Weeks of Gestation. Front. Pediatr. 9:624323. doi: 10.3389/fped.2021.624323
Received: 31 October 2020; Accepted: 18 March 2021;
Published: 29 April 2021.
Edited by:
Kamran Yusuf, University of Calgary, CanadaReviewed by:
Katrin Klebermass-Schrehof, Medical University of Vienna, AustriaSilvia Salvatore, University of Insubria, Italy
Copyright © 2021 Yoneda, Yoneda, Tsuda, Ito, Shiozaki, Niimi, Yoshida, Nakashima and Saito. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shigeru Saito, czMwc2FpdG9AbWVkLnUtdG95YW1hLmFjLmpw
 Noriko Yoneda1
Noriko Yoneda1 Akitoshi Nakashima
Akitoshi Nakashima Shigeru Saito
Shigeru Saito