
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pediatr., 10 March 2021
Sec. Genetics of Common and Rare Diseases
Volume 9 - 2021 | https://doi.org/10.3389/fped.2021.598805
Prior studies have examined the influence of MTHFR C677T on autism susceptibility, however, there are no consensus conclusions and specific analyses of a Chinese population. This meta-analysis included a false-positive report probability (FPRP) test to comprehensively evaluate the association of MTHFR C677T polymorphism with autism susceptibility among a Chinese Han population. A large-scale literature retrieval was conducted using various databases including PubMed, Embase, Wan Fang, and the Chinese National Knowledge Infrastructure (CNKI) up to July 31, 2020, with a total of 2,258 cases and 2,073 controls included. The strength of correlation was assessed by odds ratios (ORs) and 95% confidence intervals (95% CIs). MTHFR C677T showed a significant correlation with increased ASD susceptibility under all genetic models (T vs. C, OR = 1.89, 95% CI 1.28 to 2.79; TT vs. CC: OR = 2.44, 95% CI 1.43 to 4.15; CT vs. CC, OR = 1.73; 95% CI 1.19 to 2.51; CT + TT vs. CC: OR = 2.03, 95% CI 1.31 to 3.15; TT vs. CT + CC, OR = 1.95, 95% CI 1.21 to 3.13). Stratification analysis by region also revealed a consistent association in the Northern Han subgroup, but not in the Southern Han subgroup. Pooled minor allele frequency (MAF) of 30 studies were 45% in Northern Han and 39% in Southern Han. To avoid a possible “false positive report,” we further investigated the significant associations observed in the present meta-analysis using the FPRP test, which consolidated the results. In conclusion, MTHFR C677T polymorphism is associated with the increased risk of autism in China, especially in Northern Han. For those mothers and children who are generally susceptible to autism, prenatal folate and vitamin B12 may reduce the risk that children suffer from autism, especially in Northern Han populations. In the future, more well-designed studies with a larger sample size are expected.
Autism spectrum disorder (ASD) involves a constellation of neurodevelopmental disorders featuring impaired repetitive behaviors and deficits in terms of social communication, which are associated with genetic factors and other causes (1). The prevalence of autism in people under eight years old has increased from about 1/59 in 2014 (2) to about 1/54 in 2016 (3). In China, autistic children occupy about 0.7% of the total population, with a rapid upward trend shown (4). As to the consequences of ASD, not only do child patients suffer from a lower level of living quality but their families also often bear a substantial burden (5). The high heritability index is a genetic component in the etiology of ASD and the related genetic factors have the highest level of complicacy (1). On the one hand, the various symptoms of autism reveal its nature as a complex disease with multi-genetic changes (6); on the other hand, the variability of phenotype among ASDs subgroups indicates the interactions of susceptibility genes with environmental factors (7). Hence, there is an urgent need to identify the etiology or risk factors of ASD.
Methylenetetrahydrofolate reductase (MTHFR) gets involved in the process of converting homocysteine into methionine, with the latter one, as a cofactor, playing a critical role in regulating homocysteine concentration in the blood (8). Homocysteine and oxidative stress are associated with several neuropsychiatric disorders, e.g., autism (9), schizophrenia (10), depression (11), and attention deficit hyperactivity disorder (12), etc. The existing evidence suggests that several DNA sequence variants (genetic polymorphism) are associated with the MTHFR gene, with NM_005957.5(MTHFR): c.665C>T (p.Ala222Val) (C677T in short) drawing most attention as a single nucleotide polymorphism (SNP) (13). MTHFR C677T polymorphism tends to reduce the efficiency of methyl group production with possible adverse downstream effects on gene expression, and impair the efficiency of the one-carbon (C1) metabolic pathway (14).
Previous studies have mainly focused on the influence of the MTHFR C677T on autism susceptibility, but the findings are still inconclusive. For example, (15) reported a correlation between MTHFR C677T polymorphism and a higher susceptibility to ASD, but this is not consistent with the findings of Dos Santos et al. (16). A recent meta-analysis (17) suggested a significant association between them overall and by ethnicity, and thus the MTHFR C677T polymorphism could be used as a diagnostic marker of autism by ethnic background. Given that China is a vast territory characterized by prominent regional differences, it is worth conducting a subgroup analysis of the Chinese population. The present meta-analysis sought to comprehensively evaluate the genetic association of MTHFR C677T polymorphism with autism susceptibility among a Chinese population, with particular attention to the possible differences between those from the north and the south.
This study undertook a large-scale literature retrieval based on various databases including PubMed, Embase, Wan Fang, and the Chinese National Knowledge Infrastructure (CNKI) up to July 31, 2020. For minor allele frequency (MAF), the search strategy was performed as follows: (“MTHFR” OR “methylenetetrahydrofolate reductase”) AND (“polymorphism” OR “genotype”) AND (“Chinese Han” OR “China Han”). For the association between MTHFR gene C677T polymorphism and the susceptibility to ASD, the predefined search terms are as follows with no language restriction: (“MTHFR” OR “methylenetetrahydrofolate reductase”) AND (“autism” OR “autism spectrum disorders”) AND (“polymorphism” OR “genotype”). The references listed in relevant primary articles were manually checked to avoid missing any other related articles.
For MAF, any human studies with a focus on the prevalence of MTHFR C677T polymorphism and ASD in relation to Chinese Han ethnicity were included, regardless of their sample size and report type. To assess the potential genetic association, the inclusion criteria included: (1) case-control studies with the distribution of MTHFR C677T mutation frequencies in autism and non-autism patients; (2) presenting the accurate genotype or allele frequency; (3) standard diagnostic criteria for autism diagnosis; (4) research on Chinese individuals. The exclusion conditions are as follows: (1) not related to MTHFR polymorphism and autism research; (2) repeated publications; (3) previous meta-analysis, case reports, reviews, editorials, and comments; (4) not human beings model studies.
Two co-authors (Li and Liu) extracted the data independently from all included studies for analysis, including the first author's surname, publication year, country, region, control source, sample size, genotype frequency of case and control, diagnostic criteria, genotyping method and HWE for controls. When facing discrepancy, we returned to the original studies in discussion with a third reviewer (Che).
To measure the strength of correlation between MTHFR C677T polymorphism and autism risk under five genetic models, Odds ratios (ORs), and 95% confidence intervals (95% CIs) were calculated. We also performed a Chi-square test to determine the Hardy-Weinberg equilibrium (HWE) in the control groups, with P < 0.05 indicating disequilibrium. Heterogeneity across the studies was evaluated using both Cochran's Q statistic and the I2 statistic. Specifically, significant heterogeneity was indicated when P was less than 0.10 and I2 was higher than 50% and a random-effects model (the DerSimonian and Laird method) was supposed to be fitted in this case; otherwise, a fixed-effects model using the Mantel-Haenszel was the suitable choice. Furthermore, we conducted sensitivity analysis according to the HWE status of controls, and the subgroup meta-analyses by region were undertaken for particular relationships. We used Begg's funnel plot and Egger's test to detect potential publication bias. The statistical significance of the ORs was determined by Z test, with P < 0.05 indicating a significant difference. Stata 14.0 software was used for the above-mentioned statistical analyses.
To determine whether the significant associations (P < 0.05) between the MTHFR C677T polymorphism and the risk of ASD were “noteworthy,” we further calculated the FPRP value. Although it is suggested to draw on statistical power to detect an OR of 1.5 with an α-level equal to the observed P-value by Wacholder et al. (18), we decided to present the results for OR of 2 as well to make it more stringent.
Based on the estimated probability that the finding may not be a genuine association, only associations with FPRP < 0.2 were deemed noteworthy, as recommended by Wacholder et al. (18).
The procedure of study selection is shown in Figure 1, with the details of the inclusion and exclusion of studies illustrated. For pooling MAF, a total of 264 studies were identified from databases, among which 30 case-control studies were included for pooling minor allele prevalence (19–48), which all reported the MAF in non-ASD populations in Chinese Han. For the targeted gene effect, we yielded 119 papers initially and finally, six publications (49–54) were included in the present meta-analysis, with a total of 2,258 cases and 2,073 controls. Among them, four publications (50–54) concerning Northern Chinese Han, whereas the other two papers (49, 52) focus on Southern Chinese Han. Three studies (50, 51, 54) deviated from HWE. The estimation of MAF is shown in Table 1 and the characteristics of the included studies are summarized in Table 2.
Five studies (23, 26, 33, 44, 45) deviated from HWE were excluded, leaving 12 studies on Northern Han and 13 studies on Southern Han to be pooled. A high between-study heterogeneity (I2 = 97.6%, P < 0.01) was shown among all the studies and the pooled MAF estimated by a random-effect model was 42% (95% CI: 37–46%). There was also heterogeneity (I2 = 98.5%, P < 0.01) among studies on Northern Han (MAF: 45%, 95% CI: 37–52%), and heterogeneity (I2 = 91.7%, P < 0.01) was found among Southern Han studies (MAF: 39%, 95% CI: 35–43%).
Overall analysis showed that the MTHFR C677T polymorphism increased the risk of autism (Table 3) under allele model (T vs. C, OR = 1.89, 95% CI 1.28 to 2.79, Figure 2), homozygous model (TT vs. CC: OR = 2.44, 95% CI 1.43 to 4.15), heterozygous model (CT vs. CC, OR = 1.73; 95% CI 1.19 to 2.51), dominant model (CT + TT vs. CC: OR = 2.03, 95% CI 1.31 to 3.15), and recessive model (TT vs. CT + CC, OR = 1.95, 95% CI 1.21 to 3.13).
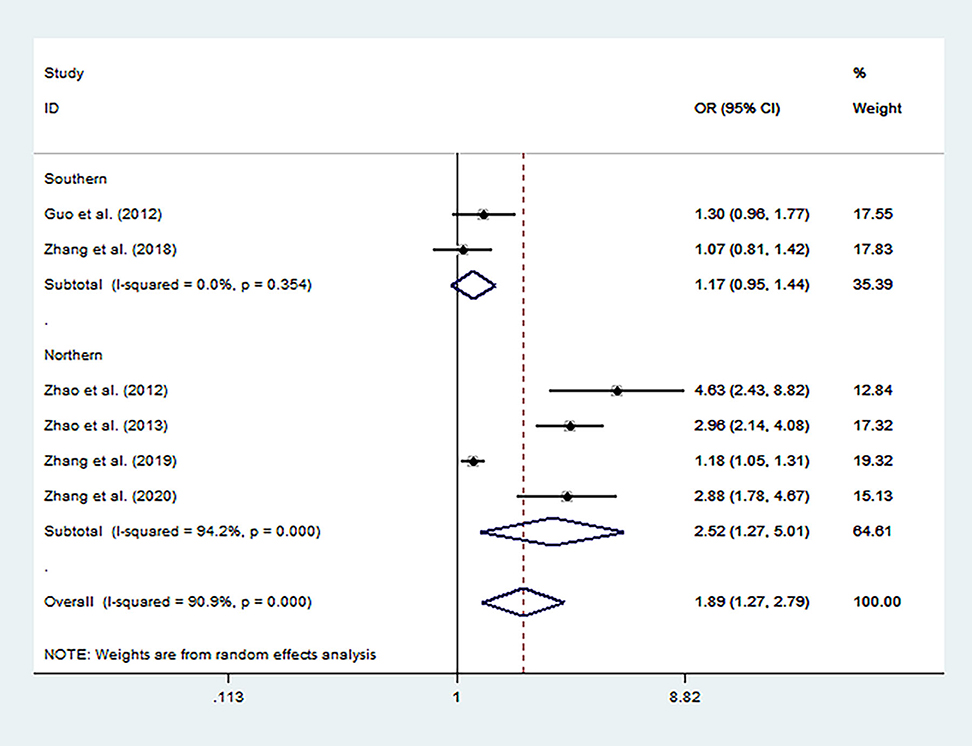
Figure 2. Forest plot for the association between MTHFR C677T polymorphism and autism susceptibility in allele genetic model stratified by region.
Sensitivity analysis was performed by removing the studies in which the controls were not consistent with HWE and then the pooled OR for the remaining studies was recalculated. The sensitivity analysis suggested similar patterns to the overall analyses (Figures 3, 4).
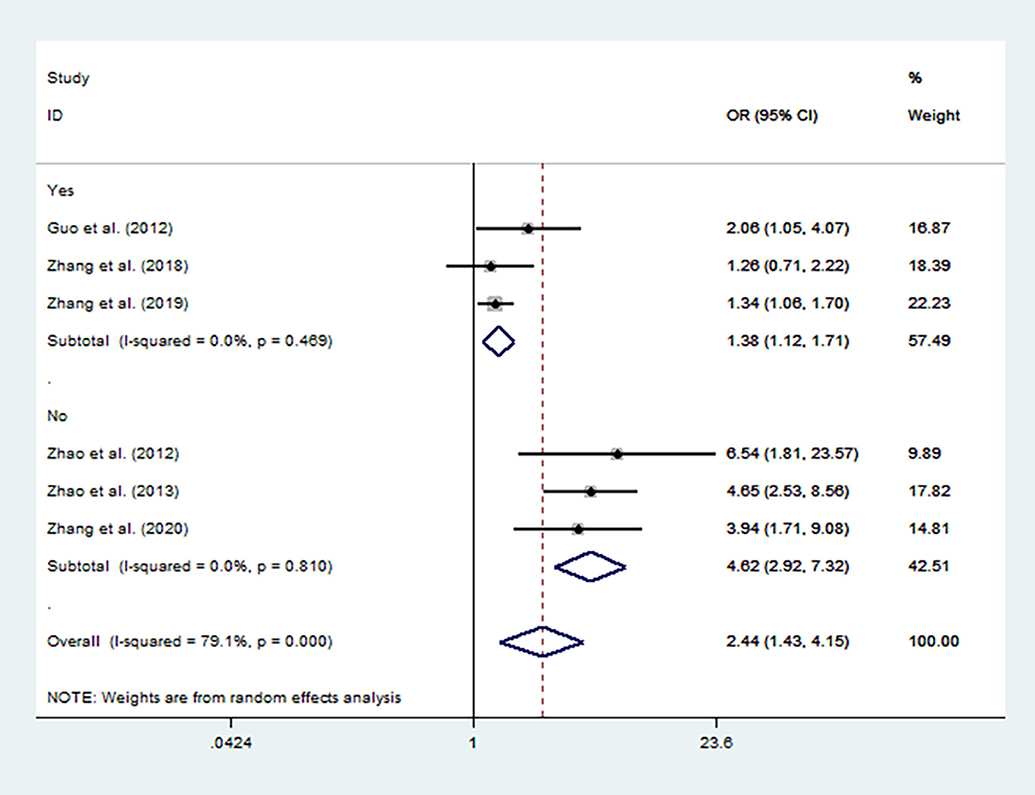
Figure 3. Forest plot for the association between MTHFR C677T polymorphism and autism susceptibility in homozygous genetic model stratified by HWE status of controls.
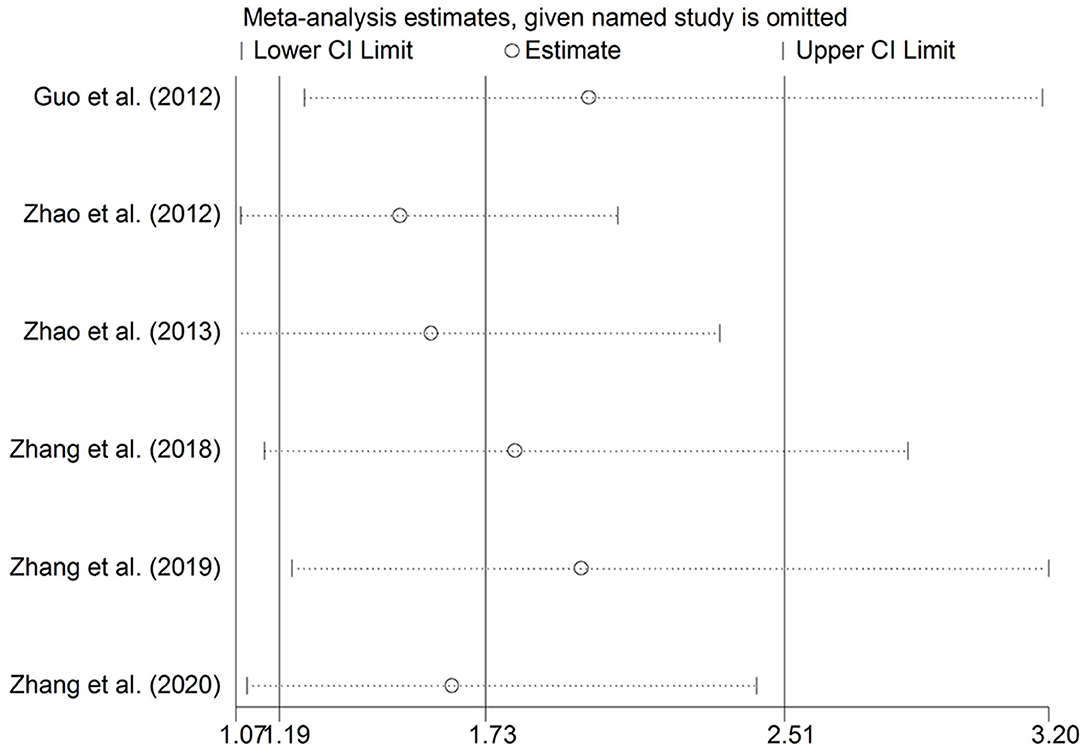
Figure 4. Sensitivity analysis through the deletion of each study to reflect the individual influence on the calculated ORs in a heterozygous genetic model of MTHFR C677T polymorphism.
Subgroup analyses showed that when stratified by region, there was a significant association between MTHFR C677T polymorphism and an increased risk of autism in the Northern Han Subgroup under five models (T vs. C: OR = 2.52, 95% CI 1.27 to 5.01; TT vs. CC: OR = 3.26, 95% CI 1.37 to 7.76; CT vs. CC: OR = 2.21; 95%CI 1.19 to 4.08; CT+TT vs. CC: OR = 2.67, 95% CI 1.30 to 5.46; TT vs. CT + CC: OR = 2.56, 95% CI 1.22 to 5.40).
By contrast, a significant correlation was absent in the Southern Han subgroup analyses in each model mentioned above (T vs. C: OR = 1.17, 95% CI 0.95 to 1.44; TT vs. CC: OR = 1.55, 95% CI 0.96 to 2.51; CT vs. CC: OR = 1.21; 95%CI 0.82 to 1.81; CT+TT vs. CC: OR = 1.27, 95% CI 0.93 to 1.75; TT vs. CT + CC: OR = 1.32, 95% CI 0.61 to 2.87).
We drew on an FPRP test to investigate whether the significant associations (P < 0.05) detected in the present study were a false positive effect. The results of the FPRP test (see Table 4) indicated that the MTHFR C677T polymorphism was associated overall with autism susceptibility in all gene models. In addition, the FPRP test suggested a truly significant association of MTHFR C677T polymorphism with autism susceptibility in Northern Han instead of Southern Han. These results showed consistent patterns with those reported in the preceding sections.
The results of Egger's linear regression tests indicated no significant publication bias (P < 0.05), and meanwhile, the Begg's funnel plots were consistent with the conclusion (Figure 5).
To our knowledge, the present meta-analysis is the first to investigate the association between MTHFR C677T polymorphism and the risk of autism in the Chinese Han population. The results suggested that the MTHFR C677T was significantly associated with the increased risk of autism under all genetic models in China, which is in accordance with previous studies. The significant association between the C>T and ASD is consistent with previous results in American (55), Indian (56), and Egyptian (57) populations. Furthermore, a strong relationship between MTHFR C677T and the risk of autism was shown among the Northern Han subgroup, but not the Southern Han subgroup. The above-mentioned results were all consolidated by the FPRP test.
Given that autism is a multifactorial disorder, epigenetic mechanisms play a vital role in the expression of autism phenotypes (58), and are affected by nutritional status as well as medication (59). As a key enzyme of folate metabolism in the process of one-carbon metabolism, the activity of Methylenetetrahydrofolate reductase (MTHFR) strongly affects the one-carbon (C1) metabolic pathway, which is central to cellular methylation reactions. In detail, MTHFR catalyzes the conversion of 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate and the latter is required for the conversion of homocysteine to methionine by methionine synthase (60). The MTHFR C677T polymorphism results in a thermolabile variant of MTHFR with a decreased enzyme activity and functions as a well-established genetic determinant of elevated plasma tHcy (total homocysteine; all the circulating forms of Hcy) levels (61). A shift in the glutathione redox ratio and redox imbalance may contribute to the etiology of autism (62). Meanwhile, MTHFR C677T can interact with other SNPs (63–65). For example, the synergistic interactions between MTHFR C677T and MTRR A66G tend to cause an increase in homocysteine, which makes the MTHFR C677T polymorphism a risk factor for autism (66).
In the subgroup analysis, a strong relationship between MTHFR C677T and the risk of autism was shown among the Northern Han subgroup, but not the Southern Han subgroup. These patterns are similar to other studies with a focus on regional subgroup differences in China. These prior studies indicate significant associations between the MTHFR C677T polymorphism and an increased risk of various diseases and disorders, including lung cancer (67), non-syndromic cleft lip and palate (68), depression (69), diabetic nephropathy (70) in the North China population, but such associations were absent (67, 68) or weaker (69, 70) in the South China population. One possible explanation for this may be related to people's different folate and vitamin B12 concentrations in the two regions. A cross-sectional survey conducted by Ren et al. (71) showed that the women in the north had less than half the folate concentration relative to the women in the south. This can be attributed to the fact that the southern region is one of the wealthiest regions in China, and there is a longer growing season with higher temperatures in the south. A survey in 2019 (72) suggested significant differences in distribution characteristics of C677T gene polymorphism of MTHFR between the northern and southern regions, and that Han nationality women in the north had a higher risk of folate in dysmetabolism than the women in the south. Furthermore, Hao et al.'s (73) study on vitamin B12 also showed a similar pattern.
Inspired by these relevant studies, the North-South difference detected in the present meta-analysis may be attributed to the influence of vitamin B12 and folate on the association between the MTHFR C677T and the risk of ASD. Vitamin B12 and folate participate in the methylation cycle as well as in DNA and RNA biosynthesis. Low folate concentrations lead to decreased methylation of proteins, phospholipids, DNA, and neurotransmitters. Al-Batayneh et al. (60) found a significant association between homozygous MTHFR C677T variant as well as T allele frequencies and vitamin B12 deficiency. Through a large-scale study (N = 365), Jacques et al. (74) found that when the plasma folate level was lower than 14.5 μmol/l, the plasma Hcy level of the MTHFR gene mutation group was significantly higher than that of the normal genotype group. Therefore, it is suggested that proper levels of vitamin B12 and folate are needed to regulate the metabolism of Hcy in MTHFR gene mutation, to maintain its balance in vivo.
Empirically, compensatory folate and vitamin B12 intake can be used to prevent the increase of Hcy level in MTHFR gene mutation. The influences of diet during the periconceptional period are of primary importance for the establishment of DNA methylation patterns and the epigenetic effects caused by these patterns have the potential to persist throughout the life span (75). Oxidative stress may function as a contributing factor to autism pathology. Folate effectively reduced oxidative stress and restored normal concentrations of antioxidant enzymes. Two large-scale case-control studies (76, 77) suggested that the risk for ASD children among the mothers with the MTHFR 677TT genotype is reduced when folate and prenatal vitamin supplements were taken periconceptionally and in the first trimester of pregnancy. According to a mouse study (78), prenatal or early postnatal supplementation of methyl-donors (e.g., folate) decreased the risk of MTHFR-deficiency mice to present ASD-like behavior.
Above all, it is worth paying more attention to genetic screening for women of childbearing age and newborn babies to assess the genetic risk of folate metabolism disorders. On an individual level, genotype/metabolic phenotype analysis tends to guide effective intervention and shed light on the foundations for individual differences in response to treatment (55). A genetic deficiency concerning the MTHFR gene may directly affect metabolite availability and control the environment of the developing embryonic brain in an indirect way (79). The abnormal metabolic profile caused by MTHFR C677T polymorphism can be reduced or counteracted by nutrition treatment (80). Furthermore, treatment for ASD children is effective in correcting metabolic derangements and potentially likely to ameliorate autistic symptoms (81). The intake dosage is also important and of note. According to a study conducted by Raghavan et al. (82), the moderate intake (3–5 times/week) of multivitamin supplements during pregnancy may reduce the risk of ASD in offspring, but very high levels of maternal plasma folate and B12 (≥90%) at birth associated with increased risk of ASD. In other words, both deficient and excessive nutrient status might be associated with an elevated risk of ASD. Proper intake doses of folate and vitamin B12 based on individual needs could not only improve lower tHcy but also avoid the potential adverse effects of excessive intake. Therefore, when the C677T gene polymorphism is detected, targeted nutrition treatment therapies can be expected, which are tailored to individual folate and vitamin B12 levels and genetic background. The above-mentioned measures are likely to reduce the prevalence of autism in China, especially in the Northern Han population.
There are some limitations in the present study. First, as one common limitation in genetic association meta-analysis, heterogeneity may function as a confounding factor in the present meta-analysis. Many factors including experimental design, genetic testing methods, the accuracy of laboratory equipment, etc., may result in heterogeneity (83). To address this issue, a sensitivity analysis was conducted by removing the studies where the controls were not consistent with HWE, with the findings indicating that the results are stable and not significantly constrained by any single study. Second, a single included study having more than half of all participants may bias the current results. Third, the lack of some factors such as oxidant proteins and anti-oxidant status blood tests in the origin articles may influence the conclusion. Finally, publication bias might exist even though no significant publication bias was observed through the Begg test and Egger test. In this regard, more well-designed studies with a large sample size are needed to elucidate the conclusions.
In summary, the present meta-analysis is the first to provide refined current evidence of MTHFR C677T polymorphism with the increased risk of autism in Chinese Han. These results suggest that the MTHFR C677T polymorphism might be a risk factor for ASD in Chinese Han, especially in the north. For those mothers and children who are generally susceptible to autism, tailored nutrition treatment of prenatal folate and vitamin B12 may reduce the risk of having children with autism, especially in the north. Further studies with greater gene-environment statistical power are encouraged to verify our conclusions, taking into account more precise analysis of factors such as age, gender, and lifestyle factors in the development of autism.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
H-FL and C-XL designed the study. C-XL, Y-GL, Y-PC, J-LO, and W-CR performed the literature search, data extraction, and statistical analysis. C-XL drafted the manuscript. Y-GL, Y-LY, and H-FL revised the manuscript. All authors reviewed and approved the final paper for submission and publication.
This work was supported by grants from The National Key Research and Development Program of China of the 13th Five-Year Plan (No. 2016YFC1306205); The Provincial key disciplines of Zhejiang Traditional Chinese Medicine (a combination of traditional Chinese and Western medicine) (No. 2017-XK-A41); Technological Research Program of Zhejiang (2015C33178); and The Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (2016148961).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Hong Weng for their assistance in editing this manuscript.
1. Grove J, Ripke S, Als TD, Mattheisen M, Walters RK, Won H, et al. Identification of common genetic risk variants for autism spectrum disorder. Nat Genet. (2019) 51:431–44. doi: 10.1038/s41588-019-0344-8
2. Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z, et al. Prevalence of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 Sites, United States, 2014. Morb Mortal Wkly Rep Surveill Summ. (2018) 67:1–23. doi: 10.15585/mmwr.mm6745a7
3. Maenner MJ, Shaw KA, Baio J, Washington A, Patrick M, DiRienzo M, et al. Prevalence of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 Sites, United States, 2016. MMWR Surveill Summ. (2020) 69:1–12. doi: 10.15585/mmwr.ss6903a1
4. Zhou H, Xu X, Yan W, Zou X, Wu L, Luo X, et al. Prevalence of autism spectrum disorder in China: a nationwide multi-center population-based study among children aged 6 to 12 years. Neurosci Bull. (2020) 36:961–71. doi: 10.1007/s12264-020-00530-6
5. Lyall K, Croen L, Daniels J, Fallin MD, Ladd-Acosta C, Lee BK, et al. The changing epidemiology of autism spectrum disorders. Annu Rev Public Health. (2017) 38:81–102. doi: 10.1146/annurev-publhealth-031816-044318
6. Ruzzo EK, Perez-Cano L, Jung JY, Wang LK, Kashef-Haghighi D, Hartl C, et al. Inherited and de novo genetic risk for autism impacts shared networks. Cell. (2019) 178:850–66.e826. doi: 10.1016/j.cell.2019.07.015
7. Agam G, Taylor Z, Vainer E, Golan H. The influence of choline treatment on behavioral and neurochemical autistic-like phenotype in Mthfr-deficient mice. Transl Psychiatry. (2020) 10:316. doi: 10.1038/s41398-020-01002-1
8. Wan L, Li Y, Zhang Z, Sun Z, He Y, Li R. Methylenetetrahydrofolate reductase and psychiatric diseases. Transl Psychiatry. (2018) 8:242. doi: 10.1038/s41398-018-0276-6
9. El-Baz F, El-Aal MA, Kamal TM, Sadek AA, Othman AA. Study of the C677T and 1298AC polymorphic genotypes of MTHFR Gene in autism spectrum disorder. Electron Phys. (2017) 9:5287–93. doi: 10.19082/5287
10. Burghardt KJ, Pilsner JR, Bly MJ, Ellingrod VL. DNA methylation in schizophrenia subjects: gender and MTHFR 677C/T genotype differences. Epigenomics. (2012) 4:261–8. doi: 10.2217/epi.12.25
11. Bousman CA, Potiriadis M, Everall IP, Gunn JM. Methylenetetrahydrofolate reductase (MTHFR) genetic variation and major depressive disorder prognosis: a five-year prospective cohort study of primary care attendees. Am J Med Genet B Neuropsychiatr Genet. (2014) 165b:68–76. doi: 10.1002/ajmg.b.32209
12. Krull KR, Brouwers P, Jain N, Zhang L, Bomgaars L, Dreyer Z, et al. Folate pathway genetic polymorphisms are related to attention disorders in childhood leukemia survivors. J Pediatr. (2008) 152:101–5. doi: 10.1016/j.jpeds.2007.05.047
13. Levin BL, Varga E. MTHFR: addressing genetic counseling dilemmas using evidence-based literature. J Genet Couns. (2016) 25:901–11. doi: 10.1007/s10897-016-9956-7
14. Yan J, Wang W, Gregory J, Malysheva O, Brenna J, Stabler S, et al. MTHFR C677T genotype influences the isotopic enrichment of one-carbon metabolites in folate-compromised men consuming d9-choline. Am J Clin Nutr. (2011) 93:348–55. doi: 10.3945/ajcn.110.005975
15. Arab A, Elhawary N. Methylenetetrahydrofolate reductase gene variants confer potential vulnerability to autism spectrum disorder in a Saudi community. Neuropsychiatr Dis Treat. (2019) 15:3569–81. doi: 10.2147/NDT.S230348
16. Dos Santos P, Longo D, Brandalize A, Schuler-Faccini L. MTHFR C677T is not a risk factor for autism spectrum disorders in South Brazil. Psychiatr Genet. (2010) 20:187–9. doi: 10.1097/YPG.0b013e32833a2220
17. Sadeghiyeh T, Dastgheib S, Mirzaee-Khoramabadi K, Morovati-Sharifabad M, Akbarian-Bafghi M, Poursharif Z, et al. Association of MTHFR 677C>T and 1298A>C polymorphisms with susceptibility to autism: a systematic review and meta-analysis. Asian J Psychiatr. (2019) 46:54–61. doi: 10.1016/j.ajp.2019.09.016
18. Wacholder S, Chanock S, Garcia-Closas M, El Ghormli L, Rothman N. Assessing the probability that a positive report is false: an approach for molecular epidemiology studies. J Natl Cancer Inst. (2004) 96:434–42. doi: 10.1093/jnci/djh075
19. Feng L, Song Z, Xin F, Hu J. Association of plasma homocysteine and methylenetetrahydrofolate reductase C677T gene variant with schizophrenia: a Chinese Han population-based case-control study. Psychiatry Res. (2009) 168:205–8. doi: 10.1016/j.psychres.2008.05.009
20. Xiao H, Xu J, Zhou X, Stankovich J, Pan F, Zhang Z, et al. Associations between the genetic polymorphisms of MTHFR and outcomes of methotrexate treatment in rheumatoid arthritis. Clin Exp Rheumatol. (2010) 28:728–33.
21. Li H, Xu WL, Shen HL, Chen QY, Hui LL, Long LL, et al. Methylenetetrahydrofolate reductase genotypes and haplotypes associated with susceptibility to colorectal cancer in an eastern Chinese Han population. Genet Mol Res. (2011) 10:3738–46. doi: 10.4238/2011.December.14.8
22. Li R, Wang R, Li Y, Li X, Feng Y, Li Y, et al. Association study on MTHFR polymorphisms and meningioma in northern China. Gene. (2013) 516:291–3. doi: 10.1016/j.gene.2012.12.019
23. Yang B, Liu Y, Li Y, Fan S, Zhi X, Lu X, et al. Geographical distribution of MTHFR C677T, A1298C and MTRR A66G gene polymorphisms in China: findings from 15357 adults of Han nationality. PLoS ONE. (2013) 8:e57917. doi: 10.1371/journal.pone.0057917
24. Zhang Y, Yan H, Tian L, Wang F, Lu T, Wang L, et al. Association of MTHFR C677T polymorphism with schizophrenia and its effect on episodic memory and gray matter density in patients. Behav Brain Res. (2013) 243:146–52. doi: 10.1016/j.bbr.2012.12.061
25. Shen X, Wu Y, Guan T, Wang X, Qian M, Lin M, et al. Association analysis of COMT/MTHFR polymorphisms and major depressive disorder in Chinese Han population. J Affect Disord. (2014) 161:73–8. doi: 10.1016/j.jad.2014.03.008
26. Tang W, Zhang S, Qiu H, Wang L, Sun B, Yin J, et al. Genetic variations in MTHFR and esophageal squamous cell carcinoma susceptibility in Chinese Han population. Med Oncol. (2014) 31:915. doi: 10.1007/s12032-014-0915-6
27. Yang B, Fan S, Zhi X, Wang D, Li Y, Wang Y, et al. Associations of MTHFR C677T and MTRR A66G gene polymorphisms with metabolic syndrome: a case-control study in Northern China. Int J Mol Sci. (2014) 15:21687–702. doi: 10.3390/ijms151221687
28. Fan SJ, Yang BY, Zhi XY, He M, Wang D, Wang YX, et al. Are MTHFR C677T and MTRR A66G polymorphisms associated with overweight/obesity risk? From a case-control to a meta-analysis of 30,327 subjects. Int J Mol Sci. (2015) 16:11849–63. doi: 10.3390/ijms160611849
29. Luo L, Chen Y, Wang L, Zhuo G, Qiu C, Tu Q, et al. Polymorphisms of genes involved in the folate metabolic pathway impact the occurrence of unexplained recurrent pregnancy loss. Reprod Sci. (2015) 22:845–51. doi: 10.1177/1933719114565033
30. Lv C, Bai Z, Liu Z, Luo P, Zhang J. Renal cell carcinoma risk is associated with the interactions of APOE, VHL and MTHFR gene polymorphisms. Int J Clin Exp Pathol. (2015) 8:5781–6.
31. Lv QQ, Lu J, Sun H, Zhang JS. Association of methylenetetrahydrofolate reductase (MTHFR) gene polymorphism with ischemic stroke in the Eastern Chinese Han population. Genet Mol Res. (2015) 14:4161–8. doi: 10.4238/2015.April.27.31
32. Wang XB, Qiao C, Wei L, Han YD, Cui NH, Huang ZL, et al. Associations of polymorphisms in MTHFR gene with the risk of age-related cataract in Chinese Han population: a genotype-phenotype analysis. PLoS ONE. (2015) 10:e0145581. doi: 10.1371/journal.pone.0145581
33. Wang Y, Chen S, Kang M, Tang W, Gu H, Yin J, et al. Genetic variations in MTHFR and gastric cardia adenocarcinoma susceptibility in the Chinese Han population. Int J Clin Exp Med. (2015) 8:18936–44.
34. Yun L, Xu R, Li G, Yao Y, Li J, Cong D, et al. Homocysteine and the C677T gene polymorphism of its key metabolic enzyme MTHFR are risk factors of early renal damage in hypertension in a Chinese Han population. Medicine. (2015) 94:e2389. doi: 10.1097/MD.0000000000002389
35. Fan S, Yang B, Zhi X, Wang Y, Wei J, Zheng Q, et al. Interactions of methylenetetrahydrofolate reductase C677T polymorphism with environmental factors on hypertension susceptibility. Int J Environ Res Public Health. (2016) 13:601. doi: 10.3390/ijerph13060601
36. Hu X, Tao C, Xie Z, Li Y, Zheng J, Fang Y, et al. The MTHFR C677T polymorphism and risk of intracerebral hemorrhage in a Chinese Han population. Med Sci Monit. (2016) 22:127–33. doi: 10.12659/MSM.896315
37. Wang Y, Zhang H, Yue S, Zhang K, Wang H, Dong R, et al. Evaluation of high resolution melting for MTHFR C677T genotyping in congenital heart disease. PLoS ONE. (2016) 11:e0151140. doi: 10.1371/journal.pone.0151140
38. Yuan L, Song Z, Deng X, Xiong W, Yang Z, Deng H. Association of the MTHFR rs1801131 and rs1801133 variants in sporadic Parkinson's disease patients. Neurosci Lett. (2016) 616:26–31. doi: 10.1016/j.neulet.2016.01.031
39. Jiao X, Luo Y, Yang B, Jing L, Li Y, Liu C, et al. The MTHFR C677T mutation is not a risk factor recognized for HBV-related HCC in a population with a high prevalence of this genetic marker. Infect Genet Evol. (2017) 49:66–72. doi: 10.1016/j.meegid.2017.01.008
40. Li X, Weng L, Han B, Dai Y, Cha L, Yan S, et al. Association of folate metabolism gene polymorphisms and haplotype combination with pulmonary embolism risk in Chinese Han population. Mamm Genome. (2017) 28:220–6. doi: 10.1007/s00335-017-9692-9
41. Liu S, Zhang C, Peng H, Sun H, Lin K, Huang X, et al. Strong association of SLC1A1 and DPF3 gene variants with idiopathic male infertility in Han Chinese. Asian J Androl. (2017) 19:486–92. doi: 10.4103/1008-682X.178850
42. Jiao X, Chen W, Zhang J, Wang W, Song J, Chen D, et al. Variant alleles of the ESR1, PPARG, HMGA2, and MTHFR genes are associated with polycystic ovary syndrome risk in a Chinese population: a case-control study. Front Endocrinol. (2018) 9:504. doi: 10.3389/fendo.2018.00504
43. Mao X, Han L. The relationship of methylenetetrahydrofolate reductase gene C677T polymorphism and ischemic stroke in Chinese Han population. Ann Clin Lab Sci. (2018) 48:242–7.
44. Wang C, Xie H, Lu D, Ling Q, Jin P, Li H, et al. The MTHFR polymorphism affect the susceptibility of HCC and the prognosis of HCC liver transplantation. Clin Transl Oncol. (2018) 20:448–56. doi: 10.1007/s12094-017-1729-8
45. Long Y, Zhao X, Liu C, Sun Y, Ma Y, Liu X, et al. A case-control study of the association of the polymorphisms of MTHFR and APOE with risk factors and the severity of coronary artery disease. Cardiology. (2019) 142:149–57. doi: 10.1159/000499866
46. Wei L, Niu F, Wu J, Chen F, Yang H, Li J, et al. Association study between genetic polymorphisms in folate metabolism and gastric cancer susceptibility in Chinese Han population: a case-control study. Mol Genet Genomic Med. (2019) 7:e633. doi: 10.1002/mgg3.633
47. Peng X, Zhou Y, Wu X, Wang X, Bai H, Li Y, et al. Association of methylenetetrahydrofolate reductase (MTHFR) variant C677T and risk of carotid atherosclerosis: a cross-sectional analysis of 730 Chinese Han adults in Chongqing. BMC Cardiovasc Disord. (2020) 20:222. doi: 10.1186/s12872-020-01505-1
48. Wang S, Zuo S, Liu Z, Ji X, Yao Z, Wang X. Association of MTHFR and RFC1 gene polymorphisms with methotrexate efficacy and toxicity in Chinese Han patients with rheumatoid arthritis. J Int Med Res. (2020) 48:300060519879588. doi: 10.1177/0300060519879588
49. Guo T, Chen H, Liu B, Ji W, Yang C. Methylenetetrahydrofolate reductase polymorphisms C677T and risk of autism in the Chinese Han population. Genet Test Mol Biomark. (2012) 16:968–73. doi: 10.1089/gtmb.2012.0091
50. Zhao D, Xia W, Sun CH, Li NN, Wu K. Association methylenetetrahydrofolate reductase gene C677T polymorphism with autism children. Chinese J Child Health Care. (2012) 20:585–90.
51. Zhao D, Sun C, Yang XW. Association of methylenetetrahydrofolate reductase in gene C677T and A1298C polymorphism among children with autism. Chin J Sch Health. (2013) 34:52–8.
52. Zhang ZY, Yu LF, Li SF, Liu J. Association study of polymorphisms in genes relevant to vitamin B12 and folate metabolism with childhood autism spectrum disorder in a Han Chinese population. Med Sci Monit. (2018) 24:370–6. doi: 10.12659/MSM.905567
53. Zhang JS, Wang F, Su Y, Lu H, Zhang T. Association study of MTHFR C677T polymorphism and birth body mass with risk of autism. Chin J Behav Med and Brain Sci. (2019) 28:673–7.
54. Zhang HC, Shang Q, Gao C, Geng XJ. Correlation between gene polymorphism of MTHFR gene C677T locus, 5-HTTLPR and autism in Han nationality children. J Clin Pathol Res. (2020) 40:80–5.
55. Boris M, Goldblatt A, Galanko J, James SJ. Association of MTHFR gene variants with autism. J Am Phys Surg. (2004) 9:106–8.
56. Divyakolu S, Tejaswini Y, Thomas W, Thumoju S, Sreekanth VR, Vasavi M, et al. Evaluation of C677T polymorphism of the methylenetetrahydrofolate reductase (MTHFR) gene in various neurological disorders. J Neurol Disord. (2013) 2:1–4. doi: 10.4172/2329-6895.1000142
57. Ismail S, Senna AA, Behiry EG, Ashaat EA, Zaki MS, Ashaat NA, et al. Study of C677T variant of methylene tetrahydrofolate reductase gene in autistic spectrum disorder Egyptian children. Am J Med Genet B Neuropsychiatr Genet. (2019) 180:305–9. doi: 10.1002/ajmg.b.32729
58. Siu MT, Weksberg R. Epigenetics of autism spectrum disorder. Adv Exp Med Biol. (2017) 978:63–90. doi: 10.1007/978-3-319-53889-1_4
59. Ranjan S, Nasser JA. Nutritional status of individuals with autism spectrum disorders: do we know enough? Adv Nutr. (2015) 6:397–407. doi: 10.3945/an.114.007914
60. Al-Batayneh KM, Zoubi MSA, Shehab M, Al-Trad B, Bodoor K, Khateeb WA, et al. Association between MTHFR 677C>T polymorphism and vitamin B12 deficiency: a case-control study. J Med Biochem. (2018) 37:141–7. doi: 10.1515/jomb-2017-0051
61. Caldeira-Araújo H, Ramos R, Florindo C, Rivera I, Castro R, Tavares de Almeida I. Homocysteine metabolism in children and adolescents: influence of age on plasma biomarkers and correspondent genotype interactions. Nutrients. (2019) 11:646. doi: 10.3390/nu11030646
62. Zhang Q, Wu H, Zou M, Li L, Li Q, Sun C, et al. Folic acid improves abnormal behavior via mitigation of oxidative stress, inflammation, and ferroptosis in the BTBR T+ tf/J mouse model of autism. J Nutr Biochem. (2019) 71:98–109. doi: 10.1016/j.jnutbio.2019.05.002
63. Liu ZZ, Zhang JT, Liu D, Hao YH, Chang BM, Xie J, et al. Interaction between maternal 5,10-methylenetetrahydrofolate reductase C677T and methionine synthase A2756G gene variants to increase the risk of fetal neural tube defects in a Shanxi Han population. Chin Med J. (2013) 126:865–9. doi: 10.3760/cma.j.issn.0366-6999.20121688
64. They-They TP, Battas O, Nadifi S. Synergistic effect of MTHFR C677T and F2 G20210A polymorphisms on ischemic stroke. Neurosci Bull. (2013) 29:725–30. doi: 10.1007/s12264-013-1381-4
65. Li WX, Dai SX, Zheng JJ, Liu JQ, Huang JF. Homocysteine metabolism gene polymorphisms (MTHFR C677T, MTHFR A1298C, MTR A2756G and MTRR A66G) jointly elevate the risk of folate deficiency. Nutrients. (2015) 7:6670–87. doi: 10.3390/nu7085303
66. Mohammad NS, Jain JM, Chintakindi KP, Singh RP, Naik U, Akella RR. Aberrations in folate metabolic pathway and altered susceptibility to autism. Psychiatr Genet. (2009) 19:171–6. doi: 10.1097/YPG.0b013e32832cebd2
67. Zhong R, Chen QL, Zhang XY, Li MM, Zhang X, Lin W. Association between methylenetetrahydrofolate reductase (MTHFR) polymorphisms and lung cancer risk in Chinese people: an updated meta-analysis. Medicine. (2019) 98:e16037. doi: 10.1097/MD.0000000000016037
68. Zhu JH, Ren AG, Hao L, Pei LJ, Liu JM, Zhu HP, et al. Variable contribution of the MTHFR C677T polymorphism to non-syndromic cleft lip and palate risk in China. Am J Med Genet A. (2006) 140:551–7. doi: 10.1002/ajmg.a.31115
69. Jiang W, Xu J, Lu XJ, Sun Y. Association between MTHFR C677T polymorphism and depression: a meta-analysis in the Chinese population. Psychol Health Med. (2016) 21:675–85. doi: 10.1080/13548506.2015.1120327
70. Xiong X, Lin XK, Xiao X, Qin DP, Zhou DY, Hu JG, et al. Association between MTHFR C677T polymorphism and diabetic nephropathy in the Chinese population: an updated meta-analysis and review. Nephrology. (2016) 21:5–12. doi: 10.1111/nep.12541
71. Ren AG, Zhang L, Hao L, Li ZW, Tian YH, Li Z. Comparison of blood folate levels among pregnant Chinese women in areas with high and low prevalence of neural tube defects. Public Health Nutr. (2007) 10:762–8. doi: 10.1017/S1368980007246786
72. Liu JF, Li ZW, Ye RW, Ren AG, Liu J. Folic acid supplementation and risk for congenital limb reduction defects in China. Int J Epidemiol. (2019) 48:2010–7. doi: 10.1093/ije/dyz130
73. Hao L, Ma J, Zhu J, Stampfer MJ, Tian Y, Willett WC, et al. High prevalence of hyperhomocysteinemia in Chinese adults is associated with low folate, vitamin B-12, and vitamin B-6 status. J Nutr. (2007) 137:407–13. doi: 10.1093/jn/137.2.407
74. Jacques P, Kalmbach R, Bagley P, Russo G, Rogers G, Wilson P, et al. The relationship between riboflavin and plasma total homocysteine in the Framingham Offspring cohort is influenced by folate status and the C677T transition in the methylenetetrahydrofolate reductase gene. J Nutr. (2002) 132:283–8. doi: 10.1093/jn/132.2.283
75. Cooney CA, Dave AA, Wolff GL. Maternal methyl supplements in mice affect epigenetic variation and DNA methylation of offspring. J Nutr. (2002) 132(8 Suppl.):2393s−400s. doi: 10.1093/jn/132.8.2393S
76. Schmidt RJ, Hansen RL, Hartiala J, Allayee H, Schmidt LC, Tancredi DJ, et al. Prenatal vitamins, one-carbon metabolism gene variants, and risk for autism. Epidemiology. (2011) 22:476–85. doi: 10.1097/EDE.0b013e31821d0e30
77. Schmidt RJ, Tancredi DJ, Ozonoff S, Hansen RL, Hartiala J, Allayee H, et al. Maternal periconceptional folic acid intake and risk of autism spectrum disorders and developmental delay in the CHARGE (CHildhood Autism Risks from Genetics and Environment) case-control study. Am J Clin Nutr. (2012) 96:80–9. doi: 10.3945/ajcn.110.004416
78. Orenbuch A, Fortis K, Taesuwan S, Yaffe R, Caudill MA, Golan HM. Prenatal nutritional intervention reduces autistic-like behavior rates among mthfr-deficient mice. Front Neurosci. (2019) 13:383. doi: 10.3389/fnins.2019.00383
79. Sadigurschi N, Golan H. Maternal and offspring methylenetetrahydrofolate-reductase genotypes interact in a mouse model to induce autism spectrum disorder-like behavior. Genes Brain Behav. (2019) 18:e12547. doi: 10.1111/gbb.12547
80. Huang X, Qin X, Yang W, Liu L, Jiang C, Zhang X, et al. MTHFR gene and serum folate interaction on serum homocysteine lowering: prospect for precision folic acid treatment. Arterioscler Thromb Vasc Biol. (2018) 38:679–85. doi: 10.1161/ATVBAHA.117.310211
81. James SJ, Melnyk S, Jernigan S, Cleves MA, Halsted CH, Wong DH, et al. Metabolic endophenotype and related genotypes are associated with oxidative stress in children with autism. Am J Med Genet B Neuropsychiatr Genet. (2006) 141b:947–56. doi: 10.1002/ajmg.b.30366
82. Raghavan R, Riley A, Volk H, Caruso D, Hironaka L, Sices L, et al. Maternal multivitamin intake, plasma folate and vitamin B levels and autism spectrum disorder risk in offspring. Paediatr Perinat Epidemiol. (2018) 32:100–11. doi: 10.1111/ppe.12414
Keywords: autism spectrum disorders, susceptibility, MTHFR, polymorphism, meta-analysis
Citation: Li C-X, Liu Y-G, Che Y-P, Ou J-L, Ruan W-C, Yu Y-L and Li H-F (2021) Association Between MTHFR C677T Polymorphism and Susceptibility to Autism Spectrum Disorders: A Meta-Analysis in Chinese Han Population. Front. Pediatr. 9:598805. doi: 10.3389/fped.2021.598805
Received: 25 August 2020; Accepted: 08 January 2021;
Published: 10 March 2021.
Edited by:
Merlin G. Butler, University of Kansas Medical Center, United StatesReviewed by:
Emanuele Micaglio, IRCCS Policlinico San Donato, ItalyCopyright © 2021 Li, Liu, Che, Ou, Ruan, Yu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hai-Feng Li, NjE5OTAwNUB6anUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.