
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr., 07 July 2021
Sec. Pediatric Cardiology
Volume 9 - 2021 | https://doi.org/10.3389/fped.2021.612644
 Ioana-Cristina Olariu1,2
Ioana-Cristina Olariu1,2 Anca Popoiu1,2
Anca Popoiu1,2 Andrada-Mara Ardelean1,2
Andrada-Mara Ardelean1,2 Raluca Isac1,2
Raluca Isac1,2 Ruxandra Maria Steflea1
Ruxandra Maria Steflea1 Tudor Olariu3
Tudor Olariu3 Adela Chirita-Emandi4,5*
Adela Chirita-Emandi4,5* Ramona Stroescu1,2
Ramona Stroescu1,2 Mihai Gafencu1,2
Mihai Gafencu1,2 Gabriela Doros1,2
Gabriela Doros1,2Background: Atrioventricular septal defect (AVSD) is a cardiac malformation that accounts for up to 5% of total congenital heart disease, occurring with high frequency in people with Down Syndrome (DS). We aimed to establish the surgical challenges and outcome of medical care in different types of AVSD in children with DS compared to those without DS (WDS).
Methods: The study included 62 children (31 with DS) with AVSD, evaluated over a 5 year period.
Results: Complete AVSD was observed in 49 (79%) children (27 with DS). Six children had partial AVSD (all WDS) and seven had intermediate types of AVSD (4 with DS). Eight children had unbalanced complete AVSD (1 DS). Median age at diagnosis and age at surgical intervention in complete AVSD was not significantly different in children with DS compared to those WDS (7.5 months vs. 8.6). Median age at surgical intervention for partial and transitional AVSDs was 10.5 months for DS and 17.8 months in those without DS. A large number of patients were not operated: 13/31 with DS and 8/31 WDS.
Conclusion: The complete form of AVSD was more frequent in DS group, having worse prognosis, while unbalanced AVSD was observed predominantly in the group without DS. Children with DS required special attention due to increased risk of pulmonary hypertension. Late diagnosis was an important risk factor for poor prognosis, in the setting of suboptimal access to cardiac surgery for patients in Romania. Although post-surgery mortality was low, infant mortality before surgery remains high. Increased awareness is needed in order to provide early diagnosis of AVSD and enable optimal surgical treatment.
Atrioventricular septal defect (AVSD) or endocardial cushion defect is a cardiac malformation characterized by a variable deficiency of the crux cordis in the developing heart. AVSD accounts for 4–5% of total congenital heart diseases (1). The International Pediatric and Congenital Cardiac Code classified AVSDs into four main groups: complete, partial (or incomplete), intermediate (or transitional), and AVSDs with ventricular imbalance (unbalanced AVSD) (2). Most AVSDs are complete (up to 75%), and account for 3% of all cardiac malformations (2, 3).
Numerous recent studies contributed to a better understanding of genetic causes in congenital heart diseases (4–6). AVSD has been observed in patients with Down syndrome (DS), left or right isomerism, Noonan Syndrome and other (7, 8). Almost half (40–45%) of children with DS have congenital heart diseases. Of these, 35–40% have AVSD (9, 10). The prevalence of complete AVSD is 0.3–0.5% of the populations without DS (WDS). The complete form of AVSD is more frequently associated with additional cardiac defects, left-sided obstructive lesions being more prevalent in children WDS (11, 12). Complete AVSD is observed in almost all patients with right isomerism and in 25% of those with left isomerism (3). The partial forms of AVSD are less frequently associated with chromosomal anomalies (13). A study by Marino Bruno et al. showed that Noonan syndrome is associated with an increased risk for partial AVSD (14). Nonetheless, AVSD can also be non-syndromic (15).
AVSD has major health implications due to the significant interatrial and interventricular systemic-pulmonary shunt leading to increased pressure in the right ventricle with volume overloading, pulmonary hypertension (PAH), heart failure and consequent failure to thrive (16–18). Therefore, precocious diagnosis and surgical treatment is crucial, in order to avoid irreversible complications. Ideally, surgical treatment of complete AVSD should be performed before the age of 6 months (1, 18, 19). In partial and transitional AVSDs surgical treatment may be delayed until the age of 1.6 years or later, if the malformation is well-tolerated (20, 21). Major risk factors for development of PAH in patients with AVSD include presence of DS and age at surgical intervention (3, 17). Because AVSD is common in DS and the onset of PAH in DS is earlier, these patients need special medical management.
We aimed to evaluate the age at diagnosis, age at the time of surgery, and postoperative evolution, in children with AVSD and DS compared to those WDS, in order to understand if specific management is warranted.
The study included 62 children diagnosed with AVSD, admitted in a tertiary clinical care center, the “Louis Turcanu” Emergency Hospital for Children, Timişoara, Romania, between 2014 and 2019. Most children were living in the western Romania, yet some were referred from other regions of the country. We compared children with DS and those WDS, in regards to type of AVSD, age at diagnosis, age at the time of surgery and evolution. AVSDs diagnosed prenatally where termination of pregnancy was chosen were not included in the study.
The echocardiografic examination is the cornerstone for diagnosis and types of AVSDs have been classified according to the International Pediatric and Congenital Cardiac Code as follows: complete AVSDs include an ostium primum defect with a non-restrictive inlet ventricular defect and a common atrioventricular valve orifice. Partial AVSDs have an isolated ostium primum defect and partitioning of the common atrioventricular valve into two separate atrioventricular orifices (cleft in the mitral valve). Intermediate or transitional AVSDs consists of an ostium primum defect in association with a restrictive inlet ventricular defect and two valvular orifices. Unbalanced AVSD is defined as malalignment of the atrioventricular valve, being unequal for both ventricles, resulting in one ventricle receiving more blood flow and the other ventricle being underdeveloped (hypoplastic) (2).
The retrospective study included the evaluation of medical records, chest radiography, 12-lead electrocardiogram (ECG), echocardiograms, intraoperative findings, and surgical outcomes of all patients with AVSD. PAH was defined as a pressure gradient estimated by Doppler on tricuspid valve regurgitation over 30 mmHg or a systolic pulmonary artery pressure of more than half systolic systemic pressure. In children presenting severe cardiac failure, blood cardiac biomarkers (troponins and N-terminal pro b-type Natriuretic Peptide NT-pro BNP) were evaluated.
The cardiac diagnoses were presented in accordance to the classification of the International Pediatric and Congenital Cardiac Code (2). The statistical analysis is descriptive. Data was analyzed using IBM-SPSS version 25 (IBM, Armonk, New York, U.S.A.). Shapiro-Wilk test was used to detect normal distribution. As variables were non-normally distributed, data is presented as median with interquartile range (IQR). Mann-Whitney test was used in continuous variables studied and Chi Square test for categorical variables to evaluate statistical significance of differences between the two groups.
As the study analyzed data that was routinely collected as part of the clinical care of the patient, the study did not require informed consent and was approved as an evaluation of health care delivery. Data were obtained by review of files and electronic medical records. Patient anonymity was preserved throughout.
Between 2014 and 2019, 62 patients diagnosed with AVSD were evaluated in our tertiary pediatric hospital. Thirty-one children were also diagnosed with DS, while thirty-one were WDS. The male-to-female distribution of AVSD was approximately equal. The male-to female ratio was: in DS 1:1.2 (14:17), in WDS 1.06:1 (16:15). Regarding the types of AVSD, 49 of 62 children had complete AVSD (27 DS, 22 WDS), 6 had partial AVSD (all WDS) and 7 had intermediate AVSD (4 DS, 3 WDS). Among the 49 children with complete AVSD, 8 presented unbalanced ventricles (1 DS, 7 WDS).
Age at diagnosis ranged between 1 day and 15 years (details presented in Table 1). In DS group median age at diagnosis was 7.0 days (IQR, 1 day−31 months) and 10.5 days (IQR, 1 day−7 months) in WDS group, p = 0.805 (Mann Whitney). Of all patients, 39 were diagnosed before 6 weeks of age (20 DS, 19 WDS). Eleven patients with AVSD were diagnosed after 1 year of age, 5 with DS (all complete AVSDs), and 6 WDS (2 complete AVSD, 3 partial and 1 transitional AVSD). Only 4 patients were diagnosed with AVSD before birth (1 DS, 3 WDS).
AVSD was associated with other cardiac (presented in Table 2) and extracardiac anomalies (presented in Table 3). In DS group 19 patients (61.3%) were found with other cardiac anomalies, in WDS group 20 (64%). ASD ostium secundum (13 vs. 6), multiple VSD (2 vs. 0), and Tetralogy of Fallot (3 vs. 0) were the most frequent in DS group, while severe cardiac anomalies such as unbalanced ventricles (1 vs. 7), double-outlet right ventricle (0 vs. 7), aortic stenosis (1 vs. 4) were more frequent among patients WDS. None of partial or transitional AVSD had unbalanced ventricles. Left side obstructive lesions were found in two children with partial or transitional AVSD, these being from the group WDS.
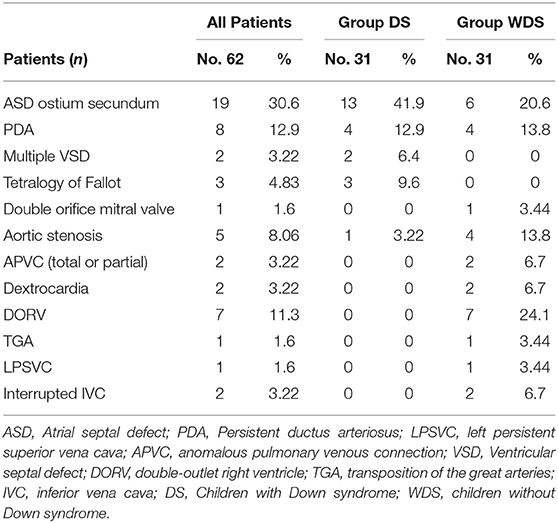
Table 2. Incidence of other cardiovascular anomalies in 62 patients with atrioventricular septal defect.
Extracardiac abnormalities were found in 25.8% (8/31) patients from DS group and 32.25% (10/31) patients in those without DS (presented in Table 3). Some of them had multiple malformations. Left or right isomerism was associated with AVSD in 8 patients, all from WDS group. One child diagnosed with AVSD, presented a complex cardiac phenotype (Tetralogy of Fallot) and a rare double aneuploidy (Down and Klinefelter syndrome, 48, XXY, +21 karyotype). He died at 7 months due to pulmonary infection.
At physical examination all children with AVSD presented systolic murmur (grade 2/6 to 6/6). Half of the children with AVSD presented signs of heart failure such as: dyspnea, feeding difficulties, poor growth, tachycardia, tachypnea, subcostal and intercostal retractions, wheezing, hepatic enlargement, poor peripheral blood perfusion, and diaphoresis. Three of them had pulmonary edema: one from DS group and two from the WDS group, all of them were under 6 months of age. Patients with AVSD had feeding problems and presented symptoms in the first few months of life. Failure to thrive was a common finding, 27 patients (43%) had malnutrition (<Percentile 5 on World Health Organization growth references) before surgical correction: 15 from group DS (12 with complete AVSD and 3 with transitional AVSD), 12 WDS (8 with complete AVSD, 2 with partial form and 2 with transitional AVSD).
Electrocardiograms (12-lead) showed sinus rhythm, left-axis-deviation (44/62, 71%), prominent P waves due to atrial enlargement, and signs of possible biventricular hypertrophy. Prolonged PR interval were detected in 6 children at diagnosis, 3 with complete AVSD, 2 partial and 1 transitional AVSD. Chest X-ray showed enlarged cardiac silhouette and increased pulmonary vascular markings in almost all children. The cardiomegaly was mild in partial AVSDs and significant in complete AVSDs.
Echocardiography showed the atrial and ventricular components of the atrioventricular septal defect with medium and large ostium primum defect in 53 children (23 DS, 30 WDS), while 29 patients had large inlet ventricular defect (>6 mm). Of them, 19 were from DS-group (17 with complete AVSD and 2 transitional AVSD) and 10 were from WDS group (8 complete and 2 transitional AVSDs).
PAH before surgery was identified in 34 children with AVSD (56%), 61% from the DS group. Moderate PAH was identified in 31% of children (12 DS, 7 WDS), while 24% (7 DS, 8 WDS) had severe PAH. One patient from DS group that was diagnosed late with AVSD presented Eisenmenger disease. Cardiac catheterization was performed in those with late diagnosis in order to establish the severity of PAH.
Cardiac biomarkers were determined in patients with complex AVSD and heart failure. Half of children with AVSD presented heart failure (16 DS, 18 WDS). Pathological values of NT-pro BNP and troponins were detected in patients with severe cardiac involvement (5 DS, 8 WDS). NT-pro BNP values were elevated, up to 50 times higher than normal, proportional to the degree of heart failure. Values of NT-pro BNP ranged from 207.5 to 17,482 pg/ml with a mean of 4,912 pg/ml.
Of the 62 children with AVSD, 41 had cardiac surgery. Of them, 18 patients were in the DS group and 23 in WDS group (Table 4). Median age at surgical intervention in complete AVSD was 7.5 months in DS group and 8.6 months in WDS (p = 0.764). Median age at surgical intervention for partial and transitional AVSDs was 10.5 months (IQR, 5–18 months) for DS and 17.8 months (IQR, 9–19 months) for WDS patients. Ten children (16%) were diagnosed late (6 DS−19.3%, 4 WDS−13.7%). In these children, irreversible PAH was already present. Eleven patients died before surgical intervention, 7 from DS group (22.5%), and 4 from WDS group (13.7%). No deaths were reported in the perioperative period.
Postoperative complications following surgical repair of AVSD were: left atrioventricular valve (AV valve) regurgitation, left ventricular outflow tract obstruction, left AV valve stenosis, residual VSD, late-onset complete heart block and pulmonary vascular disease. In the DS group we identified one patient with severe left AV valve insufficiency and DS, who was a candidate for a second surgery (unfortunately pulmonary infection lead to exitus). Another child presented left ventricular outflow tract obstruction. Three patients were left with small residual VSDs with insignificant rest shunt. A child with complete heart block required pacemaker at 12 days after surgery. Severe PAH was identified in 3 children with complete AVSD, one presenting unbalanced ventricles. These patients required endothelin receptor antagonist treatment up to 2 years after surgery.
In the WDS group, 4 children presented severe left AV valve insufficiency (only 1 required corrective surgery after first intervention). Another child had severe left AV valve stenosis that required re-intervention within 1 month after the first intervention. One other child with complete heart block required pacemaker. Only one child had persistent severe PAH after surgery. This child had a complex malformation with unbalanced ventricles, partial anomalous pulmonary venous drainage and heterotaxy syndrome, and he received treatment with phosphodiesterase type 5 inhibitors (sildenafil). Other important postoperative complications in children with complete AVSD were: endocarditis in two children (1 DS, 1 WDS), secondary ischemic stroke after cerebral embolization (a patient with DS, totally recovered) in one patient and protein-losing enteropathy after Fontan intervention (another patient with heterotaxy syndrome, WDS). Partial and transitional AVSDs had good postoperative evolution, without major complications. Postoperative evolution of patients with AVSD was favorable, with the exception of one child with DS, severe PAH and severe left AV valve insufficiency, who died 1 year after surgery (at the age of 2 years).
We aimed to establish if a particular medical management is required in children with DS and AVSD by evaluation of 31 children with DS compared to 31 children without DS, in regards, to type of AVSD, age at diagnosis, age at the time of surgery and postoperative evolution, in a Romanian setting.
The prevalence of complete AVSD was almost 80%, slightly more than in other published reports (2, 3). Complete AVSD was the most common form in both groups. Conversely, partial AVSD was identified only in the group WDS in our cohort, while transitional AVSD were distributed similarly in both groups as showed by other reports (2, 3). Almost 13% of AVSD had unbalanced ventricles, compared to a prevalence of 10% in literature (22, 23). Unbalanced AVSDs are frequently associate with left or right isomerism, required a special approach, and these comorbidities had a major impact on the medical outcome (24–26).
The detection rate of DS in pregnancy may be 90–95%, which leads to an earlier detection of congenital heart diseases, especially of AVSD. In this case, most families choose to termination of pregnancy (9, 27, 28). However, in this cohort, only four children with AVSD were diagnosed during pregnancy and the parents decided to continue the pregnancy. In Romania there are still a large number of pregnant women who do not attend assessment during pregnancy. Nonetheless, our study design did not include an evaluation of cases diagnosed prenatally, where families opted for termination of pregnancy. After birth, the age at diagnosis for AVSD was similar for both groups. Both genders were equally affected.
Age at diagnosis was late for several children in this cohort, despite the recommendations that routine cardiac screening should be performed for all newborn babies with DS. UK and Ireland Down Syndrome Medical Interest Group′s (DSMIG) guidelines recommend that the cardiac status of all children with DS should be established before 6 weeks of age, by clinical, ECG and cardiac ultrasound evaluation (29). Possibly, improved awareness for AVSD complications in primary care is needed, in order to improve referral to tertiary care facilities in Romania.
AVSD has been associated with other heart abnormalities in more than half of children, while extracardiac abnormalities have been found in 25–30% of them, similarly to other reports in literature (27, 30, 31). Digestive system and musculoskeletal anomalies were more frequent in DS group, while isomerism was identified only in the group WDS. Knowledge regarding the existence of extracardiac abnormalities is important as they require a multidisciplinary approach.
The optimal surgical time for complete AVSD is between the 3rd and 6th month of life, before PAH is established (1, 3, 16). Importantly, eleven children in our study died before surgery, more from the DS group, suggesting early diagnosis and follow-up is needed, according to AVSD severity. Most of these cases presented too late at the hospital. There was no difference in surgical technique between AVSD in DS and WDS group (32). Several studies demonstrate that DS patients need reoperation for left AV valve regurgitation later than those WDS (18, 19, 33, 34).
Children with partial and transitional AVSD are largely asymptomatic so referral for surgical repair may be delayed to preschool or older age (20, 21, 35). In a study made by Minich et al. median age at surgery was 1.8 years (21). Patients with partial or transitional AVSDs in our group had no major postoperative complications. Special attention is needed for partial or transitional AVSDs that associates left-side obstructive lesions or unbalanced ventricles, especially those WDS.
Clinical symptoms in AVSD occur in infancy as a result of high pulmonary blood flow associated with PAH (3, 17). Patients with no ventricular component or a small ventricular defect and good AV valve function were asymptomatic in our cohort. Failure to thrive, congestive heart failure and frequent pulmonary infections, were invariably observed children from literature and in this study (3). In selected cases, patients with cardiac failure benefit from determining cardiac biomarkers (36). Pathological values were detected in complex AVSD with severe cardiac involvement in this study.
PAH before surgery was identified in more than 60% from the DS group in this study. After surgery, 3 out of 4 patients that remained with severe PAH had DS, suggesting that special attention is needed in postoperative care. Children with DS and large left-to-right shunt were shown to develop PAH much earlier than children without DS with similar defects (37).
In children with unrepaired complete AVSD, irreversible pulmonary vascular disease becomes increasingly common with age, and affects almost all patients older than 2 years of age (3). A study by Sharma et al. showed that 5 out of 10 children with DS develop PAH and the risk increases to almost 8 out of 10 children with DS and AVSD (37). Early correction of AVSDs can help prevent the development of irreversible pulmonary vascular disease (17). In our study, one patient with Down syndrome presented Eisenmenger syndrome, due to the fact that he was diagnosed late (age 3 years). Early recognition of lesions allows appropriate and timely treatment. Survival following AVSD repair is over 85% at 10 years and over 82% at 20 years after surgery (33, 38). Several studies have showed that the complications risk after AVSD surgical repair in DS are not increased, with the exception of the risk for PAH (16, 18, 39). Life expectancy of patients with DS has increased significantly in developed countries, with a current expected age of 60 years as compared to only 12 years life expectancy in the 1940s (7, 40).
There were several limitations in our study. Our study has a relatively small sample size thus results may not enable accurate identification of significant differences between groups. Therefore, larger studies are needed to further evaluate differences in medical outcome between groups in a similar setting. As several children were diagnosed late in this cohort, the results cannot be generalized for other groups or other countries. Social and educational status of families was not evaluated in this study. Late diagnosis and surgical treatment were caused largely by a particular medical care setting in Romania in former years, which included: low number of pediatric cardiologists and pediatric cardiovascular surgeons, few tertiary pediatric cardiovascular surgery clinics, coupled with low referral rates of newborns to tertiary clinics, as well as family's fear of surgery. Nonetheless, these results are needed to understand morbidity and mortality rates in AVSD, in children with or without DS, in a developing economy. Increased awareness health risks associated with AVSD could bring improved referral rates to tertiary care cardiology clinics.
The complete form of AVSD was more frequent in DS group, having worse prognosis, while unbalanced AVSD was observed predominantly in the group WDS. Age at diagnosis ranged between 1 day and 15 years. There were no significant differences between the age at diagnosis and age at surgical intervention in children with DS or WDS, however, postoperative care for children with DS required special attention due to risk of PAH. The postoperative results were encouraging; however, a large number of children with AVSD did not receive surgical treatment. Late diagnosis was an important risk factor for poor prognosis, in the setting of suboptimal access to cardiac surgery for patients in Romania. Increased awareness is needed in order to provide early diagnosis of AVSD and enable optimal surgical treatment, before occurrence of irreversible PAH. This way, both increased life expectancy and increased quality of life is expected for these people.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
I-CO, GD, and AP: conceptualization. AP and RS: methodology. AC-E and TO: software. GD, AC-E, and AP: validation. GD and I-CO: formal analysis. I-CO, A-MA, RI, and RS: investigation. AP and GD: resources. A-MA, RI, RMS, and TO: data curation. I-CO: writing. GD, AP, and MG: supervision. All authors have read and agreed to the published version of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Baffa JM Atrioventricular Septal Defect MSD Manual Professional Version. Last full review/revision (2018).
2. Chaudhry A, Urbinelli L, Veldtman GR. Atrioventricular septal defects. In: Gatzoulis MA, Webb GD, Daubeney PEF, editors. Diagnosis and Management of Adult Congenital Heart Disease. 3rd ed. Philadelphia, PA: Elsevier Inc. (2018). p. 326–38. doi: 10.1016/b978-0-7020-6929-1.00031-9
3. Calabrò R, Limongelli G. Complete atrioventricular canal. Orphanet J Rare Dis. (2006) 1:8. doi: 10.1186/1750-1172-1-8
4. Muntean I, Togănel R, Benedek T. Genetics of congenital heart disease: past and present. Biochem Genet. (2017) 55:105–23. doi: 10.1007/s10528-016-9780-7
5. Zaidi S, Brueckner M. Genetics and genomics of congenital heart disease. Circ Res. (2017) 120:923–40. doi: 10.1161/CIRCRESAHA.116.309140
6. Pan X, Hu X, Zhang Y, Feng K, Wang SP, Chen L, et al. Identifying patients with atrioventricular septal defect in down syndrome populations by using self-normalizing neural networks and feature selection. Genes. (2018) 9:208. doi: 10.3390/genes9040208
7. Pfitzer C, Helm PC, Rosenthal LM, Berger F, Bauer UMM, Schmitt KR. Dynamics in prevalence of Down syndrome in children with congenital heart disease. Eur J Pediatr. (2018) 177:107–15. doi: 10.1007/s00431-017-3041-6
8. Gómez O, Martinez JM. Atrioventricular septal defect. In: Copel JA, D'Alton ME, Feltovich H, Gratacós E, Krakow D, Odibo AO, Platt LD, Tutschek B, editors. Obstetric Imaging: Fetal Diagnosis and Care. 2nd ed. Philadelphia, PA: Elsevier Inc. (2018). p. 360–4. doi: 10.1016/B978-0-323-44548-1.00076-0
9. Craig B. Atrioventricular septal defect: from fetus to adult. Heart. (2006) 92:1879–85. doi: 10.1136/hrt.2006.093344
10. Bergström S, Carr H, Petersson G, Stephansson O, Bonamy AK, Dahlström A, et al. Trends in congenital heart defects in infants with down syndrome. Pediatrics. (2016) 138:e20160123. doi: 10.1542/peds.2016-0123
11. Digilio MC, Marino B, Toscano A, Giannotti A, Dallapiccola B. Atrioventricular canal defect without down syndrome: a heterogeneous malformation. Am J Med Genet. (1999) 16:85:140–6.
12. De Biase L, Di Ciommo V, Ballerini L, Bevilacqua M, Marcelletti C, Marino B. Prevalence of left-sided obstructive lesions in patients with atrioventricular canal without Down's syndrome. J Thorac Cardiovasc Surg. (1986) 91:467–9. doi: 10.1016/S0022-5223(19)36064-7
13. Paladini D, Volpe P, Sglavo G, Russo MG, De Robertis V, Penner I, et al. Partial atrioventricular septal defect in the fetus: diagnostic features and associations in a multicenter series of 30 cases. Ultrasound Obstet Gynecol. (2009) 34:268–73. doi: 10.1002/uog.7317
14. Marino B, Digilio MC, Toscano A, Giannotti A, Dallapiccola B. Congenital heart diseases in children with Noonan syndrome: an expanded cardiac spectrum with high prevalence of atrioventricular canal. J Pediatr. (1999) 135:703–6. doi: 10.1016/S0022-3476(99)70088-0
15. Hartman RJ, Riehle-Colarusso T, Lin A, Frías JL, Patel SS, Duwe K, et al. Descriptive study of nonsyndromic atrioventricular septal defects in the National Birth Defects Prevention Study, 1997–2005. Am J Med Genet Part A. (2011) 155A:555–64. doi: 10.1002/ajmg.a.33874
16. Lange R, Guenther T, Busch R, Hess J, Schreiber C. The presence of Down syndrome is not a risk factor in complete atrioventricular septal defect repair. J Thorac Cardiovasc Surg. (2007) 134:304–10. doi: 10.1016/j.jtcvs.2007.01.026
17. Marder L, Tulloh R, Pascall E. Cardiac problems in down syndrome. Paediatr Child Health. (2015) 25:23–9. doi: 10.1016/j.paed.2014.07.011
18. Al-Hay AA, MacNeill SJ, Yacoub M, Shore DF, Shinebourne EA. Complete atrioventricular septal defect, down syndrome, and surgical outcome: risk factors. Ann Thorac Surg. (2003) 75:412–21. doi: 10.1016/S0003-4975(02)04026-2
19. Formigari R, Di Donato RM, Gargiulo G, Di Carlo D, Feltri C, Picchio FM, et al. Better surgical prognosis for patients with complete Atrioventricular Septal Defect and Down's Syndrome. Ann Thorac Surg. (2004) 78:666–72. doi: 10.1016/j.athoracsur.2003.12.021
20. Giamberti A, Marino B, Duccio di Carlo Iorio FS, Formigari R, De Zorzi A, et al. Partial Atrioventricular Canal with congestive heart failure in the first year of life: surgical options. Ann Thorac Surg. (1996) 62:151–4. doi: 10.1016/0003-4975(96)00262-7
21. Minich LL, Atz AM, Colan SD, Sleeper LA, Mital S, Jaggers J, et al. Partial and transitional atrioventricular septal defect outcomes. Ann Thorac Surg. (2010) 89:530–6. doi: 10.1016/j.athoracsur.2009.10.047
22. Corno A, Marino B, Catena G, Marcelletti C. Atrioventricular septal defects with severe left ventricular hypoplasia. Staged palliation. J Thorac Cardiovasc Surg. (1988) 96:249–52. doi: 10.1016/S0022-5223(19)35266-3
23. Overman DM, Baffa JM, Cohen MS, Mertens L, Gremmels DB, Jegatheeswaran A, et al. Unbalanced atrioventricular septal defect: definition and decision making. World J Pediatr Congenit Heart Surg. (2010) 1:91–6. doi: 10.1177/2150135110363024
24. Meza JM, Devlin PJ, Overman DM, Gremmels D, Baffa G, Cohen MS, et al. The congenital heart surgeon's society complete atrioventricular septal defect cohort: baseline, preintervention echocardiographic characteristics. Semin Thorac Cardiovasc Surg. (2019) 31:80–6. doi: 10.1053/j.semtcvs.2018.02.004
25. Makrexeni ZM. Down syndrome with unbalanced complete atrioventricular septal defect and truncus arteriosus. Cardiovasc Pharm Open Access. (2017) 6:224–6. doi: 10.4172/2329-6607.1000224
26. Sano S, Fujii Y, Arai S, Kasahara S, Tateishi A. Atrioventricular valve repair for patient with heterotaxy syndrome and a functional single ventricle. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. (2012) 15:88–95. doi: 10.1053/j.pcsu.2012.01.014
27. Mureşan D, Mărginean C, Zaharie G, Stamatian F. Complete atrioventricular septal defect in the era of prenatal diagnosis. Med Ultrason. (2016) 18:500–7. doi: 10.11152/mu-879
28. Flanders L, Tulloh R. Cardiac problems in down syndrome. Paediatr Child Health. (2011) 21:25–31. doi: 10.1016/j.paed.2010.09.005
29. DSMIG. Down Syndrome Medical Interest Group. Available online at: http://www.dsmig.org.uk/information-resources/guidance-for-essential-medical-surveillance/neonatal-guidelines-amended-7-1-18-nb-edit-26-9-18-new-address-2019/
30. Stoll C, Dott B, Alembik Y, Roth MP. Associated congenital anomalies among cases with Down syndrome. Eur J Med Genet. (2015) 58:674–80. doi: 10.1016/j.ejmg.2015.11.003
31. Digilio MC, Marino B. What is new in genetics of congenital heart defects? Front Pediatr. (2016) 4:120. doi: 10.3389/fped.2016.00120
32. Atz AM, Hawkins JA, Lu M, Cohen MS, Colan SD, Jaggers J, et al. Surgical management of complete atrioventricular septal defect: associations with surgical technique, age, trisomy 21. J Thorac Cardiovasc Surg. (2011) 141:1371–9. doi: 10.1016/j.jtcvs.2010.08.093
33. Schleiger A, Miera O, Peters B, Schmitt KRL, Kramer P, Buracionok J, et al. Long-term results after surgical repair of atrioventricular septal defect. Interact Cardiovasc Thorac Surg. (2019) 28:789–96. doi: 10.1093/icvts/ivy334
34. El-Najdawi EK, Driscoll DJ, Puga FJ, Dearani JA, Spotts BE, Mahoney DW, et al. Operation for partial atrioventricular septal defect: a forty-year review. J Thorac Cardiovasc Surg. (2000) 119:880–90. doi: 10.1016/S0022-5223(00)70082-1
35. Devlin PJ, Backer CL, Eltayeb O, Mongé MC, Hauck AL, Costello JM. Repair of partial atrioventricular septal defect: age and outcomes. Ann Thorac Surg. (2016) 102:170–7. doi: 10.1016/j.athoracsur.2016.01.085
36. Doros G, Olariu C, Ardelean AM, Stroescu R, Gafencu M. Relevance of the cardiac biomarkers in children with heart disease admitted for severe cardiac pathology. Rev Chim. (2017) 68:748–53. doi: 10.37358/RC.17.4.5545
37. Sharma M, Khera S, Sondhi V, Devgan A. A study to determine the prevalence of pulmonary arterial hypertension in children with Down syndrome and congenital heart disease. Med J Armed Forces India. (2013) 69:241–5. doi: 10.1016/j.mjafi.2012.11.013
38. Ginde S, Lam J, Hill GD, et al. Long-term outcomes after surgical repair of complete atrioventricular septal defect. The Journal of Thoracic and Cardiovascular Surgery. (2015) 150(2):369–374. doi: 10.1016/j.jtcvs.2015.05.011
39. St. Louis JD, Jodhka U, Jacobs JP, He X, Hill KD, Pasquali SK, et al. Contemporary outcomes of complete atrioventricular septal defect repair: analysis of the Society of Thoracic Surgeons Congenital Heart Surgery Database. J Thorac Cardiovasc Surg. (2014) 148:2526–31. doi: 10.1016/j.jtcvs.2014.05.095
Keywords: atrioventricular septal defect, Down syndrome, congenital heart disease, pulmonary hypertension, risk factors
Citation: Olariu I-C, Popoiu A, Ardelean A-M, Isac R, Steflea RM, Olariu T, Chirita-Emandi A, Stroescu R, Gafencu M and Doros G (2021) Challenges in the Surgical Treatment of Atrioventricular Septal Defect in Children With and Without Down Syndrome in Romania-A Developing Country. Front. Pediatr. 9:612644. doi: 10.3389/fped.2021.612644
Received: 30 September 2020; Accepted: 07 June 2021;
Published: 07 July 2021.
Edited by:
Antonio Francesco Corno, Children's Memorial Hermann Hospital, United StatesReviewed by:
Bruno Marino, Sapienza University of Rome, ItalyCopyright © 2021 Olariu, Popoiu, Ardelean, Isac, Steflea, Olariu, Chirita-Emandi, Stroescu, Gafencu and Doros. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Adela Chirita-Emandi, YWRlbGEuY2hpcml0YUB1bWZ0LnJv
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.