- 1Department of Pediatrics, University of Alberta, Edmonton, AB, Canada
- 2NICU, Northern Alberta Neonatal Program of Alberta Health Services, Edmonton, AB, Canada
- 3School of Public Health, University of Alberta, Edmonton, AB, Canada
- 4Wascana Children's Program, Regina, SK, Canada
- 5PICU Stollery Children's Hospital, Edmonton, AB, Canada
- 6Complex Pediatric Therapies Developmental Assessment Clinic at the Glenrose Rehabilitation Hospital of Alberta Health Services, Edmonton, AB, Canada
Background: Compared with those born at term gestation, infants with complex congenital heart defects (CCHD) who were delivered before 37 weeks gestational age and received neonatal open-heart surgery (OHS) have poorer neurodevelopmental outcomes in early childhood. We aimed to describe the growth, disability, functional, and neurodevelopmental outcomes in early childhood of preterm infants with CCHD after neonatal OHS. Prediction models were evaluated at various timepoints during hospitalization which could be useful in the management of these infants.
Study Design: We studied all preterm infants with CCHD who received OHS within 6 weeks of corrected age between 1996 and 2016. The Western Canadian Complex Pediatric Therapies Follow-up Program completed multidisciplinary comprehensive neurodevelopmental assessments at 2-year corrected age at the referral-site follow-up clinics. We collected demographic and acute-care clinical data, standardized age-appropriate outcome measures including physical growth with calculated z-scores; disabilities including cerebral palsy, visual impairment, permanent hearing loss; adaptive function (Adaptive Behavior Assessment System-II); and cognitive, language, and motor skills (Bayley Scales of Infant and Toddler Development-III). Multiple variable logistic or linear regressions determined predictors displayed as Odds Ratio (OR) or Effect Size (ES) with 95% confidence intervals.
Results: Of 115 preterm infants (34 ± 2 weeks gestation, 2,339 ± 637 g, 64% males) with CCHD and OHS, there were 11(10%) deaths before first discharge and 21(18%) deaths by 2-years. Seven (6%) neonates had cerebral injuries, 7 had necrotizing enterocolitis; none had retinopathy of prematurity. Among 94 survivors, 9% had cerebral palsy and 6% had permanent hearing loss, with worse outcomes in those with syndromic diagnoses. Significant predictors of mortality included birth weight z-score [OR 0.28(0.11,0.72), P = 0.008], single-ventricle anatomy [OR 5.92(1.31,26.80), P = 0.021], post-operative ventilation days [OR 1.06(1.02,1.09), P = 0.007], and cardiopulmonary resuscitation [OR 11.58 (1.97,68.24), P = 0.007]; for adverse functional outcome in those without syndromic diagnoses, birth weight 2,000–2,499 g [ES −11.60(−18.67, −4.53), P = 0.002], post-conceptual age [ES −0.11(−0.22,0.00), P = 0.044], post-operative lowest pH [ES 6.75(1.25,12.25), P = 0.017], and sepsis [ES −9.70(−17.74, −1.66), P = 0.050].
Conclusions: Our findings suggest preterm neonates with CCHD and early OHS had significant mortality and morbidity at 2-years and were at risk for cerebral palsy and adverse neurodevelopment. This information may be important for management, parental counseling and the decision-making process.
Introduction
Infants with complex congenital heart defects (CCHD) who have open-heart surgery (OHS) during the neonatal period are at risk for mortality and neurodevelopmental morbidity (1, 2). Advancements in obstetric and neonatal care have improved the overall outcomes of preterm infants (born at <37 [+0] weeks gestation) significantly with high survival rates without neurodevelopmental impairment in early childhood (3). However, when compared with term neonates, preterm infants generally have more adverse short- and long-term neurodevelopmental outcomes depending on the degree of prematurity (3, 4). It has been shown that delivery before 39–40 weeks of gestational age (GA) is associated with higher mortality and worse neurodevelopmental outcomes for those infants with CCHD having cardiac surgery (5–12).
Despite the adverse outcome compared with that of term infants, there is little information available regarding “gestation-related details” and predictors of outcomes in early childhood of preterm neonates with CCHD and early OHS at “different time points during hospitalization.” This information is important for management of the infants, parental counseling and informing the decision-making process. We therefore aimed to describe the growth, disability, functional, and neurodevelopmental outcomes in early childhood of preterm infants with CCHD who had OHS with cardiopulmonary bypass (CPB) by 6 weeks corrected age. The secondary aims of the study included the identification of risk factors for mortality and adverse functional outcomes at each of five time points that we believe are important moments of clinical decision-making and family counseling: (i) before and at birth, (ii) pre-operative, (iii) day 1 post-operatively, (iv) post-operatively day 5, and (v) at first hospital discharge.
Methods
We studied all preterm infants with CCHD who received OHS with CPB at 6 weeks of corrected age or less at the Stollery Children's Hospital in Edmonton, Alberta, Canada, between 1996 and 2016. Demographic, clinical and outcomes data were obtained from the Western Canadian Complex Pediatric Therapies Follow-up Program. This program maintains a prospectively collected registry and database including demographic, acute-care clinical and long-term neurodevelopmental outcomes in all infants who have complex cardiac surgery (CCS) at 6 weeks of age or less (13). The clinical information includes pre-operative, intra-operative, and post-operative data of these infants during the first hospital stay when the OHS was performed. Details of the methodology of this program have been previously published (13, 14). All neonates had standard genetic testing and geneticist assessment as appropriate. As per neuroimaging study protocol in these preterm infants, all had pre-operative cranial ultrasound examination with magnetic resonance imaging as indicated by clinical and neurologic findings. The occurrence of necrotizing enterocolitis and retinopathy of prematurity for this project was obtained by retrospective chart review. Consents were obtained from the patient's legal guardian. The study was approved by institutional ethics boards at all six follow-up sites.
Outcomes
Disability and developmental assessments at a corrected age of 2 years were completed by multidisciplinary teams at the follow-up referral sites, of Winnipeg, Manitoba; Regina and Saskatoon, Saskatchewan; Calgary, Alberta; Vancouver, British Columbia, and the Glenrose Rehabilitation Hospital, Edmonton, Alberta, Canada. Standardized age-appropriate outcome measures included physical growth with calculated z-scores (15, 16), a functional measure (17) and a neurodevelopmental test of cognitive, language, and motor skills (18). Pediatricians experienced in developmental follow-up examined each child for evidence of cerebral palsy (defined as a group of permanent disorders of the development of movement and posture, causing activity limitation, that are attributed to non-progressive disturbances that occurred in the developing fetal or infant brain) (19) or visual impairment (defined as corrected visual acuity in the better eye of <20/60). Permanent hearing impairment was defined as responses in the better ear of >40 dB at any frequency from 250 to 4,000 Hz (13).
A parent completed questionnaire, the Adaptive Behavior Assessment System, second edition (ABAS-II) determined child functional abilities (17). The ABAS-II measures realistic, independent behaviors of young children and the effectiveness of interactions with others, while considering the community context. Thus, this rating scale measures daily living skills, that is, what children can do, without the assistance of others. The ABAS-II for children under age 6 years consists of nine skill areas grouped into three composite domains: Conceptual—communication, functional pre-academics, and self-direction; Practical—home living, health and safety, community use and self-care; and Social—leisure and social. A separate rating for motor skills is added to the nine skill areas to determine the overall score, the General Adaptive Composite (GAC) score. Age-based United States population norms for children have mean of 100 and a standard deviation (SD) of 15; skill areas have a mean (SD) of 10(3) (17). The ABAS-II GAC was used as the main outcome measure for analysis as it was available for all children.
Certified pediatric psychologists and psychometrists administered the Bayley Scales of Infant and Toddler Development, third edition (Bayley-III) (18). The Bayley-III is a technically sound instrument of early childhood development, with strong internal consistency, high test–retest stability and employs a representative sample based on United States demographics. This study corrected for age up to 2 years as recommended (18). The Cognitive Scale is made up of largely non-verbal activities involving memory, problem solving, and manipulation of the physical world. The Language Scale is composed of Receptive and Expressive Communication subtests. The Motor Scale includes Fine Motor (visual–motor integration, visual–spatial, and fine-motor control skills), and the Gross Motor (large body movements, mobility, and complex movements) subtests. The population mean(SD) is 100(15) (18). As our study spans over 2 decades and the Bayley-III was published in 2006, only 71 of the 94 survivors have these results and hence this measure was not used as the main outcome measure.
Anonymous data were returned to the central site for adjudication by one of the investigators (13). Maternal education was indicated by years of schooling. The Blishen Index, population mean (SD) of 43 (13) and based on employment of the main wage earner of each family, was used to reflect socioeconomic status (20).
Statistical Analysis
CCHD were coded according to cardiac anatomy, particularly the presence of single ventricle shown to be predictive of perioperative mortality (1), and perioperative physiology as described previously (13). Mortality and outcomes were analyzed as categorical or continuous variables.
Objective 1: First, we conducted a descriptive analysis to explore the distribution of mortality and each neurodevelopmental outcome using mean, SD, median, and interquartile range (IQR). Student t, Fisher's Exact and Chi-square tests were used to compare groups.
Objective 2: To determine potential predictors of adverse outcomes [mortality, and ABAS-II-GAC scores], we used univariate and multiple variable regression analyses.
a. Univariate analyses: Using logistic regression, we compared the demographic and clinical variables at each time point between the surviving and non-surviving groups of infants during the first hospital stay for OHS. Using linear regression, we analyzed predictors of the ABAS-II GAC scores as a continuous variable. Following these comparisons, risk factors with P < 0.1 were selected for the multiple variable regression models.
b. Multiple variable analyses: The effect of risk factors for mortality and ABAS-II GAC scores was further examined in multiple variable models using five clinical time points for analyses. We chose these five periods as they are important time points informing clinical management decisions and family counseling. The five time points were: (i) before and at birth, (ii) pre-operative, (iii) post-operative day 1, (iv) post-operative day 5, and (v) before hospital discharge. Predictive analyses were performed at each time point with the retainment of risk factors which had P < 0.1 in the previous clinical period. Because of the large association of chromosomal abnormalities with adverse outcome, the prediction of the ABAS-II GAC scores was further completed with the non-syndromic 76 children. Only potential predictor variables that occurred in 10% of more of the total population were entered in the regressions. In view of the small number of infants in some GAs, we also analyzed the effects of GA on outcomes using three different categorical bands (28–31, 32–34, and 35–37 weeks).
Continuous variables were tested for the normality of their distribution and are presented as means (SD) or medians (IQR). Odds Ratio (OR) and Effect Size (ES) are reported with 95% confidence intervals [CI]. Significance was determined as P ≤ 0.05. Data analyses were performed using R software V3.6.1 (21).
Results
General Description
From September 1996 to December 2016, all 115 preterm infants with CCHD who received OHS at ≤6 weeks corrected age at the Stollery Children's Hospital were registered. These 115 children made up 10.7% of all children at age 6 weeks and under having complex cardiac surgery with CPB at this institution during this time. The demographic and clinical characteristics are shown in Table 1. Of these infants, 11 (9.6%) and 21 (18.3%) died before first hospital discharge and by the age of 2 years corrected age, respectively. The reasons for death before first discharge included multiple organ dysfunction (9), brain hemorrhage (1) and septic shock (1). The causes of additional deaths included cardiac failure with chronic illness (5), thrombo-embolic event (1), probable non-accidental injury (1), sepsis (1) and unknown (2). Prior to the first surgery, 7 (6.1%) neonates had cerebral injuries known to be risk factors for disability [including infarction (3), periventricular leukomalacia (2), and grade 3–4 intraventricular hemorrhage (2)]. In addition, 7 (6.1%) preterm infants had necrotizing enterocolitis. None had retinopathy of prematurity.
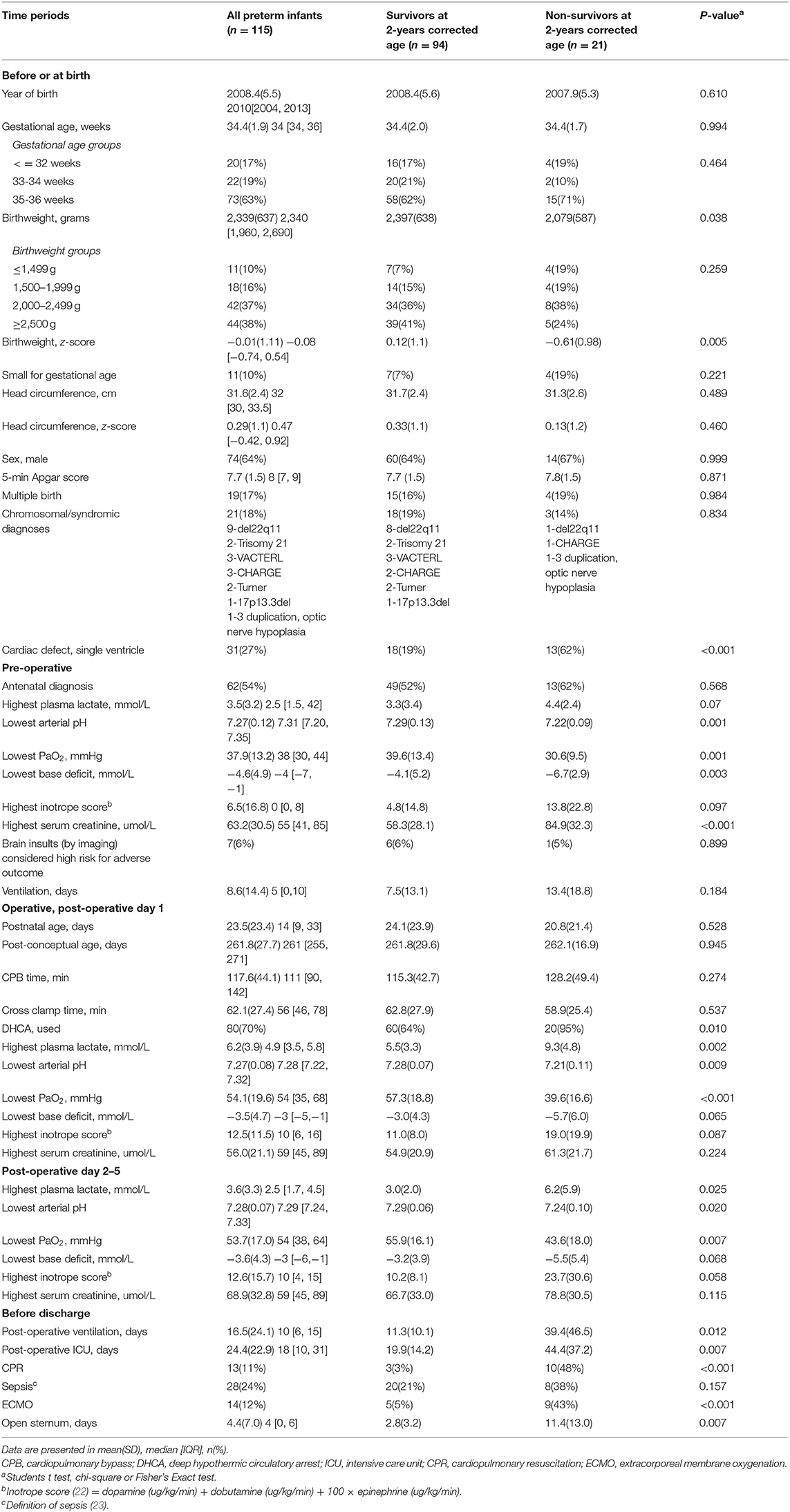
Table 1. Clinical characteristics of 115 preterm infants with complex congenital heart defects who had open heart surgery at ≤6 weeks corrected postnatal age from September 1996 to December 2016.
Mortality Prediction
Following the five different time points when important parental counseling sessions may occur, Table 2 shows that among the risk factors studied at the before and at birth time point, birthweight z-scores and primary cardiac defect of single ventricle were associated with death and these factors remained significant when including variables from all time points. Other risk factors included the lowest arterial pH at the pre-operative time point, lowest arterial pH and highest lactate level post-operatively, and cardiopulmonary resuscitation (CPR) and days of post-operative ventilation before discharge. GA, postnatal age and the post-conceptual age of surgery were not statistically significantly different between survivors and non-survivors (Table 1).
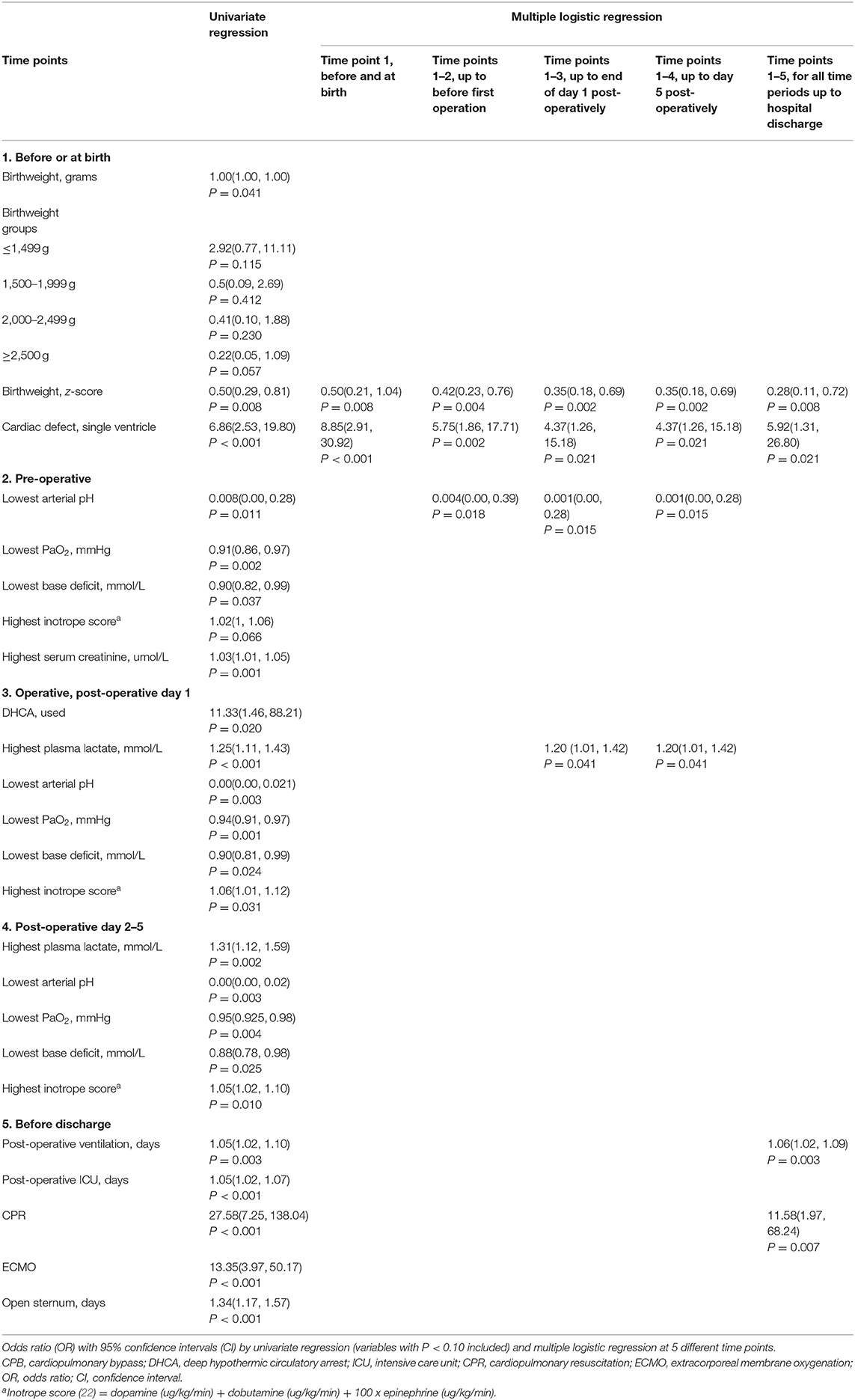
Table 2. Predictive factors of death by 2-years corrected age in 115 preterm infants with complex congenital heart defects who had early open heart surgery from September 1996 to December 2016.
Growth, Disability, and Neurodevelopmental Outcomes
All 94 (100%) surviving infants received neurodevelopmental evaluation at 2 years corrected age. The demographic and clinical characteristics of these surviving preterm infants with (n = 18, 19.1%) and without (n = 76, 80.9%) syndromic diagnoses are shown in the Table 3. The physical growth and neurodevelopmental outcomes are shown in Table 4. Although growth, functional, and neurodevelopmental parameters of survivors are within the population normative range, the means are shifted to the left of population norms. Family socioeconomic status, years of mother's schooling, as well as 2-year growth, did not differ between those with and without syndromes (Table 4). Eight (10.5%) of the 76 survivors without identified syndromic diagnoses had spastic cerebral palsy; of these, three had pre-operative brain insult with risk for motor disability. Visual impairment occurred equally in each group: in the syndromic group one child had bilateral optic nerve colobomata; in the non-syndromic group one child had cortical visual impairment. Of the five infants with permanent sensorineural hearing loss, only one had a syndromic abnormality. Those infants with syndromic diagnoses had worse functional and cognitive outcomes compared with those without syndromes (Table 4).
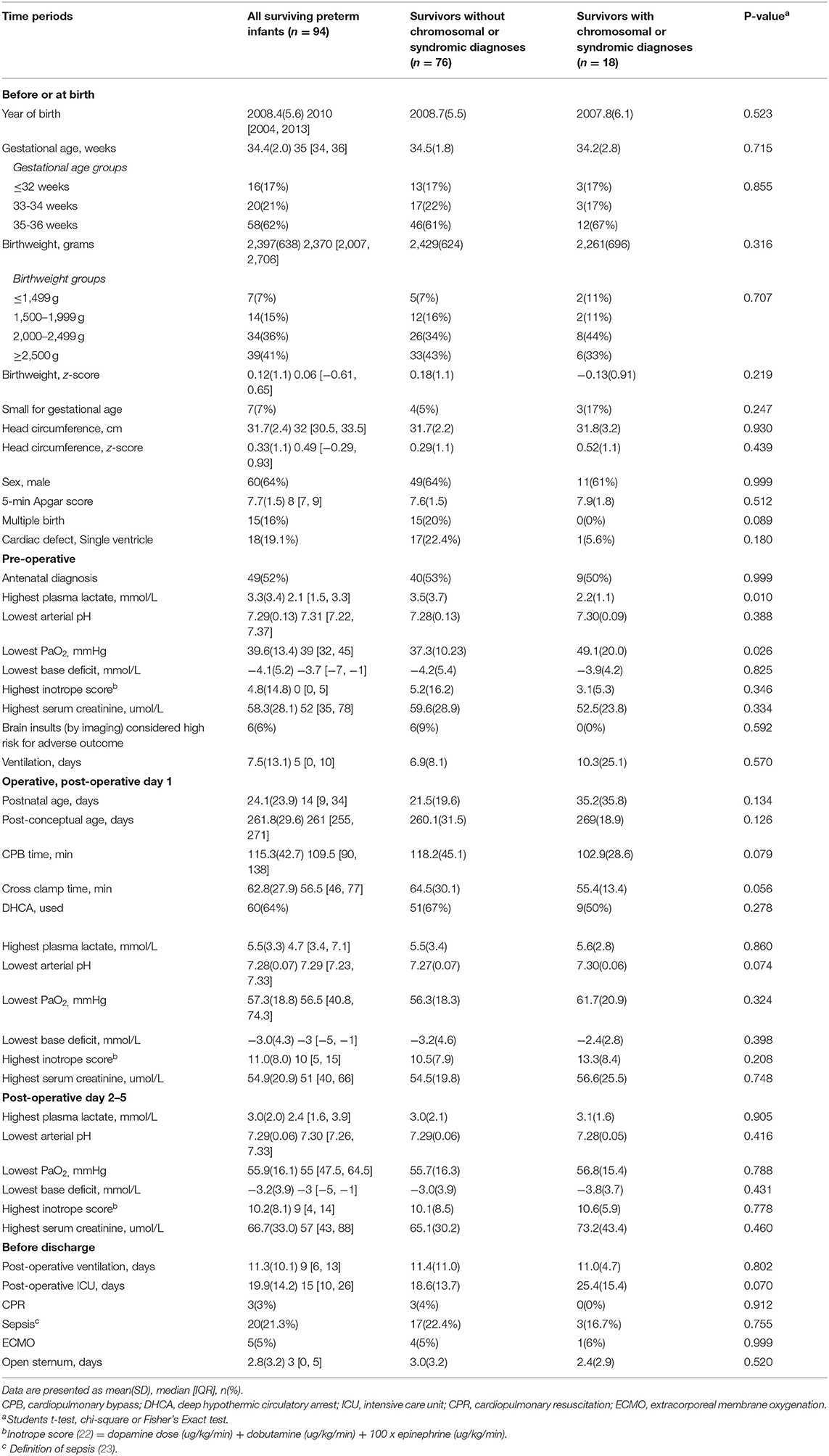
Table 3. Clinical characteristics of 94 surviving preterm infants after open heart surgery at 6 weeks or less corrected postnatal age from September 1996 to December 2016.
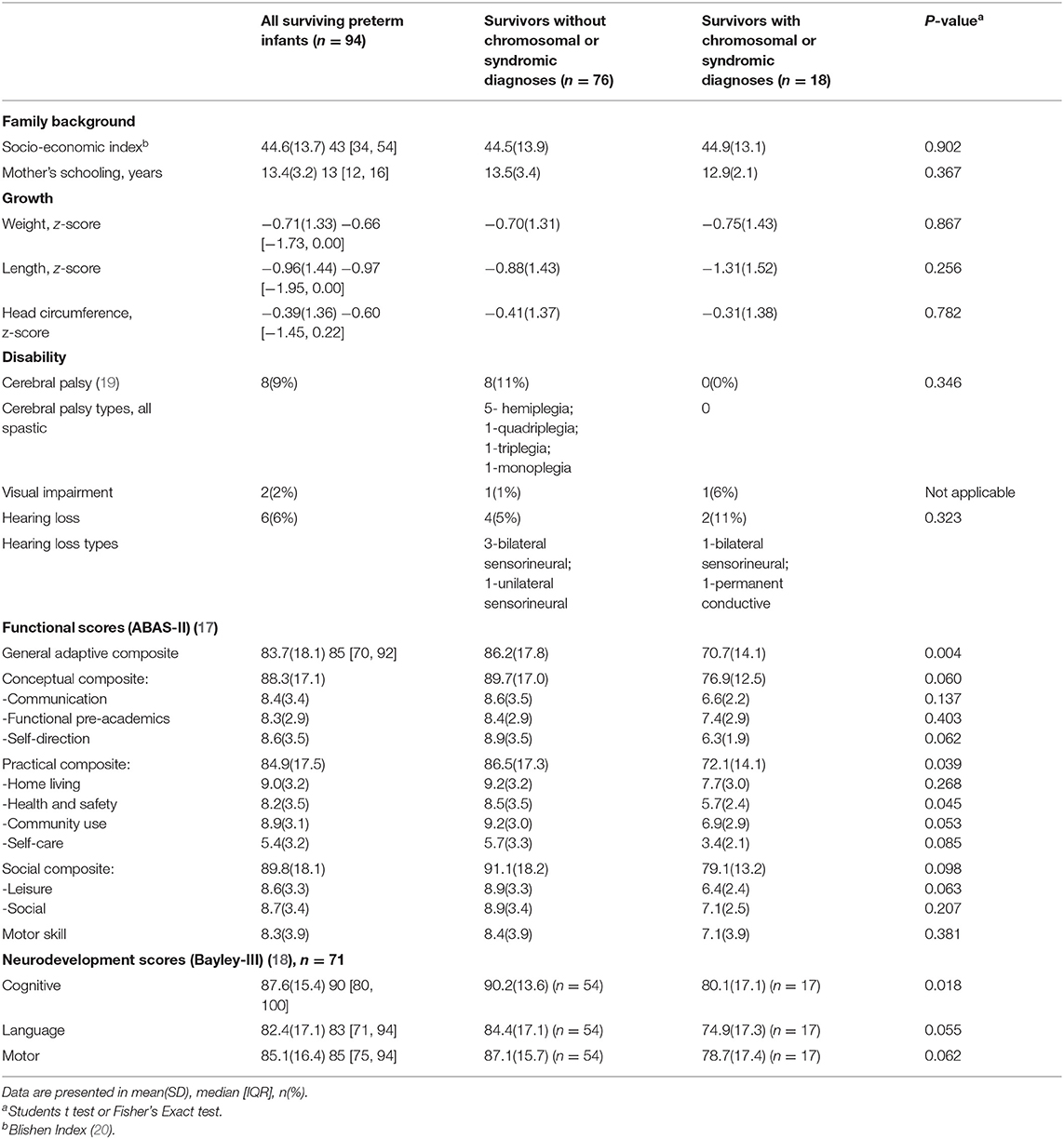
Table 4. Growth and neurodevelopmental outcomes at 2-years corrected age for 94 surviving preterm infants after early open heart surgery from September 1996 to December 2016.
Functional Outcome Prediction
As there was a significant contribution of chromosomal/syndromic diagnoses to ABAS-II GAC at 2 years corrected age in the 94 surviving preterm infants [ES(95%CI) −13.8(−22.5, −5.1)], we sought possible predictors of outcome including only the 76 infants without syndromic abnormalities (Table 5). We did not evaluate the effects of pre-operative brain injury because of the small number of infants affected (n = 6). Of the birthweight groups, those with a weight of 2,000–2,499 g (n = 26) had ABAS-II GAC scores significantly below the others (n = 50), [76.9 ± 13.7 vs. 90.5± 16.6, t = 3.593, P = 0.001]. This birth weight of 2,000–2,499 g was associated with the ABAS-II GAC at each of the 5 different time points. The adjusted R2 of the variance in ABAS-II GAC scores at 2 years accounted for in the multiple logistic models was 0.263 before discharge from the hospital (Table 5). The explained variance was 0.137 at birth with the predictor birthweight of 2,000–2,499 g alone. Other statistically significant predictive risk factors also included post-conceptual age of surgery, lowest arterial pH on day 2–5 post-operatively, and sepsis. GA, birthweight z-sores and postnatal age of surgery were not found to be independent variables associated with functional outcome.
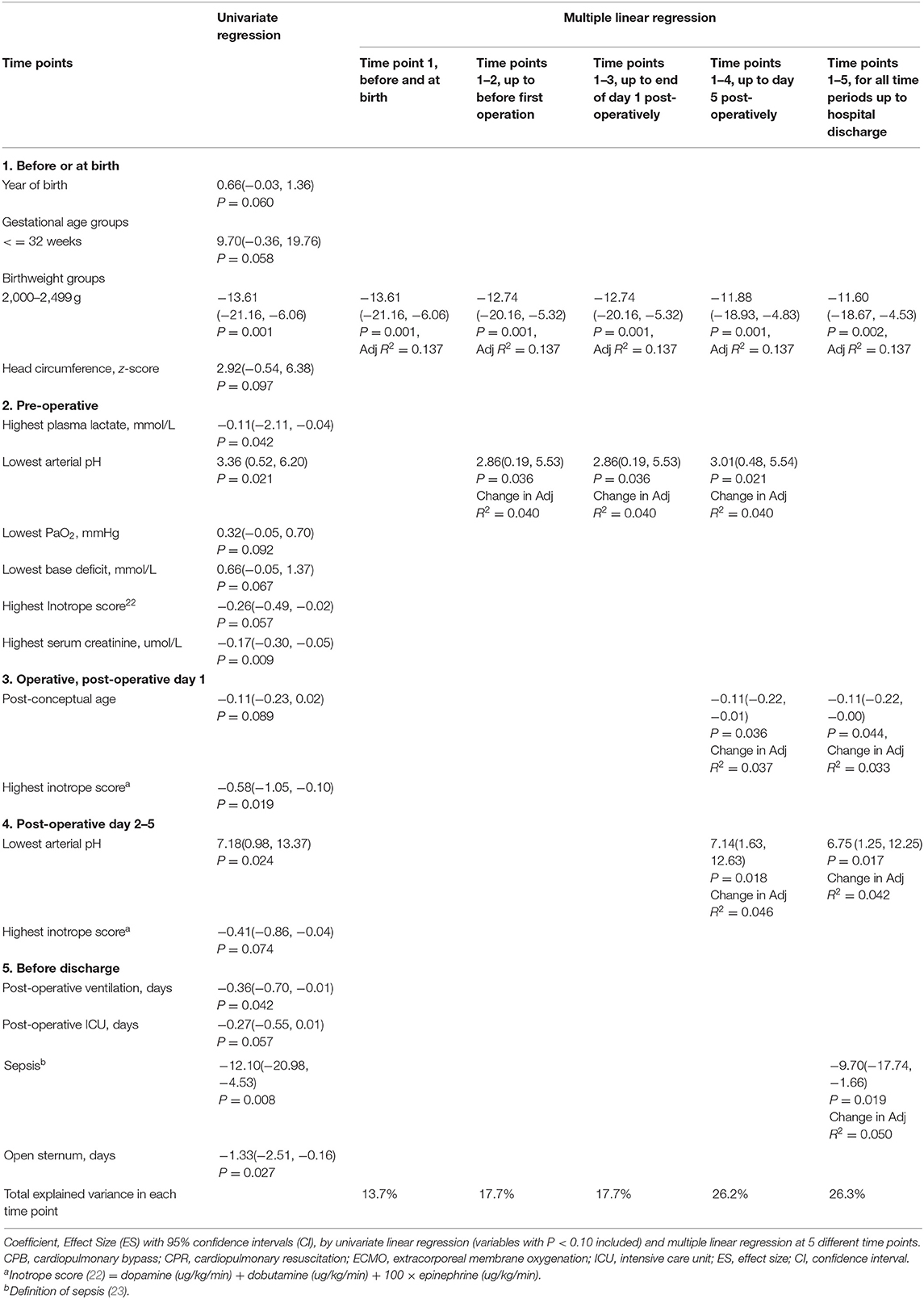
Table 5. Predictive factors of General Adaptive Composite (ABAS-GAC) at 2-years of corrected age for 76 preterm infants without chromosomal/syndromic abnormalities with complex congenital heart defects who had early open heart surgery from September 1996 to December 2016.
Discussion
This study adds “gestation-related information” to the mortality and developmental outcome information of preterm neonates with CCHD (5–12, 24, 25), and may be useful for the in-hospital management of patients and families including but not limited to (a) the timing of surgical correction, (b) the identification and correction of risk factors that are associated with adverse neurodevelopmental outcomes, (c) parental counseling on the course, care plan, possible outcomes, and importance of follow-up and early interventions, and (d) informing decision-making so clinicians have a better understanding of the course and outcome of these critically-ill preterm neonates.
The preterm neonates of this study with CCHD (mean GA of 34.4 weeks; mean birthweight of 2,339 g), show a high mortality (18.3%) at 2 years of corrected age. When compared with preterm infants of lower GA (31–32 weeks) in the Canadian Neonatal Network, those with CCHD have more neonatal short-term complications including brain injuries (6.1% vs. 1–2% of PVL and parenchymal lesions) and necrotizing enterocolitis (6.1 vs. 1%), but not of retinopathy of prematurity (0 vs. 0% of Stage 3/4/5) (26). The prevalence of cerebral injuries with known association with disability in this group of moderate-late preterm and modestly low birthweight infants was high, 7 (6.1%). Chronic lung disease and patent ductus arteriosus were not examined in this cohort because the respective conditions were difficult to diagnose in surviving neonates with co-morbid cardiopulmonary condition of CCHD after OHS. Growth of these 2-year-old children was within normal ranges, although skewed to the left of population norms.
Consistent with previous reports (13, 27, 28), we have shown the primary cardiac defect of single ventricle and the peri-operative risk factor of CPR are important determinants of mortality. While GA and body weight did not predict death, the intra-uterine growth of the fetus as reflected by low birthweight z-scores was critical for this prediction. As part of the consideration of termination of pregnancy for a fetus with CCHD, it is important to examine fetal growth rather than GA alone, in addition to the compounding effect of CCHD. Postnatal age and post-conceptual age of surgery were not related to mortality. This finding should be interpreted with caution because of the small study population. We speculate that postnatal transition of cardiopulmonary systems may affect the tolerance of OHS, a pathophysiological phenomenon which is similar to the delay of surgery in infants with congenital diaphragmatic hernia (29). Therefore, based on the relation between outcome and postnatal and post-conceptual age, we suggest the OHS should be postponed to the time after feto-neonatal transition with the stabilization of cardiopulmonary status.
This study provides information about the early childhood outcomes that may be useful for counseling parents of preterm neonates with CCHD. Dysmaturation and dysregulation of cortical neuronal development due to reduced cerebral oxygenation in CCHD might be responsible for neurodevelopmental adversity (30, 31). Cerebral palsy was found in 10.5% for those without syndromic diagnoses; this suggests higher rates of cerebral palsy than for preterm survivors of the same GA without CCHD (32, 33) and higher than those of mostly term infants with CCHD (34). This study also suggests that sensorineural hearing loss was higher than that for preterm infants without CCHD (35, 36) and similar to term infants with CCHD (37). Vision loss was not increased for these preterm children over other preterm rates (35, 36). This study did not compare CCHD children with and without prematurity, however, mean scores for functional and neurodevelopmental outcomes of preterm neonates with CCHD in this study tend to be within the lower range of published results for preterm neonates (33, 35, 36). Particularly, low scores for self-care skills and language abilities indicate the need for early developmental testing and appropriate interventions for these children (38–40).
Among the predictive factors, the presence of syndromic diagnoses adversely affected neurodevelopmental outcome, as previously reported (41). Within birthweight subgroups, a birthweight of 2,000–2,499 g had a negative (unexpected) correlation with ABAS-II GAC in 76 preterm neonates without syndromic abnormalities. While Dimmick et al. (42) observed that low birthweight was associated with adverse outcomes in infants with CCHD, we do not know the exact reason to explain this relationship, which warrants replication to determine its importance. Due to the retrospective study design and small sample size, risk factors including CPR, inotrope scores, GA, birthweight z-scores and postnatal age of surgery had modest or no effects on the ABAS-II-GAC. Older post-conceptual age, low post-operative pH and sepsis added to the prediction. We have previously shown the significant effect of sepsis on adverse outcomes after early cardiac surgery (23). Using multiple variable analyses for prediction of ABAS-II GAC at 2 years corrected age, the cumulative variance explained was 26.3% in preterm infants without syndromic diagnoses. This highlights the possible importance of post-discharge risk factors in subsequent neurodevelopment, in addition to possible risk factors during pregnancy and hospitalization that we did not study.
Our study has several limitations. The research objective was retrospectively determined. Single center study and changes in clinical practice and surgical interventions over a span of 25 years, some antenatal risk factors were missed. This includes but limits to the study of cardiovascular and cerebrovascular state. Small sample size limited us from finding statistically significant relationships. In particular, there were only 6 infants with brain injuries identified in the pre-operative period and thus we could not study its predictive role in neurodevelopment in the multiple variable model. However, information on brain injury before surgery would be helpful in counseling. We did not have detailed information of the maternal conditions associated with the preterm births. Future investigations should address if these conditions may have impact on in utero growth or adverse post-natal effects on the infants which are independent of the gestational age. Given these limitations, our findings should be interpreted with caution and generalization to other centers. The use in parental counseling may be limited. Further multicenter prospective study will be needed to confirm our findings. Strengths of this study include the prospective inception cohort design with most data collected prospectively, and the detailed neurodevelopmental and functional outcomes determined prospectively without any loss to follow-up of survivors. Of note, the infants who did not survive were sicker than the survivors. While it may be interesting to study the prediction of a combined group of adverse outcomes including non-survivors and disabled survivors, the current study design was to study the prediction of mortality and neurodevelopmental outcomes in a separate manner.
Conclusions
Preterm neonates with CCHD and early OHS had significant mortality and morbidity. Early outcomes suggest more cerebral palsy and lower functional and neurodevelopmental scores than occurring with prematurity alone. In this preterm group, predictive factors for mortality included birthweight z-scores, cardiac lesion of single ventricle, prolonged post-operative ventilation and the need for CPR. In addition to syndromic diagnoses, predictive factors for adverse functional outcome (ABAS-II GAC) included birth weight 2,000–2,499 g, older post-conceptual age of surgery, post-operative acidosis, and sepsis. These findings may aid clinicians in discussing outcomes with families. While parents are anxious and stressed regarding the prognosis of these infants with CCHD, it may be difficult to explain to the parents and counsel them regarding the impact of birth weight category and post-conceptual age on the outcomes. Nevertheless, the information may help explain the management of these infants including the timing of delivery and surgery. Future studies may assist in providing better counseling. Furthermore, these findings emphasize the importance of long-term follow-up of survivors to detect neurodevelopmental concerns that can be addressed by early intervention programs, ensuring these vulnerable children can reach their full potential.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Institutional ethics boards at all six follow-up sites (University of British Columbia, University of Alberta, University of Calgary, University of Regina, University of Saskatchewan, and University of Manitoba). Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author Contributions
P-YC conceptualized and designed the study, drafted the initial manuscript, reviewed, and revised the manuscript. CR designed the data collection instruments, conceptualized and designed the study, supervised data collection, critically reviewed, and revised the manuscript. MH and ID carried out the statistical analyses, and critically reviewed and revised the manuscript. HS and AJ conceptualized and designed the study, supervised data collection, and critically reviewed the manuscript for important intellectual content. GB conceptualized and designed the study, coordinated data collection, and critically reviewed the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Funding
The Western Canadian Complex Pediatric Therapies Follow-up Program has been supported over the years by contributions from the following: Alberta Health, Stollery Children's Hospital, Women and Children's Health Research Institute, referral-site follow-up clinics (Saskatoon and Regina SK, Winnipeg MB, Calgary AB, Vancouver BC), and the Glenrose Rehabilitation Hospital Research Trust, with ongoing funding by the Glenrose Rehabilitation Hospital. These funding agencies had no role in the design and conduct of the study; collection, analysis or interpretation of the data; preparation, writing, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
ABAS-II, Adaptive Behavior Assessment System, second edition; CCHD, complex congenital heart defects; CI, confidence interval; CPB, cardiopulmonary bypass; CPR, cardiopulmonary resuscitation; ES, effect size; GA, gestational age; GAC, General Adaptive Composite; IQR, interquartile range; OHS, open heart surgery; OR, odds ratio; SD, standard deviation.
References
1. O'Brien SM, Clarke DR, Jacobs JP, Jacobs ML, Lacour-Gayet FG, Pizarro C, et al. An empirically based tool for analyzing mortality associated with congenital heart surgery. J Thorac Cardiovasc Surg. (2009) 138:1139–53. doi: 10.1016/j.jtcvs.2009.03.071
2. Gaynor JW, Wernovsky G, Jarvik GP, Bernbaum J, Gerdes M, Zackai E, et al. Patient characteristics are important determinants of neurodevelopmental outcomes at one year of age after neonatal and infant cardiac surgery. J Thorac Cardiovasc Surg. (2007) 133:1344–53. doi: 10.1016/j.jtcvs.2006.10.087
3. Behrman RE, Stith Butler A. Institute of Medicine Committee on Understanding Premature Birth and Assuring Healthy Outcomes Board on Health Sciences Outcomes: Preterm Birth: Causes, Consequences, and Prevention. Washington, DC: The National Academies Press (2007).
4. Raju TN, Higgins RD, Stark AR, Leveno KJ. Optimizing care and outcome for late-preterm (near-term) infants: a summary of the workshop sponsored by the National Institute of Child Health and Human Development. Pediatrics. (2006) 118:1207–14. doi: 10.1542/peds.2006-0018
5. Chang AC, Hanley FL, Lock JE, Castaneda AR, Wessel DL. Management and outcome of low birth weight neonates with congenital heart disease. J Pediatr. (1994) 124:461–6. doi: 10.1016/S0022-3476(94)70376-0
6. Dees E, Lin H, Cotton RB, Graham TP, Dodd DA. Outcome of preterm infants with congenital heart disease. J Pediatr. (2000) 137:653–9. doi: 10.1067/mpd.2000.108568
7. Costello JM, Pasquali SK, Jacobs JP, He X, Hill KD, Cooper DS, et al. Gestational age at birth and outcomes after neonatal cardiac surgery: an analysis of the Society of Thoracic Surgeons Congenital Heart Surgery Database. Circulation. (2014) 129:2511–7. doi: 10.1161/CIRCULATIONAHA.113.005864
8. Goff DA, Luan X, Gerdes M, Bernbaum J, D'Agostino JA, Rychik J, et al. Younger gestational age is associated with worse neurodevelopmental outcomes after cardiac surgery in infancy. J Thorac Cardiovasc Surg. (2012) 143:535–42. doi: 10.1016/j.jtcvs.2011.11.029
9. Costello JM, Polito A, Brown DW, McElrath TF, Graham DA, Thiagarajan RR, et al. Birth before 39 weeks' gestation is associated with worse outcomes in neonates with heart disease. Pediatrics. (2010) 126:277–84. doi: 10.1542/peds.2009-3640
10. Cnota JF, Gupta R, Michelfelder EC, Ittenbach RF. Congenital heart disease infant death rates decrease as gestational age advances from 34 to 40 weeks. J Pediatr. (2011) 159:761–5. doi: 10.1016/j.jpeds.2011.04.020
11. Curzon CL, Milford-Beland S, Li JS, O'Brien SM, Jacobs JP, Jacobs ML, et al. Cardiac surgery in infants with low birth weight is associated with increased mortality: analysis of the Society of Thoracic Surgeons Congenital Heart Database. J Thorac Cardiovasc Surg. (2008) 135:546–51. doi: 10.1016/j.jtcvs.2007.09.068
12. Hickey EJ, Nosikova Y, Zhang H, Caldarone CA, Benson L, Redington A, et al. Very low-birth-weight infants with congenital cardiac lesions: is there merit in delaying intervention to permit growth and maturation? J Thorac Cardiovasc Surg. (2012) 143:126–36. doi: 10.1016/j.jtcvs.2011.09.008
13. Robertson CM, Joffe AR, Sauve RS, Rebeyka IM, Phillipos EZ, Dyck JD, et al. Outcomes from an interprovincial program of newborn open heart surgery. J Pediatr. (2004) 144:86–92. doi: 10.1016/j.jpeds.2003.09.048
14. Cheung PY, Chui N, Joffe AR, Rebeyka IM, Robertson CM; Western Canadian Complex Pediatric Therapies Project Follow-up Group. Postoperative lactate concentrations predict the outcome of infants aged 6 weeks or less after intracardiac surgery: a cohort follow-up to 18 months. J Thorac Cardiovasc Surg. (2005) 130:837–43. doi: 10.1016/j.jtcvs.2005.04.029
15. Centers for Disease Control and Prevention National Center for health Statistics. CDC Growth Charts, United States. (2009) Available online at: http://www.cdc.gov/growth~charts/ (accessed April, 2020).
16. Fenton TR, Kim JH. PediTools: clinical tools for pediatric providers, based on the CDC growth calculator for 0–36 months for percentiles and Z-scores. BMC Pediatrics. (2013) 13:59. doi: 10.1186/1471-2431-13-59
17. Harrison PL, Oakland T. Manual of the Adaptive Behaviour Assessment System II. Psychological Corp, Harcourt Assessment Company (2003).
18. Bayley N. Bayley Scales of Infant and Toddler Development. 3rd ed. San Antonio, TX: Harcourt Assessment Inc. (2006).
19. Rosenbaum P, Paneth N, Leviton A, Goldstein M, Bax M. A report: the definition and classification of cerebral palsy. Dev Med Child Neurol. (2007) 49(Suppl.109):8–14.
20. Blishen B, Carroll W, Moore C. The 1981 socioeconomic index for occupations in Canada. Can Rev Social Anthropol. (1987) 24:465–88. doi: 10.1111/j.1755-618X.1987.tb00639.x
21. R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing (2019). Available online at: https://www.R-project.org/ (accessed April, 2020)
22. Wernovsky G, Wypij D, Jonas RA, Mayer JE Jr, Hanley FL, Hickey PR, et al. Post-operative course and hemodynamic profile after the arterial switch operation in neonates and infants. A comparison of low-flow cardiopulmonary bypass and circulatory arrest. Circulation. (1995) 92:2226–35. doi: 10.1161/01.CIR.92.8.2226
23. Sidhu N, Joffe AR, Doughty P, Vatanpour S, Dinu I, Alton G, et al. Sepsis after cardiac surgery early in infancy and adverse 4.5-year neurodevelopmental outcomes. J Am Heart Assoc. (2015) 4:e001954. doi: 10.1161/JAHA.115.001954
24. Chu PY, Li JS, Kosinski AS, Hornik CP, Hill KD. Congenital heart disease in premature infants 25-32 weeks' gestational age. J Pediatr. (2017) 181:37–41. doi: 10.1016/j.jpeds.2016.10.033
25. Lytzen R, Vejlstrup N, Bjerre J, Petersen OB, Leenskjold S, Dodd JK, et al. Live-born major congenital heart disease in Denmark: Incidence, detection rate, and termination of pregnancy rate from 1996 to 2013. JAMA Cardiol. (2018) 3:829–37. doi: 10.1001/jamacardio.2018.2009
26. Canadian Neonatal Network™. Annual Report 2018 (2019). Available online at: http://www.canadianneonatalnetwork.org/Portal/LinkClick.aspx?fileticket=PvniYH94zm0%3d&tabid=39 (accessed April, 2020)
27. Alsoufi B, Slesnick T, McCracken C, Ehrlich A, Kanter K, Schlosser B, et al. Current outcomes of the Norwood operation in patients with single-ventricle malformations other than hypoplastic left heart syndrome. World J Pediatr Congenit Heart Surg. (2015) 6:46–52. doi: 10.1177/2150135114558069
28. Kuraim GA, Garros D, Ryerson L, Moradi F, Dinu IA, Guerra GG, et al. Predictors and outcomes of early post-operative veno-arterial extracorporeal membrane oxygenation following infant cardiac surgery. J Intensive Care. (2018) 6:56. doi: 10.1186/s40560-018-0326-4
29. Puligandla PS, Skarsgard ED. The Canadian Pediatric Surgery Network congenital diaphragmatic hernia evidence review project: developing national guidelines for care. Paediatr Child Health. (2016) 21:183–6. doi: 10.1093/pch/21.4.183
30. Leonetti C, Back SA, Gallo V, Ishibashi N. Cortical dysmaturation in congenital heart disease. Trends Neurosci. (2019) 42:192–204. doi: 10.1016/j.tins.2018.12.003
31. Clouchoux C, du Plessis AJ, Bouyssi-Kobar M, Tworetzky W, McElhinney DB, Brown DW, et al. Delayed cortical development in fetuses with complex congenital heart disease. Cereb Cortex. (2013) 23:2932–43. doi: 10.1093/cercor/bhs281
32. Robertson CMT, Watt MJ, Yasui Y. Changes in the prevalence of cerebral palsy for children born very prematurely within a population-based program over 30 years. JAMA. (2007) 297:2733–40. doi: 10.1001/jama.297.24.2733
33. Allotey J, Zamora J, Cheong-See F, Kalidindi M, Arroyo-Manzano D, Asztalos E, et al. Cognitive, motor, behavioural and academic performances of children born preterm: a meta-analysis and systematic review involving 64061 children. Br J Obstet Gynaecol. (2018) 125:16–25. doi: 10.1111/1471-0528.14832
34. Ricci MF, Andersen JC, Joffe AR, Watt MJ, Moez EK, Dinu IA, et al. Chronic neuromotor disability after complex cardiac surgery in early life. Pediatrics. (2015) 36:e922–33. doi: 10.1542/peds.2015-1879
35. Radic JA, Vincer M, McNeely PD. Outcomes of intraventricular hemorrhage and posthemorrhagic hydrocephalus in a population-based cohort of very preterm infants born to residents of Nova Scotia from 1993 to 2010. J Neurosurg Pediatr. (2015) 15:580–8. doi: 10.3171/2014.11.PEDS14364
36. Synnes A, Luu TM, Moddemann D, Church P, Lee D, Vincer M, et al. Determinants of developmental outcomes in a very preterm Canadian cohort. Arch Dis Child Fetal Neonatal Ed. (2017) 102:F235–4. doi: 10.1136/archdischild-2016-311228
37. Bork KT, To BP, Leonard NJ, Douglas CM, Dinon DA, Leonard EE, et al. Prevalence of childhood permanent hearing loss after early complex cardiac surgery. J Pediatr. (2018) 198:104–9. doi: 10.1016/j.jpeds.2018.02.037
38. Alton GY, Taghados S, Joffe AR, Robertson CM, Dinu I, Western Canadian Pediatric Therapies Follow-Up Group. Prediction of preschool functional abilities after early complex cardiac surgery. Cardiol Young. (2015) 25:655–62. doi: 10.1017/S1047951114000535
39. Martin BJ, De Villiers Jonker I, Joffe AR, Bond GY, Acton BV, et al. Hypoplastic left heart syndrome is not associated with worse clinical or neurodevelopmental outcomes than other cardiac pathologies after the Norwood-Sano operation. Pediatr Cardiol. (2017) 38:922–31. doi: 10.1007/s00246-017-1598-5
40. Ricci MF, Martin BJ, Joffe AR, Dinu IA, Alton GY, Guerra GG, et al. Deterioration of functional abilities in children surviving the Fontan operation. Cardiol Young. (2018) 28:868–75. doi: 10.1017/S1047951118000537
41. Gaynor JW, Stopp C, Wypij D, Andropoulos DB, Atallah J, Atz AM, et al. Neurodevelopmental outcomes after cardiac surgery in infancy. Pediatrics. (2015) 135:816–25. doi: 10.1542/peds.2014-3825
Keywords: prediction model, neurodevelopmental outcome, mortality, prematurity, cardiac surgery
Citation: Cheung P-Y, Hajihosseini M, Dinu IA, Switzer H, Joffe AR, Bond GY, Robertson CMT and on behalf of the Western Canadian Complex Pediatric Therapies Follow-up Program (2021) Outcomes of Preterm Infants With Congenital Heart Defects After Early Surgery: Defining Risk Factors at Different Time Points During Hospitalization. Front. Pediatr. 8:616659. doi: 10.3389/fped.2020.616659
Received: 12 October 2020; Accepted: 11 December 2020;
Published: 28 January 2021.
Edited by:
Offer Erez, Soroka Medical Center, IsraelReviewed by:
Puneet Kumar Arora, Children's Hospital of Wisconsin, United StatesLai Wen Yu, University of Hong Kong, China
Copyright © 2021 Cheung, Hajihosseini, Dinu, Switzer, Joffe, Bond and Robertson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Po-Yin Cheung, cG95aW4mI3gwMDA0MDt1YWxiZXJ0YS5jYQ==
 Po-Yin Cheung
Po-Yin Cheung Morteza Hajihosseini
Morteza Hajihosseini Irina A. Dinu3
Irina A. Dinu3 Gwen Y. Bond
Gwen Y. Bond