
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
OPINION article
Front. Pediatr. , 24 June 2021
Sec. Pediatric Cardiology
Volume 9 - 2021 | https://doi.org/10.3389/fped.2021.471558
 Stefan Kurath-Koller1*
Stefan Kurath-Koller1* Martin Koestenberger1
Martin Koestenberger1 Georg Hansmann2
Georg Hansmann2 Massimiliano Cantinotti3
Massimiliano Cantinotti3 Cecille Tissot4
Cecille Tissot4 Hannes Sallmon5
Hannes Sallmon5Point of care ultrasound is essential in any emergency department (ED) and intensive care unit (ICU) and represents a well-established procedure in ICUs and EDs all over the world. In terms of cardiac evaluation, focused cardiac ultrasound (FOCUS) is recommended by a consensus statement of the American Society of Echocardiography (ASE) and the American College of Emergency Physicians (ACEP), outlining the important role of FOCUS in clinical medicine (1). The principal role for FOCUS is the time-sensitive hemodynamic assessment of the acutely ill patient (2, 3).
Neonatologists and pediatric intensivists may benefit from using FOCUS to assess a variety of objectives (e.g., guiding therapy or assess pericardial effusion) (4, 5). In any case, obtaining clearly defined echocardiographic views is required in order to perform adequate assessment. In expert hands, subcostal area views constitute an easily accessible option to perform a structured echocardiographic evaluation of the heart with the advantage of achieving clear views quickly, especially in neonates and infants, in whom cardiac structures are in proximity to the echocardiography probe (4). Herein, we provide a literature review of the current state of subcostal imaging supported by technical information on how to acquire subcostal window views.
FOCUS (also termed point-of-care cardiac ultrasound or bedside cardiac ultrasound, among others) represents a focused examination of the cardiovascular system, performed by a physician who uses ultrasound as an adjunct to the physical examination. The aim is to recognize specific sonographic signs that represent a narrow list of potential diagnoses in specific clinical settings (6).
FOCUS can be a helpful tool in the hands of trained non-specialists (i.e., pediatric intensive care specialists, neonatologists, or pediatric emergency physicians). Its goal is the early identification of limited hemodynamic changes, thus expediting establishment of initial diagnoses and clinical decision making (7). Trained non-specialists should be able to asses and focus on left ventricular systolic function, volume status/response to fluid resuscitation, pericardial effusion/cardiac tamponade, and right ventricular systolic function and pulmonary hypertension (7–10). By incorporating a few prechosen echocardiographic views, several conditions of interest can easily be confirmed or ruled out by targeted FOCUS examination (Table 1). Subsequent referral for a comprehensive echocardiographic study should be considered to delineate and quantify all findings, including incidental findings, which may unrecognized during FOCUS (7). Throughout FOCUS, abnormal findings are classified as present or absent using a predefined specific imaging protocol. The performing physician must adhere to this predefined protocol in order to provide specific diagnosis in a timely fashion while avoiding exaggerated examination. This approach allows for the rapid bedside detection of some selected pathologic conditions, which then must be interpreted in concert with all physical findings and the patient's history.
FOCUS aims to support and guide clinical decision-making. For example, in a patient presenting with acute shortness of breath or palpitations, FOCUS may detect impaired systolic contractility, thereby aiding decision making regarding pharmacological or interventional treatment (11).
Subcostal area views may allow for estimation of LV and RV function. This can be achieved using eyeballing of LV contraction in the subcostal short axis view, RV dimension measurement, or S-TAPSE determination (12, 13). However, except for RV dimension estimation and S-TAPSE, currently no normative values regarding left and right ventricular function parameters (e.g., ejection fraction, aortic, or pulmonary VTI) are available in the pediatric population.
Pericardial effusion (PE) resulting in cardiac tamponade is a critical condition which may be easily diagnosed using FOCUS. Pericardial tamponade is a clinical diagnosis supported by an echocardiographic view of pericardial fluid/blood in addition to clinical signs (e.g., arterial hypotension, tachycardia, or distended neck veins) (1). FOCUS will reveal PE with early diastolic RV collapse (1, 14, 15) and is highly accurate in diagnosing PE (sensitivity 96%, specificity 98%) (1, 15). For life-saving pericardiocentesis ultrasound may be used to guide needle insertion resulting in less complication and higher success rates, when compared to intervention without ultrasound based guidance (1, 14, 16). Subcostal area views may provide a quick assessment of a pericardial effusion and support guidance to needle insertion during pericardiocentesis. Furthermore, impaired RV function may be quantified by determination of S-TAPSE and RV dimensions (12, 13).
Evaluation of RV function often is crucial to estimate disease severity when treating children suffering from congenital heart disease (CHD) (2, 3). RV dimensions may be assessed using different projections (apical four chamber/five chamber view, parasternal long/short axis), and according to adult recommendations measurements of both, linear dimensions and areas should be achieved for proper functional evaluation of the RV (5, 17, 18). Recently, Cantinotti et al. (12) published nomograms for echocardiographic RV subcostal view dimensions in children. Estimation of RV function from subcostal area views may be performed using RV dimension measurements as well as S-TAPSE as a function of RV systolic shortening (13).
For echocardiographic evaluation and confirmation of PH the following parameters should be applied: estimation of systolic pulmonary artery pressure (PAP) by estimation of RV systolic pressure (RVSP) using the maximum velocity of the tricuspid regurgitation (TR) flow by continuous wave (CW)-Doppler; mean PAP and end-diastolic PAP by CW-Doppler velocity of pulmonary regurgitation (PR) flow; determination of RV longitudinal systolic function by tricuspid annular plane systolic excursion (TAPSE); RV strain and strain rate measurements; 3D echocardiographic RV volume determination; RV systolic to diastolic duration ratio; tissue Doppler velocities; RV/LV diameter ratio; eccentricity index, PA acceleration time (PAAT) (19).
Subcostal area views may allow an estimation of PH using S-TAPSE, tricuspid, and pulmonary valve regurgitation and RV dimensions (12, 13, 20). However, as sufficient literature supporting evaluation of PH from subcostal area views is lacking, assessment of PH should not be performed from subcostal area views alone, but by comprehensive echocardiographic evaluation as recently described (20).
Acute PH in newborn patients (persistent pulmonary hypertension of the newborn–PPHN, or persistent fetal circulation–PFC), is associated with high mortality despite ongoing advances in neonatal medicine. These infants are typically very sick and benefit from time-sensitive optimization of cardiorespiratory support. Clinical presentation may be misleading and mimic cyanotic congenital heart disease. In this case, FOCUS enables to rule out underlying major CHD, diagnose PH and assess its severity. Furthermore, it allows for efficacy monitoring of specific therapies (21, 22).
By using subcostal area views an estimation of PH may be possible using RV dimension measurements, assessment of shunt direction via foramen ovale (often bidirectional in the case of PPHN), TR jet velocity (used to estimate RVSP), S-TAPSE (determination of systolic RV function), or PR jet velocity (used to estimate mPAP and dPAP) (12, 13). However, as previously mentioned, assessment of PH should not be made from subcostal area views alone but requires a comprehensive echocardiographic evaluation (20). Subcostal area views may, however, be used as a last resort in situations of severe over-inflation, pneumothorax, following sternotomy, or others.
Following initial management, however, all patients with abnormal findings not previously documented should be referred to a comprehensive echocardiographic examination by a pediatric cardiologist. FOCUS performed by non-experts is not intended to completely evaluate a symptomatic cardiac patient.
Recently, FOCUS was demonstrated to be of use in assessment of pediatric septic shock, determining fluid responsiveness, obstructive physiology, and myocardial dysfunction (23).
FOCUS is both, sensitive and specific for identifying pericardial effusion and left ventricular systolic dysfunction when performed by non-experts with appropriate training (24). The latter, of course, is of major importance and needs to be updated regularly on a structured basis (25, 26).
General advantages of cardiac ultrasound, e.g., being a noninvasive risk-free method, performed serially and in real time, do of course also apply to FOCUS (7). Using the subcostal window views provides good spatial appreciation of cardiac structures, especially in congenital heart disease. Furthermore, it may provide improved imaging quality when transthoracic imaging is suboptimal, e.g., with pneumothorax or in infants after cardiac surgery with bandages in place. Especially in small children, toddlers and newborn/premature infants subcostal window views provide quick access and good imaging quality for FOCUS. Subcostal window views allow excellent assessment of pericardial effusions, left- and right ventricular function, venous filling, and volume status. Exploring the subcostal window views, one may assess the majority of cardiac anatomy, with restricted access to the great vessels (except for their most proximal part).
Due to spatial conditions, subcostal window views are less useful in adolescents, especially when obese. Even though transthoracic imaging can also be difficult in this particular patient group, it seems superior in image acquisition and quality when compared to subcostal window views. In the postoperative infant with mediastinal and pericardial drainages in place, usually placed in the subxiphoid region, obtaining subcostal window views can be challenging. A clear disadvantage of the subcostal approach lies within the assessment of the great arteries. While the proximal parts including the aortic and pulmonary valve are properly assessable, spatial distance to the ultrasound probe interferes with the assessment of more distal parts, including the aortic arch and pulmonary artery branches. These limitations also exist for assessment of persistent ductus arteriosus (PDA), one major issue in neonatal intensive care. Especially, hemodynamic significance of a PDA should not be assessed from subcostal area views as adequate visualization of the aortic isthmus is rarely possible to rule out coarctation and/or interrupted aortic arch.
Guidelines on FOCUS in pediatric patients were published by the American Society of Echocardiography in 2013 (6). In 2014, the American Society of Echocardiography also published evidence-based recommendations for FOCUS (26). Several articles report on the use of FOCUS in pediatric emergency care (27), pediatric critical care (28), and neonatology (29). However, the subcostal window views are alluded but remain to be described in detail by current publications. The majority of reports on subcostal window views originate from the 80s of the 20th century, reporting assessment of atrial septum defects, transposition of great arteries, or right ventricular wall thickness (28, 29).
More recent literature provides normative values and z-scores on right ventricular structure and function, including end diastolic basal-apical and latero-lateral diameters, end diastolic and end systolic area, four chamber end diastolic and end systolic area and length, end diastolic basal (RV1) and mid-cavity (RV2) diameters (12), as well as subcostal tricuspid annular plane systolic excursion (13).
To date, no true comparative studies outlining the advantages and disadvantages of subcostal window views over transthoracic views exist. Future studies on this topic are warranted. Despite one study comparing 3-D echo from apical, parasternal, and subcostal view to display the four chambers of the heart found subcostal window views to be inferior as compared to the apical views (30), 3-D studies remain beyond the goals of FOCUS.
Proper orientation of the acquired image on the screen is important (e.g., the LV should be positioned on the right side of the screen for subcostal views). Sweeps will provide detailed imaging and allow for assessment of the atrial and ventricular septum, atrioventricular valves, chambers, and systemic venous return. Furthermore, to assess right and left ventricular outflow tracts, rotating the transducer probe provides suitable imaging. Echocardiography is a skill dependent diagnostic tool and it is essential to outline that proper education and frequent training is required, even more so in infants and children. Therefore, “skill level” is of outmost importance and should be considered when reporting research results on FOCUS applications (31). Hébert et al. (32) emphasize the importance of expert training and need for competency based assessment.
There are some differences between the adult and pediatric population, e.g., which transducer to use in obtaining best views.
Low-frequency phased array transducers are commonly employed in transthoracic echocardiography (TTE) (1, 14, 33–35). Low frequency probes focus at 12–16 cm. It may be necessary to have the patient inhale and hold their breath in order to obtain the short axis images.
Using transducers with different frequencies is necessary in pediatric patients, ideally ranging from 12 to 4 MHz, as the patient population ranges from preterm infants to adolescents and young adults. Thereby imaging at different depths can be achieved. High frequency transducers come with high resolution, focusing at a depth of 4 to 5 cm, while lower frequency transducers focus at a greater depth and provide lower resolution. Therefore, using high frequency transducers in neonates and infants, in whom subcostal area views succeed best, is warranted (4).
In larger children and adolescents, similar to adults, it may be necessary to have the patient inhale and hold their breath in order to obtain the short axis images. However, in neonates and infants these views can be achieved quite easily and independently from respiration due to the proximity of the heart. In pediatric and neonatal intensive care patients, and in some children suffering from lung disease, as well as when the acoustic window is narrow and poor, the subcostal area approach may yield the best images of the heart (4).
Adequate knowledge and skill level can be acquired through courses on FOCUS, endorsed by national or international associations such as the AEPC (Association for European Pediatric and Congenital Cardiology) or AAP (American Academy of Pediatrics). Experts in the field of echocardiography are encouraged to teach FOCUS essentials to e.g., pediatric intensivists and neonatologists. In combination with a structured echocardiographic education and training, this should assure a high quality of FOCUS. Appropriate boundaries, e.g., when to consult an echocardiography expert and scope of competencies should be regulated by local echo-labs or pediatric cardiologists. However, assessments of global cardiac function and pericardial effusions may be adequately achieved by properly trained pediatric intensivists or neonatologists.
The following section provides an overview on specific subcostal window views.
a) How to obtain (Figure 1):
Place the transducer just inferior to the xiphoid process, with the transducer dot pointing at 3 o'clock.
b) What to identify:
Inferior vena cava (IVC), abdominal aorta, spine, liver, both hemidiaphragms.
c) Interpretation:
This view shows the descending aorta to the left and the IVC to the right of the spine. Aortic flow should be visualized using color Doppler with a pulsating pattern in 2D echocardiography. The liver is displayed to the right. This enables determination of normal abdominal visceral situs or otherwise situs anomalies, and levocardia when the cardiac apex is located to the left. Both hemidiaphragms are seen, moving symmetrically with respiration. Moreover, focusing on the hemidiaphragms fluid in the pleural cavity (pleural effusions) may be identified.
a) How to obtain (Figure 2):
Place the transducer below the xiphoid process and slide a bit to the patients' right. The transducer dot points at 1 o'clock. Slide until you get a clear view on the IVC running longitudinally by the liver, entering the right atrium (RA).
b) What to identify:
IVC, hepatic veins, RA, interatrial septum (IAS), left atrium (LA).
c)Interpretation:
IVC filling and collapsibility can be assessed (for details see below under “Volume Assessment”) and the venous flow pattern is displayed at the IVC-RA junction. Furthermore hepatic vein distension or reverse flow may be noted in case of elevated RA pressure, i.e., right heart failure or pulmonary hypertension (PH). In newborns and infants it is possible to display the IAS and the LA. A persistent foramen ovale (PFO) or atrial septal defect (ASD) may be seen. Using color Doppler determination of left-to-right or right-to-left shunt flow is possible.
a) How to obtain (Figure 3):
Tilt the transducer to the patients' left until the abdominal aorta is displayed longitudinally.
b) What to identify:
Abdominal and descending aorta, superior mesenteric artery (SMA), RA.
c)Interpretation:
Using color Doppler the abdominal and descending aorta may be identified showing a pulsatile flow pattern. Diastolic flow may be seen in upstream narrowing of the aorta, e.g., coarctation. Reverse flow pattern may be seen in significant aortic regurgitation (AR) or persistent ductus arteriosus (PDA). Furthermore, pulsatile flow and Doppler tracings of the SMA allow for resistance index (RI) calculation and estimation of visceral organ perfusion (1).
Place the transducer just inferior to the xyphoid process, positioning the index marker to the patient's left side and angling the transducer superiorly toward the left thorax. With the transducer dot pointing at 4 o'clock, tilting cranially for the subxiphoid four chamber view should be performed as described above. This represents the top view in subxiphoid long axis.
a) How to obtain (Figure 4):
Place the transducer just inferior to the xyphoid process, positioning the index marker to the patient's left side and angling the transducer superiorly toward the left thorax. Transducer dot pointing at 4 o'clock, tilting cranially.
b) What to identify:
RA, LA, right ventricle (RV), left ventricle (LV), IAS, interventricular septum (IVS), tricuspid valve (TV), mitral valve (MV), (left upper pulmonary vein LUPV).
c) Interpretation:
The subcostal four chamber view provides an overall view of the heart. It is one of the best views for detecting pericardial effusions and both atrial and ventricular septal defects because the jets usually arise relatively perpendicular to the septae and parallel to the ultrasound beam. Also, the subcostal four chamber view is one of the best views for assessing the functional status of an ASD closure device. Moreover, ventricular or atrial enlargement may be identified as well as tricuspid or mitral regurgitation. Estimation of left and right ventricular function using “eyeballing” is possible. LV or RV hypertrophy or dilation may be seen.
a) How to obtain (Figure 5):
From the subxiphoid long axis four chamber view, as described above, tilt the transducer caudally until you visualize the coronary sinus (CS).
b) What to identify:
RA, CS, LA, RV, LV, IVS, IAS in part.
c) Interpretation:
Coronary sinus (CS) blood flow can be identified using color Doppler. Prominent CS flow may be seen in partial anomalous pulmonary venous return (PAPVR), total anomalous pulmonary venous return (TAPVR), CS ASD, persistent left superior vena cava (PLSVC) draining to the dilated CS, or high RA pressure as seen in some cases of PH (2). A CS ASD due to unroofing of the CS might be suspected by color Doppler visualizing of a left-to-right or right-to-left shunt flow between LA and CS. Again a VSD or ASD might be visualized.
a) How to obtain (Figure 6):
From the subxiphoid four chamber view, tilt the transducer cranially until you visualize the left ventricular outflow tract (LVOT) and the aortic valve (AV).
b) What to identify:
SVC, RA, TV, RV, LV, AoV, ascending aorta, IVS, pulmonary artery (PA).
c) Interpretation:
The SVC entering the RA and associated flow can be visualized and SVC stenosis may be suspected if elevations of flow velocity are present. TV regurgitation may be visualized as well as a muscular or sub aortic VSD using color Doppler. A left ventricular outflow tract (LVOT) narrowing might be suspected by visualizing turbulences using color Doppler as well as obstructing tissue in 2D echocardiography. Aortic regurgitation (AR) may be seen using color Doppler, and additional LV hypertrophy may be suspected in 2D echocardiography. Using spectral Doppler, aortic velocity time integrals (AoVTI) may be measured and allow for serial monitoring of LV output (3). However, normative values for measurements from the subxiphoid area views currently do not exist for the pediatric population.
a) How to obtain (Figure 7):
From the subxiphoid four chamber view, as described above, rotate the transducer counterclockwise until you visualize the tricuspid valve, RV, RVOT and PV including the main pulmonary artery (MPA). Transducer dot pointing at 2 o'clock.
b) What to identify:
RA, TV, RV, RVOT, PV, MPA, IVS, LV in part.
c) Interpretation:
This view is best obtained in neonates and young infants and used to assess the right heart structures. TR, inflow via the tricuspid valve, hypertrophy or dilation of the RV, obstruction or dilation of the RVOT, and PR or pulmonary stenosis (sub-/supra-/valvular) may be identified. Tilting a bit more cranially may allow for visualization of the main pulmonary artery and its branches and thus evaluation for PDA or LPA/RPA stenosis. This “right heart view” is of particular value when assessing newborns or infants for Ebstein anomaly, Tetralogy of Fallot, or hypertrophied RV and allows for simultaneous imaging of RV function and PA flow (4). Moreover, pulmonary artery velocity time integrals (PaVTI) may be measured using pulsed wave Doppler to estimate pulmonary blood flow and RV output. Pulmonary artery acceleration time (PAAT) should be calculated and has been shown to be useful for evaluation of children and adult patients with PH (5, 11).
a) How to obtain (Figure 8):
From the subcostal four chamber view, rotate the transducer clockwise until the heart is visualized in its short axis. Tilt the transducer, in order to make the view of the left ventricle as circular as possible. Usually the transducer dot points at 6 o'clock.
b) What to identify:
LV, RV, papillary muscles.
c) Interpretation:
This view allows for visualization of a round left ventricle in short axis. In case of elevated right ventricular pressure (e.g., pulmonary hypertension or pulmonary stenosis) the septal wall appears flattened and may form a D-shape (12). Muscular ventricular septal defects may be seen using color Doppler and RV size may be estimated. LV hypertrophy may be seen. Using plane area measurement, LV ejection fraction may be estimated using fractional shortening (FS) (13).
a) How to obtain (Figure 9):
From the subxiphoid short axis view as described above tilt the transducer toward the patients' right side until you visualize the TV opening and closing within the RV and the right ventricular outflow tract (RVOT) and pulmonary valve (PV).
b) What to identify:
TV, RV, RVOT, PV, LV, MV in part.
c) Interpretation:
Pulmonary valve (PV) regurgitation may be seen as well as turbulent inflow via the tricuspid valve (TV) in case of e.g., TV stenosis. Using color Doppler, a muscular ventricular septal defect (VSD), especially an LV to RVOT shunt, as well as subvalvular and valvular PV stenosis may be identified e.g., in Tetralogy of Fallot.
a) How to obtain (Figure 10):
Tilt the transducer further to the patients' right until you visualize the IVS and superior vena cava (SVC) entering the RA.
b) What to identify:
RA, IVC, SVC, LA, IAS, right pulmonary artery (RPA).
c) Interpretation:
IVC and SVC inflow into the RA can be assessed and distinctive features of either of the central veins can be suspected by increased flow velocity using spectral Doppler. In particular, this view allows for evaluation of IVC/SVC inflow after atrial surgery involving patch closure of the IAS, which may lead to traction and aberration of the venous course. Furthermore, a PFO or ASD may be identified using color Doppler. Interruption of the IVC with azygous continuation can also be visualized from this view.
Subcostal window views intend to aid physicians performing FOCUS investigations. However, currently in children they cannot be recommended for exclusive use to determine cardiac anatomy and function due to lack of normative values in the pediatric cohort.
Outlining each the subcostal window views in detail above, we further provide a more comprehensive overview within the following tables (Tables 2, 3).
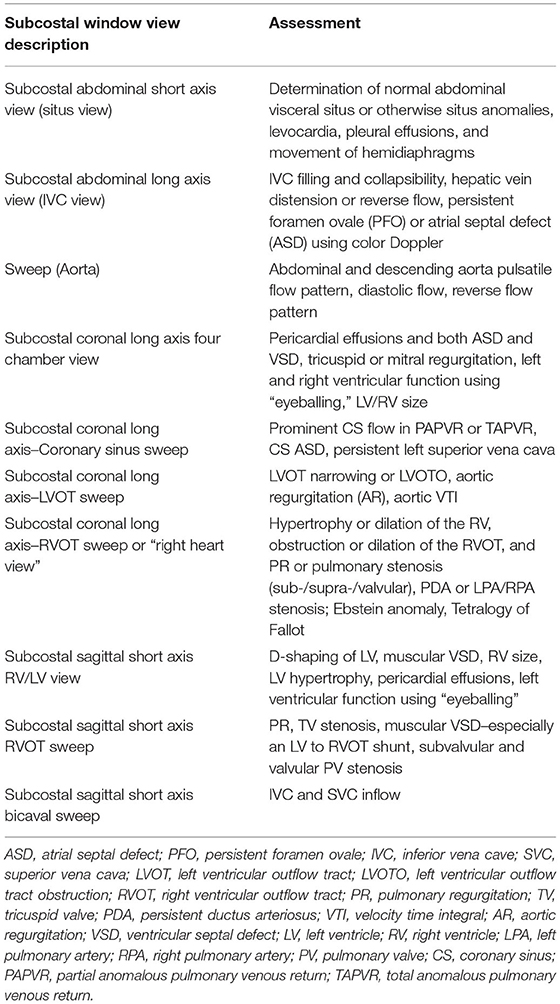
Table 2. List of available subcostal window views with assessable structures and functional parameters given for each view.
The use of FOCUS is well-established in pediatric emergency medicine, cirtical care and neonatal intensive care. Guidelines on the use of FOCUS exist but remain to be updated. As for all examiner-based techniques, education, and training to acquire and maintain a certain level of skill is of outmost importance, especially since FOCUS is usually performed by non-expert echocardiographers. Following a standardized protocol in order to assure quick and complete assessment of a limited number of diagnoses makes FOCUS highly valuable in clinical practice. Incorporation of subcostal window views into FOCUS may aid formulation of initial diagnosis and fast implementation of treatment. Since normative values for subcostal window views in pediatric patients, remain scarce, interpretation of findings from these views must be made with caution. It should be kept in mind that axis and angulation will differ to parasternal views, disabling the use of normative values derived from transthoracic views. Future research providing additional normative values and z-scores using a subcostal approach are required to improve the diagnostic value of subcostal window views.
SK-K and MK drafted the initial manuscript and reviewed and revised the manuscript. GH, HS, CT, and MC critically reviewed the manuscript for important intellectual content and revised the manuscript. CT recorded echocardiographic figures. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Labovitz AJ, Noble VE, Bierig M, Goldstien SA, Jones R, Kort S, et al. Focused cardiac ultrasound in the emergent setting: a consensus statement of the American Society of Echocardiography and American College of Emergency Physicians. JASE. (2010) 23:1225–30. doi: 10.1016/j.echo.2010.10.005
2. American College of Emergency Physicians. Use of Ultrasound Imaging by Emergency Physicians. Policy 400121. Available online at: https://www.acep.org (accessed May 09, 2019).
3. American College of Emergency Physicians. Emergency Ultrasound Guidelines.s. (2008). Available online at: https://www.acep.org (accessed May 09, 2019).
4. Tissot C, Muehlethaler V, Sekarski N. Basics of functional echocardiography in children and neonates. Front Pediatr. (2017) 5:235. doi: 10.3389/fped.2017.00235
5. Tissot C, Singh Y, Sekarski N. Echocardiographic evaluation of ventricular function-for the neonatologist and pediatric intensivist. Front Pediatr. (2018) 6:79. doi: 10.3389/fped.2018.00079
6. Spencer KT, Kimura BJ, Korcarz CE, Pellikka PA, Rahko PS, Siegel RJ. Focused cardiac ultrasound: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. (2013) 26:567–81. doi: 10.1016/j.echo.2013.04.001
7. Gaspar HA, Morhy SS. The role of focused echocardiography in pediatric intensive care: a critical appraisal. Biomed Res Int. (2015) 2015:596451. doi: 10.1155/2015/596451
8. Riera A, Weeks B, Emerson BL, Chen L. Evaluation of a focused cardiac ultrasound protocol in a pediatric emergency department. Pediatr Emerg Care. (2021) 37:1941–8. doi: 10.1097/PEC.0000000000001495
9. Noorbakhsh KA, Bell-Cheddar YR, Marin JR. Focused cardiac ultrasound in pediatric pulmonary hypertension. Pediatr Emerg Care. (2019) 35:316–8. doi: 10.1097/PEC.0000000000001700
10. Dessie A, Leung S, D'Amico B, Fischer KA, Binder Z, Abo A. Focused cardiac ultrasound to expedite diagnosis of pulmonary hypertension in children in the emergency department. Am J Emerg Med. (2020) 38:629–37. doi: 10.1016/j.ajem.2019.11.015
11. Sabia P, Abbott RD, Afrookteh A, Keller MW, Touchstone DA, Kaul S. Importance of two-dimensional echocardiographic assessment of left ventricular systolic function in patients presenting to the emergency room with cardiac-related symptoms. Circulation. (1991) 84:1615–24. doi: 10.1161/01.CIR.84.4.1615
12. Cantinotti M, Giordano R, Scalese M, Franchi E, Corana G, Assanta N, et al. Nomograms for echocardiographic right ventricular sub-costal view dimensions in healthy Caucasian children: a new approach to measure the right ventricle. J Cardiol. (2018) 71:181–6. doi: 10.1016/j.jjcc.2017.07.015
13. Kurath-Koller S, Avian A, Cantinotti M, Burmas A, Grangl G, Schweintzger S, et al. Normal pediatric values of the subcostal tricuspid annular plane systolic excursion (S-TAPSE) and its value in pediatric pulmonary hypertension. Can J Cardiol. (2019) 35:899–906. doi: 10.1016/j.cjca.2019.01.019
14. Kobner S. PV Card: Focused Echocardiography Ultrasound. ALiEM. (2015). Available online at: https://www.aliem.com/pv-card-focused-echocardiography-ultrasound/ (accessed April 28, 2017).
15. Whitson MR, Mayo PH. Ultrasonography in the emergency department. Crit Care. (2016) 20:227. doi: 10.1186/s13054-016-1399-x
16. Tayal V, Kline J. Emergency echocardiography to detect pericardial effusion in patients in PEA and near-PEA states. Resuscitation. (2003) 59:315–8. doi: 10.1016/S0300-9572(03)00245-4
17. American College of Emergency Physicians. Emergency Ultrasound Imaging Compendium. (2006). Available online at: https://www.acep.org
18. Society for Academic Emergency Medicine. Ultrasound Position Statement. Available online at: https://www.saem.org
19. Koestenberger M, Apitz C, Abdul-Khaliq H, Hansmann G. Transthoracic echocardiography for the evaluation of children and adolescents with suspected or confirmed pulmonary hypertension. Expert consensus statement on the diagnosis treatment of paediatric pulmonary hypertension. The European Paediatric Pulmonary Vascular Disease Network, endorsed by ISHLT D6PK. Heart. (2016) 102(Suppl. 2):ii14–22. doi: 10.1136/heartjnl-2014-307200
20. Hansmann G, Koestenberger M, Alastalo TP, Apic C, Austin EA, Bonnet D, et al. Updated consensus statement on the diagnosis and treatment of pediatric pulmonary hypertension. The European Pediatric Pulmonary Vascular Disease Network (EPPVDN), endorsed by AEPC, ESPR ISHLT. J Heart Lung Transplant. (2019) 38:879–901. doi: 10.1016/j.healun.2019.06.022
21. Nagiub M, Lee S, Guglani L. Echocardiographic assessment of pulmonary hypertension in infants with bronchopulmonary dysplasia: systematic review of literature and a proposed algorithm for assessment. Echocardiography. (2015) 32:819–33. doi: 10.1111/echo.12738
22. Jone PN, Ivy PD. Echocardiography in pediatric pulmonary hypertension. Front Pediatr. (2014) 2:1–15. doi: 10.3389/fped.2014.00124
23. Arnoldi S, Glau CL, Walker SB, Himebauch AS, Parikh DS, Udeh SC, et al. Integrating focused cardiac ultrasound into pediatric septic shock assessment. Pediatr Crit Care Med. (2021) 22:262–74. doi: 10.1097/PCC.0000000000002658
24. Miller AF, Arichai P, Gravel CA, Vieira RL, Neal JT, Neuman MI, et al. Use of cardiac point-of-care ultrasound in the pediatric emergency department. Pediatr Emerg Care. (2020). doi: 10.1097/PEC.0000000000002271. [Epub ahead of print].
25. Gaspar HA, Brunow de Carvalho W, Delgado AF. How to train and maintain pediatric intensivists updated in focused cardiac ultrasound? Pediatr Crit Care Med. (2016) 17:1015. doi: 10.1097/PCC.0000000000000901
26. Via G, Hussain A, Wells M, Reardon R, ElBarbary M, Noble VE, et al. International evidence-based recommendations for focused cardiac ultrasound. J Am Soc Echocardiogr. (2014) 27:683.e1–33. doi: 10.1016/j.echo.2014.05.001
27. Shopp L, Thoe J, Patel J, Maiers J, Cordes T, Nti BK. Interpretation of emergency department focused cardiac ultrasonography by pediatric emergency medicine physicians and pediatric cardiologists. Pediatrics. (2021) 147:485–6. doi: 10.1542/peds.147.3_MeetingAbstract.485
28. Mortera C, Rissech M, Payola M, Miro C, Perich R. Cross sectional subcostal echocardiography: atrioventricular septal defects and the short axis cut. Br Heart J. (1987) 58:267–73. doi: 10.1136/hrt.58.3.267
29. Marino B, de Simone G, Pasquini L, Giannico S, Marcelletti C, Ammirati A, et al. Complete transposition of the great arteries: visualization of left and right outflow tract obstruction by oblique subcostal two-dimensional echocardiography. Am J Cardiol. (1985) 55:1140–5. doi: 10.1016/0002-9149(85)90651-4
30. Ostenfeld E, Shahgaldi K, Winter R, Willenheimer R, Holm J. Comparison of different views with three-dimensional echocardiography: apical views offer superior visualization compared with parasternal and subcostal views. Clin Physiol Funct Imaging. (2008) 28:409–16. doi: 10.1111/j.1475-097X.2008.00823.x
31. Hu K, Gupta N, Teran F, Saul T, Nelson BP, Andrus P. Variability in interpretation of cardiac standstill among physician sonographers. Ann Emerg Med. (2017) 71:193–8. doi: 10.1016/j.annemergmed.2017.07.476
32. Hébert A, Lavoie PM, Giesinger RE, Ting JY, Finan E, Singh Y, et al. Evolution of training guidelines forechocardiography performed by the neonatologist: toward hemodynamic consultation. J Am Soc Echocardiogr. (2019) 32:785–90. doi: 10.1016/j.echo.2019.02.002
33. Price S, Uddin S, Quinn T. Echocardiography in cardiac arrest. Curr Opin Crit Care. (2010) 16:211–5. doi: 10.1097/MCC.0b013e3283399d4c
34. Neskovic AN, Flachskampf FA, Picard MH editors. Emergency Echocardiography. London: Taylor and Francis. (2005). p. 187. doi: 10.3109/9780203414699
Keywords: echocardiography, intensive & critical care, neonatal intensive care, subcostal approach, emergency & critical care
Citation: Kurath-Koller S, Koestenberger M, Hansmann G, Cantinotti M, Tissot C and Sallmon H (2021) Subcostal Echocardiographic Imaging in Neonatal and Pediatric Intensive Care. Front. Pediatr. 9:471558. doi: 10.3389/fped.2021.471558
Received: 11 May 2019; Accepted: 18 May 2021;
Published: 24 June 2021.
Edited by:
Ruth Heying, University Hospital Leuven, BelgiumReviewed by:
Ioannis Germanakis, University of Crete, GreeceCopyright © 2021 Kurath-Koller, Koestenberger, Hansmann, Cantinotti, Tissot and Sallmon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stefan Kurath-Koller, c3RlZmFuLmt1cmF0aEBtZWR1bmlncmF6LmF0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.