- 1Department of Pediatrics, University General Hospital of Heraklion, Crete, Greece
- 2Department of Ophthalmology, University General Hospital of Heraklion, Crete, Greece
- 3Institute of Molecular Biology and Biotechnology (IMBB), Foundation for Research and Technology - Hellas (FORTH), Crete, Greece
Guillain-Barre Syndrome (GBS), a common cause of acute flaccid paralysis, is characterized by a rapidly progressive, usually symmetric weakness of the extremities. Headache and intracranial hypertension (ICHT) are very rare complications of GBS. Herein we report our current case of an obese girl with typical signs of GBS associated with autonomic dysfunction, cranial nerve deficits and increased intracranial pressure (ICP). We also perform a systematic study presenting and discussing previous case reports of GBS associated with ICHT, papilledema or hydrocephalus, highlighting the differences of the current case compared to previous studies. Although intracranial hypertension is a rare complication of pediatric GBS, clinicians should promptly detect it. Obesity may be a predisposing factor, given the strong association between idiopathic intracranial hypertension (IIH) and weight gain. Neurological evaluation, fundus examination and low threshold for intracranial imaging should be an integral part of medical practice in case of obesity, headache or visual changes in GBS patients.
Introduction
Guillain–Barre syndrome (GBS), a potentially life-threatening postinfectious condition is an acute immune-mediated polyradiculoneuropathy characterized by a rapidly progressive, usually bilateral weakness of the limbs, hypo- or areflexia, often accompanied by sensory symptoms, cranial nerve and autonomic dysfunction (1–4). Molecular mimicry and cross-reactive immune response play a crucial role in its pathogenesis. Intravenous immunoglobulin (IVIg) and plasma exchange are effective treatments in GBS. Although medical treatment can improve or stabilize patients, subsequent deterioration may happen after initial improvement, suggesting a severe disease with slow recovery phase and poor prognosis. Among the uncommon symptoms and complications of GBS are headache, papilledema and intracranial hypertension (5–9).
In this study, we describe a case of an obese girl presenting symptoms of intracranial hypertension (ICHT) secondary to GBS. A systematic review of previous case reports associated with GBS and intracranial pressure (ICP) is included, with the aim to highlight this rare clinical manifestation of GBS in children and analyse the differences of this case compared to previous studies (Table 1). Furthermore, clinicians should be alerted for punctual diagnosis and repeated ophthalmologic reassessments in order to exclude ICHT upon headache and/or visual changes in GBS setting.
Case Report
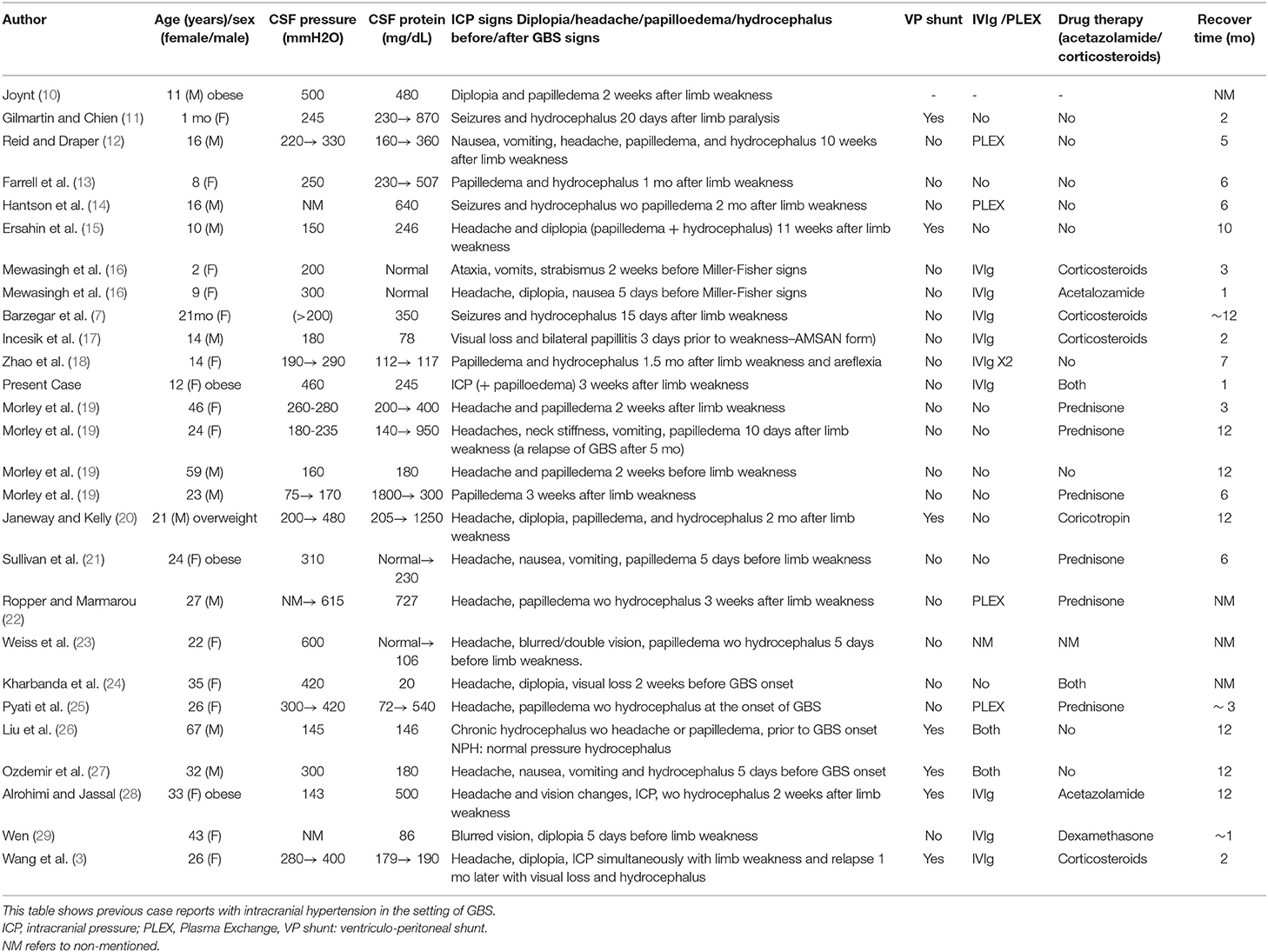
We describe the case of a fully immunized, obese 12-years-old girl (BW 68 kg, BMI 32.3), who was admitted to our hospital in 2018 with a 2-days progressive ascending weakness and aching in both legs, which had slightly been spread to her arms. An acute self-limited respiratory infection with diarrhea and a meningococcal vaccination, 2 and 4 weeks earlier respectively, were preceded the onset of weakness (Figure 1).
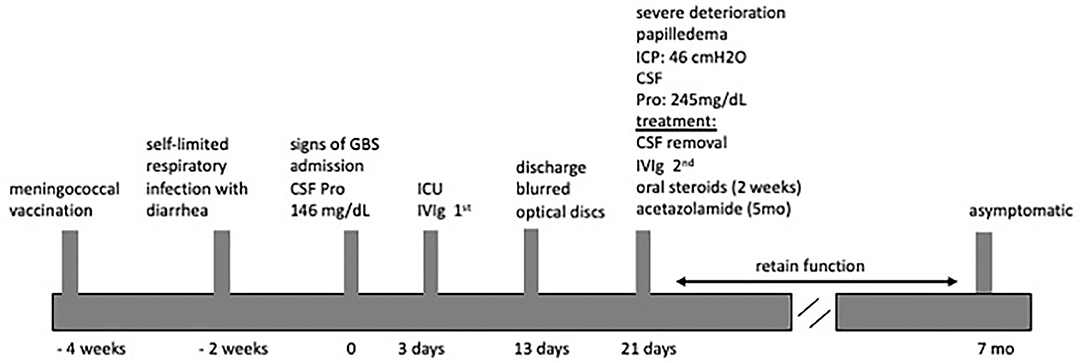
Physical examination revealed paraparesis, areflexia, and weakness restricted to her legs. However, over the course of her illness moderate arm weakness was observed. Deep tendon reflexes were absent while sensory examination was normal. Muscle strength was 3/5 proximally and 1-2/5 distally of all limbs using the Medical Research Council scale (30). Cranial nerve examination revealed bilateral facial weakness and incomplete eyelid closure while bilateral fundoscopy had normal findings. Brain/spine MRI was unremarkable (Figure 2). CSF analysis revealed cytoalbuminologic dissociation with white blood cell (WBC) of 5 × 106/L, protein at 146 mg/dl and glucose 68 mg/dL (blood glucose 87 mg/dL). On laboratory examination, routine hematological, biochemical, urine, and stool analysis were normal. Serologic tests for cytomegalovirus, herpes simplex virus, Epstein–Barr virus, coxsackie viruses, influenza A and B, enteroviruses and adenovirus, hepatitis A, B, HIV, Cambylobacter jejuni and Mycoplasma pneumoniae were negative. Thyroid-stimulating hormones and T3/T4, anti-nuclear antibody, anti-tissue and anti-neutrophil cytoplasmic antibodies were normal. Moreover, the antibodies against gangliosides (GM1, GQ1B, GD1B, GT1B, GD1a, GM2, and MAG) were negative. She was immunized for rubella and measles in early childhood (Table 2). Given her vaccination status and virologic investigation of stool samples, we excluded poliovirus as a possible cause. This case was recorded to the National Poliovirus/Enterovirus Reference Laboratory (Hellenic Pasteur Institute), responsible for the investigation of Acute Flaccid Paralysis (AFP). Electromyography (EMG) and nerve conduction studies confirmed the diagnosis of GBS, revealing a severe demyelinating motor polyneuropathy (Table 2).
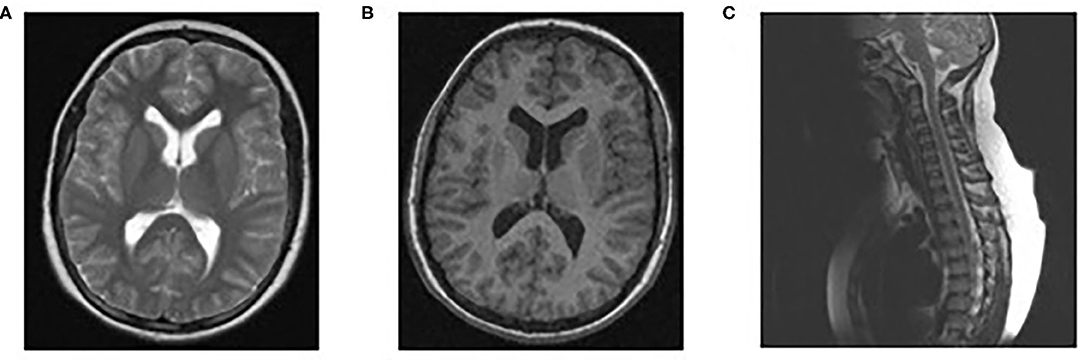
Figure 2. Normal findings of brain/spinal MRI. MRI brain scan T1 (B) and T2 (A), MRI spine sagittal (C).
Due to the rapidly progressing limb weakness and incipient respiratory failure, she was admitted to ICU for monitoring and supportive treatment, 3 days after the onset of her symptoms. In accordance with the diagnosis of GBS, she was directly treated with intravenous immune globulin at the dose of 0.4 g/kg daily for 5 days. Notably, during her hospitalization she also suffered from hypertension (SBP: 145–170 mmHg, DBP: 70–100 mmHg) with mild tachycardia, which was confronted with a selective β1-receptor antagonist.
The patient 10 days after IVIg treatment had a gradual recovery with substantial motor improvement, therefore she was discharged but closely monitored for repeated reassessments, given that 1 day before her discharge, she started complaining about mild headache. Fundoscopy for the first time showed blurred optic discs.
A week later (18 days after IVIg treatment), severe deterioration of her physical status was noticed as she presented bilateral and relatively symmetric weakness of all limb muscles and suffered from severe headaches. Additionally, neurological evaluation revealed cranial nerve involvement with dysarthria and voice hoarseness, along with bilateral facial weakness, strabismus, weak gag reflex, and tongue paresis coupled with respiratory muscles weakness. Ocular examination showed decreased visual acuity with right esotropia and presence of bilateral optic disc edema with peripapillary flame-shaped hemorrhages (Figure 3). Although treated for hypertension, pathologic values of BP sustained (SBP/DBP: 140/90 mmHg).
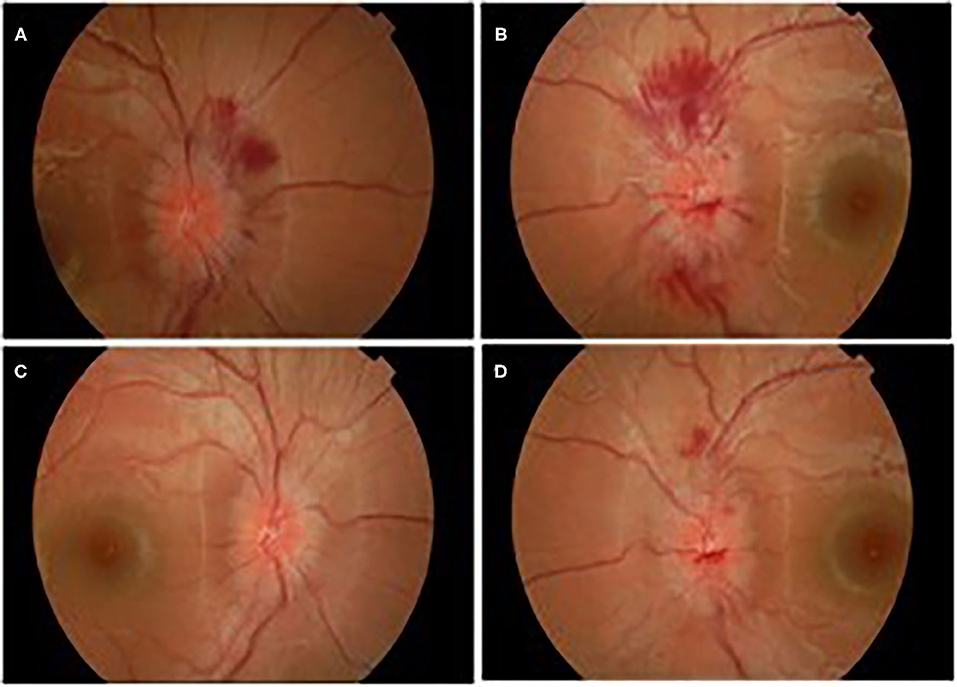
Figure 3. Ocular edema with flamed shaped hemorrhages OD (A), OS (B), and gradual improvement after treatment OD (C), OS (D).
Brain MRI and MRA scan was not feasible due to patient's non-cooperation and sedation was considered inappropriate for her safety. Therefore, we performed urgent brain CT scan with intravenous contrast dye administration, which was unremarkable, excluding the possibility of venus thrombosis, cerebral bleeding, displacement of midline brain structures and abnormal perimedullary spaces (Figure 4). Subsequent lumbar puncture revealed a CSF pressure peaked at 46 cm H2O. The CSF contained 10 × 106/L WBC and an increased protein level (245 mg/dL), thus a total of 30 ml CSF was removed. CSF removal was performed in consultation with neurosurgeon and continuous vitals monitoring of our patient. Due to her motor deterioration and secondary ICHP, she was promptly treated for a second time with 5 days IVIG administration (0.4 g/kg per day) and oral steroids (prednisolone: 2 mg/kg per day for 2 weeks). She was also treated with acetazolamide (250 mg four times per day for 5 months). While she was in hospital, she started having a gradual recovery, without any clinical deterioration. Continuous ophthalmologic and clinical reassessments showed a gradual and significant improvement in papilledema and visual acuity over the following 2 months. Eventually, with the aid of rehabilitation team and dietary she continued to retain function, lose weight, and remain asymptomatic over the subsequent 7 months (Figure 1).
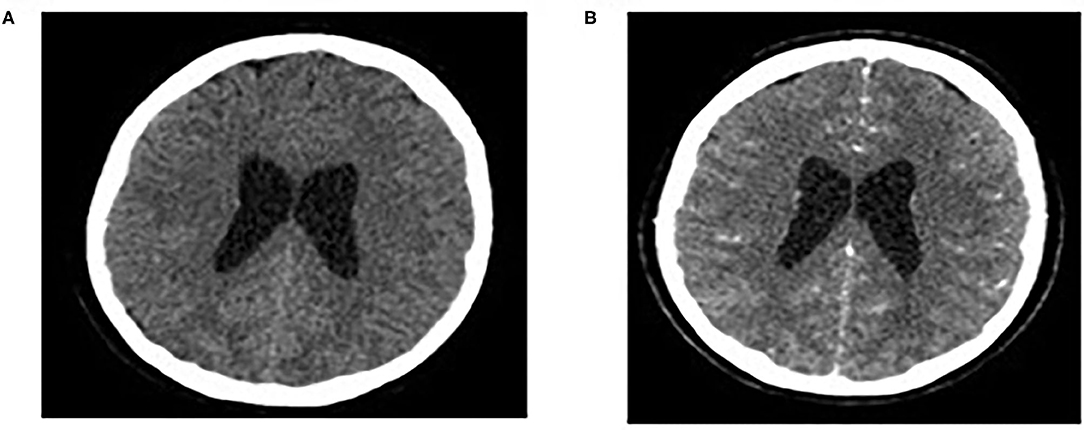
Figure 4. Normal findings of brain CT scan: CT brain scan without/with intravenous contrast dye administration (A,B).
Discussion
GBS is the most common cause of acute flaccid paralysis in healthy infants and children (31, 32). ICHT, a pathological feature that is reported in a few scattered cases of GBS, occurs in 4–6% of children with GBS and the exact mechanism remains elusive (9–13, 19–22). In this systematic study, we present and discuss previous case reports of GBS associated with ICHT, papilledema or hydrocephalus (Table 1). We also report our current case of an obese girl with typical signs of GBS associated with cranial nerve deficits, autonomic dysfunction and increased intracranial pressure (ICP), highlighting the differences of this case compared to previous reports.
The results derived from our systemic research analysis display that the peak age incidence is 20–40 years, and there is no significant difference between sexes. To be more concise, 9/27 patients were children (average age 10 years old). The duration of the illness from onset of symptoms to complete recovery, ranged from 1 month to 2 years, with an average of about 7 months. Although weight as a parameter is not always documented, we noticed that 5/27 patients are obese, suggesting an increased possibility of overweight and obese patients to develop ICP in GBS setting. Respiratory insufficiency and mechanical support are reported in 9/27 patients (Table 1) (7, 14–16, 18, 23–29).
Diagnosis of idiopathic intracranial hypertension requires papilledema, normal neurologic exam other than cranial nerve abnormalities, normal cerebrospinal fluid (CSF) composition, elevated lumbar puncture opening pressure (≥280 mm CSF in children) and normal neuroimaging (Friedman DI, et al. Neurology 2013; 81: 1–7). Thus, in the current case, intracranial pressure is secondary to GBS leading to papilledema, headache, decreased vision acuity and worsen GBS symptoms with bilateral and relatively symmetric weakness of all limb muscles. In fact, papilledema with or without hydrocephalus is rarely reported in GBS (7, 13, 20). It appears mainly after the established limb weakness and is associated with elevated CSF protein similar to our case (12). However, it is also reported that some patients presented headache, vision disturbances, ICHT and papilledema days before GBS onset (6/27 case reports) (Table 1).
Papilledema takes time to develop and has a low sensitivity for ICHT (33). However, when present, papilledema can be a specific indicator of elevated ICP. Nowadays, there are more accurate non-invasive techniques of ICP assessment, such as sonographic measurement of the optic nerve sheath diameter (ONSD) and Transcranial Doppler (TCD) (34). The reliability of both techniques depends on the knowledge of limitations and requires experienced and skilled operators.
Although the mechanism of ICHT is not well-understood, edema of the spinal nerve rootlets seen in GBS, causes decreased proteins absorption and, thereby, elevated CSF protein levels. It is speculated that increased CSF protein concentration slows reabsorption in the arachnoid granulations resulting in raised ICP (protein absorption theory) (19, 35). The aforementioned analysis underscores a tight correlation between papilledema development and the occurrence of increased pressure of the CSF. Intriguingly, in several cases the association between development of the papilledema and variations in the CSF protein levels was quite puzzling, suggesting that certain findings seem inconsistent with protein absorption theory. Moreover, what is striking about trying to explain this theory, is that although ICHT is a rare complication of GBS, high levels of CSF protein are common enough. Additionally, there are few case reports presenting GBS patients with normal CSF protein levels. These data suggest an alternative explanation of increased ICP, implicating intrinsic cerebral edema rather than impairment of CSF reabsorption. CSF dynamic studies demonstrated high venous pressure at points of CSF outflow (8, 12, 14, 21).
The potential role of underlying immunological disturbance with activation of the classical and alternative complement pathway resulted in CSF accumulation via either impaired CSF absorption at the arachnoid villi or, alliteratively, increased production of CSF at the choroid plexus. It is suggested that GBS patients, who have a relapsing course and develop high ICP, are immunologically different from those with conventional symptoms of polyneuropathy alone (8, 12). The exact mechanism of the development of high ICP during GBS remains elusive. In the present case, we did not check complement components C4 and factor B apart from C3 that was quiet high (Table 2).
Regarding treatment, both intravenous immunoglobulin (IVIg) or plasma exchange (PLEX) are proven effective (36–38). IVIg seems to be effective in children with GBS and is preferred over PLEX because it is easier to be administrated and possibly better tolerated in young children (2, 39). Importantly, PLEX can have more adverse effects and complications in children than in adults due to citrate toxicity and higher vascular volume shifts (40). A recent study comparing PLEX and IVIg as a first line treatment for children with severe GBS requiring mechanic ventilation (MV) revealed that PLEX is superior to IVIg regarding the duration of MV but not the PICU stay or the short term neurological outcome (41). In our experience, IVIg administration is first line treatment for GBS and a choice of availability and avoidance of a prolong stay in PICU.
It has been also reported that patients with IIH or GBS have increased levels of IL-17 in both CSF and plasma (42). IVIg seems to exert its therapeutic effects on GBS by downregulating IL-17 (43). Besides IVIg and PLEX, no other procedures or drugs have been proven effective in GBS treatment (2). Although corticosteroids would be expected to be beneficial in GBS, it has been reported that they are ineffective for treating GBS and there was no significant difference between methylprednisolone—IVIg and IVIg group alone (36, 44). The lack of efficiency might be related to their adverse effects on denervated muscle or macrophage activity (45). Congruently, studies in animal models have shown that corticosteroids may reduce the recruitment of macrophages that play a crucial role for nerve regeneration, thus delay disease recovery (46).
GBS is usually a monophasic disease, but secondary deterioration after initial stabilization or improvement is possible in 5–10% of treated GBS patients (47, 48). Therefore, it is suggested that a second course of IVIg treatment especially in patients with a bad prognosis, could be effective (49, 50). Indeed, in the current case, we proceeded in a second course of IVIg treatment. Actually, the patient presented slow, but gradual improvement, upon the first IVIg administration. However, 2 weeks later, our patient suffered from a severe deterioration of her clinical status characterized by papilledema, reduced visual acuity, diplopia and persistent headaches. We suppose that our patient's overweight status was probably involved in the pathogenesis of ICP, as obesity is implicated in the development of ICP (51–54).
Although pain may be a heralding feature of GBS, it is widely documented that headache is a rare symptom. A large prospective study of pain in GBS, demonstrated 2% prevalence of headache (55). Most case reports correlate headache and GBS with posterior reversible encephalopathy syndrome [PRES], an increasingly recognized dysautonomia-related GBS complication (56). Less frequent causes of headache in GBS are secondary intracranial hypertension, cerebral venous sinus thrombosis and aseptic meningitis after IVIg administration. In the current case report, headache coincided with ICHT. Although headache could be an adverse effect of IVIg treatment, the remarkable clinical response after CSF removal argued against the possibility of post IVIg aseptic meningitis. Additionally, second lumbar puncture showed increased ICP with normal cells and elevated CSF protein levels. Moreover, urgent brain CT scan with intravenous contrast dye administration, which was unremarkable, excluded venus thrombosis.
In total, our patient was treated with two courses of IVIg, carbonic anhydrase inhibitor and steroids for facing GBS itself and intracranial hypertension. Additionally, a selective β1 receptor antagonist was used for blood hypertension. Although corticosteroids have been recommended in the past for ICHT, the long-term use should be avoided because of their side effects. Corticosteroids are indicated only on a short-term basis in patients with fulminant disease accompanied by severe papilledema and compromised visual function. Although their pathophysiological mechanism remains elusive, it is suggested that they reduce ICP primarily in vasogenic edema due to their beneficial effect on the blood vessel (57–59). In the current case, however, we used steroids empirically for short-term treatment in the setting of ICHP, acute visual loss and deteriorating clinical status. Carbonic anhydrase inhibitors can provide symptomatic relief of raised intracranial pressure by promoting the reduced CSF production at the choroid plexus. Acetazolamide is considered as the first-line medication for IIH (60). Eventually, our patient started having a gradual and significant recovery without reporting any symptomatic and functional deterioration. 7 months later the clinical evolution is excellent with complete ophthalmological and neurological recovery.
Headache, papilledema and ICHT in the setting of GBS are sparse, but potentially severe events and require further investigation. Obesity may be a predisposing factor, thus, physicians should be more aware of ICHT in an obese GBS patient. Neurological evaluation, fundus examination and low threshold for intracranial imaging should be an integral part of medical practice in case of obesity, headache, or visual changes in GBS patients. More research is needed to identify specific and potential therapeutic interventions against ICHT in GBS, in order to alleviate symptoms and improve outcome.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by Scientific Advisory Board University General Hospital of Heraklion, Crete, Greece. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin. Written informed consent was obtained from the participants' legal guardian/next of kin for the publication of this case report.
Author Contributions
CD wrote the manuscript. PV supervised the project. All authors have participated to this case.
Funding
KP was funded by a grant from the Hellenic Foundation for Research and Innovation (HFRI) and the General Secretariat for Research and Technology (GSRT).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Zochodne DW. Autonomic involvement in Guillain-Barre syndrome: a review. Muscle Nerve. (1994) 17:1145–55. doi: 10.1002/mus.880171004
2. Leonhard SE, Mandarakas MR, Gondim FaA, Bateman K, Ferreira MLB, Cornblath DR, et al. Diagnosis and management of Guillain-Barre syndrome in 10 steps. Nat Rev Neurol. (2019) 15:671–83. doi: 10.1038/s41582-019-0250-9
3. Wang T, Wang Z, Guo Z. Headache and intracranial hypertension in Guillain-Barre syndrome: a case report and literature review. Int J Neurosci. (2019) 129:1179–82. doi: 10.1080/00207454.2019.1645139
4. Van Der Meche FG, Van Doorn PA, Meulstee J, Jennekens FG, Centre GB-CGOTDNRS. Diagnostic and classification criteria for the Guillain-Barre syndrome. Eur Neurol. (2001) 45:133–9. doi: 10.1159/000052111
5. Gilpin S, Moersch FP, Kernoham JW. Polyneuritis: a clinical and pathologic study of a special group of cases frequently referred to as instances of neuronitis. Archiv Neurol Psychiatry. (1936) 35:937–63. doi: 10.1001/archneurpsyc.1936.02260050011001
6. Friedman DI, Potts E. Headache associated with miller fisher syndrome. Headache. (2007) 47:1347–8. doi: 10.1111/j.1526-4610.2007.00935.x
7. Barzegar M, Malaki M. Guillain-Barre syndrome presenting with severe hydrocephalus in a child. J Pediatr Neurosci. (2013) 8:175–6. doi: 10.4103/1817-1745.117868
8. Farmakidis C, Inan S, Milstein M, Herskovitz S. Headache and pain in Guillain-Barre syndrome. Curr Pain Headache Rep. (2015) 19:40. doi: 10.1007/s11916-015-0508-x
9. Taylor E, Mcdonald C. The syndrome of polyneuritis with facial diplegia. Archiv Neurol Psychiatry. (1932) 27:79. doi: 10.1001/archneurpsyc.1932.02230130085006
10. Joynt RJ. Mechanism of production of papilledema in the Guillain-Barre syndrome. Neurology. (1958) 8:8–12. doi: 10.1212/WNL.8.1.8
11. Gilmartin RC, Ch'ien LT. Guillain-Barre syndrome with hydrocephalus in early infancy. Arch Neurol. (1977) 34:567–9. doi: 10.1001/archneur.1977.00500210069013
12. Reid AC, Draper IT. Pathogenesis of papilloedema and raised intracranial pressure in Guillain-Barre syndrome. Br Med J. (1980) 281:1393–4. doi: 10.1136/bmj.281.6252.1393
13. Farrell K, Hill A, Chuang S. Papilledema in Guillain-Barre syndrome. A case report. Arch Neurol. (1981) 38:55–7. doi: 10.1001/archneur.1981.00510010081018
14. Hantson P, Horn JL, Deconinck B, Mahieu P, Mathurin P, Dooms G, et al. Hydrocephalus in Guillain-Barre syndrome. Eur Neurol. (1991) 31:426–7. doi: 10.1159/000116709
15. Ersahin Y, Mutluer S, Yurtseven T. Hydrocephalus in Guillain-Barre syndrome. Clin Neurol Neurosurg. (1995) 97:253–5. doi: 10.1016/0303-8467(95)00041-H
16. Mewasingh LD, Sekhara T, Dachy B, Djeunang MC, Dan B. Benign intracranial hypertension: atypical presentation of Miller Fisher syndrome? Pediatr Neurol. (2002) 26:228–30. doi: 10.1016/S0887-8994(01)00362-9
17. Incecik F, Herguner OM, Besen S, Yar K, Altunbasak S. Guillain-Barre syndrome with hyperreflexia and bilateral papillitis in a child. J Pediatr Neurosci. (2016) 11:71–3. doi: 10.4103/1817-1745.181264
18. Zhao PP, Ji QK, Sui RB, Zhang R, Zhang LJ, Xu ZX, et al. Increased intracranial pressure in Guillain-Barre syndrome: a case report. Medicine. (2018) 97:e11584. doi: 10.1097/MD.0000000000011584
19. Morley JB, Reynolds EH. Papilloedema and the Landry-Guillain-Barre syndrome. Case reports and a review. Brain. (1966) 89:205–22. doi: 10.1093/brain/89.2.205
20. Janeway R, Kelly DLJr. Papilledema and hydrocephalus associated with recurrent polyneuritis. Guillian-Barre type Arch Neurol. (1966) 15:507–14. doi: 10.1001/archneur.1966.00470170061006
21. Sullivan RLJr, Reeves AG. Normal cerebrospinal fluid protein, increased intracranial pressure, and the Guillain-Barre syndrome. Ann Neurol. (1977) 1:108–9. doi: 10.1002/ana.410010113
22. Ropper AH, Marmarou A. Mechanism of pseudotumor in Guillain-Barre syndrome. Arch Neurol. (1984) 41:259–61. doi: 10.1001/archneur.1984.04050150037012
23. Weiss GB, Bajwa ZH, Mehler MF. Co-occurrence of pseudotumor cerebri and Guillain-Barre syndrome in an adult. Neurology. (1991) 41:603–4. doi: 10.1212/WNL.41.4.603
24. Kharbanda PS, Prabhakar S, Lal V, Das CP. Visual loss with papilledema in Guillain-Barre syndrome. Neurol India. (2002) 50:528–9.
25. Pyati S, Razis PA, Desai P. Headache in guillain-barre syndrome. J Neurosurg Anesthesiol. (2004) 16:294–5. doi: 10.1097/00008506-200410000-00007
26. Liu CY, Kao CD, Chen JT, Yeh YS, Wu ZA, Liao KK. Hydrocephalus associated with Guillain-Barre syndrome. J Clin Neurosci. (2006) 13:866–9. doi: 10.1016/j.jocn.2005.11.035
27. Ozdemir O, Calisaneller T, Sonmez E, Caner H, Altinors N. Atypical presentation of Guillain-Barre syndrome with acute hydrocephalus. Acta Neurochir. (2008) 150:87–8. doi: 10.1007/s00701-007-1405-9
28. Alrohimi A, Jassal R. Headache in Guillain-Barre syndrome: diagnostic and management implications. Can J Neurol Sci. (2018) 45:240–2. doi: 10.1017/cjn.2017.247
29. Wen HJ. Acute bilateral vision deficit as the initial symptom in Guillain-Barre syndrome: a case report. Exp Ther Med. (2018) 16:2712–6. doi: 10.3892/etm.2018.6465
30. Paternostro-Sluga T, Grim-Stieger M, Posch M, Schuhfried O, Vacariu G, Mittermaier C, et al. Reliability and validity of the Medical Research Council (MRC) scale and a modified scale for testing muscle strength in patients with radial palsy. J Rehabil Med. (2008) 40:665–71. doi: 10.2340/16501977-0235
31. Stapleton FB, Skoglund RR, Daggett RB. Hypertension associated with the Guillain-Barre syndrome. Pediatrics. (1978) 62:588–90.
32. Asbury AK. New concepts of Guillain-Barre syndrome. J Child Neurol. (2000) 15:183–91. doi: 10.1177/088307380001500308
33. Rangwala LM, Liu GT. Pediatric idiopathic intracranial hypertension. Surv Ophthalmol. (2007) 52:597–617. doi: 10.1016/j.survophthal.2007.08.018
34. Zhang X, Medow JE, Iskandar BJ, Wang F, Shokoueinejad M, Koueik J. Webster, Invasive and noninvasive means of measuring intracranial pressure: a review. Physiol Meas. (2017) 38:R143–82. doi: 10.1088/1361-6579/aa7256
35. Denny-Brown DE. The changing pattern of neurologic medicine. N Engl J Med. (1952) 246:839–46. doi: 10.1056/NEJM195205292462201
36. Hughes RA. Systematic reviews of treatment for inflammatory demyelinating neuropathy. J Anat. (2002) 200:331–9. doi: 10.1046/j.1469-7580.2002.00041.x
37. Raphael JC, Chevret S, Hughes RA, Annane D. Plasma exchange for Guillain-Barre syndrome. Cochrane Database Syst Rev. (2002) 2:CD001798. doi: 10.1002/14651858.CD001798
38. Korinthenberg R, Schessl J, Kirschner J, Monting JS. Intravenously administered immunoglobulin in the treatment of childhood Guillain-Barre syndrome: a randomized trial. Pediatrics. (2005) 116:8–14. doi: 10.1542/peds.2004-1324
39. Verboon C, Van Doorn PA, Jacobs BC. Treatment dilemmas in Guillain-Barre syndrome. J Neurol Neurosurg Psychiatry. (2017) 88:346–52. doi: 10.1136/jnnp-2016-314862
40. Michon B, Moghrabi A, Winikoff R, Barrette S, Bernstein ML, Champagne J, et al. Complications of apheresis in children. Transfusion. (2007) 47:1837–42. doi: 10.1111/j.1537-2995.2007.01405.x
41. El-Bayoumi MA, El-Refaey AM, Abdelkader AM, El-Assmy MM, Alwakeel AA, El-Tahan HM. Comparison of intravenous immunoglobulin and plasma exchange in treatment of mechanically ventilated children with Guillain Barre syndrome: a randomized study. Crit Care. (2011) 15:R164. doi: 10.1186/cc10305
42. Edwards LJ, Sharrack B, Ismail A, Tench CR, Gran B, Dhungana S, et al. Increased levels of interleukins 2 and 17 in the cerebrospinal fluid of patients with idiopathic intracranial hypertension. Am J Clin Exp Immunol. (2013) 2:234–44.
43. Li S, Jin T, Zhang HL, Yu H, Meng F, Concha Quezada H, et al. Circulating Th17, Th22, and Th1 cells are elevated in the Guillain-Barre syndrome and downregulated by IVIg treatments. Mediators Inflamm. (2014) 2014:740947. doi: 10.1155/2014/740947
44. Van Koningsveld R, Schmitz PI, Meche FG, Visser LH, Meulstee J, Van Doorn PA, et al. Effect of methylprednisolone when added to standard treatment with intravenous immunoglobulin for Guillain-Barre syndrome: randomised trial. Lancet. (2004) 363:192–6. doi: 10.1016/S0140-6736(03)15324-X
45. Donaghy M, Mills KR, Boniface SJ, Simmons J, Wright I, Gregson N, et al. Pure motor demyelinating neuropathy: deterioration after steroid treatment and improvement with intravenous immunoglobulin. J Neurol Neurosurg Psychiatry. (1994) 57:778–83. doi: 10.1136/jnnp.57.7.778
46. Wang YZ, Lv H, Shi QG, Fan XT, Li L, Yi Wong AH, et al. Action mechanism of corticosteroids to aggravate Guillain-Barre syndrome. Sci Rep. (2015) 5:13931. doi: 10.1038/srep13931
47. Dimachkie MM, Barohn RJ. Guillain-Barre syndrome and variants. Neurol Clin. (2013) 31:491–510. doi: 10.1016/j.ncl.2013.01.005
48. Doets AY, Jacobs BC, Van Doorn PA. Advances in management of Guillain-Barre syndrome. Curr Opin Neurol. (2018) 31:541–50. doi: 10.1097/WCO.0000000000000602
49. Farcas P, Avnun L, Frisher S, Herishanu YO, Wirguin I. Efficacy of repeated intravenous immunoglobulin in severe unresponsive Guillain-Barre syndrome. Lancet. (1997) 350:1747. doi: 10.1016/S0140-6736(97)24050-X
50. Van Doorn PA, Ruts L, Jacobs BC. Clinical features, pathogenesis, and treatment of Guillain-Barre syndrome. Lancet Neurol. (2008) 7:939–50. doi: 10.1016/S1474-4422(08)70215-1
51. Ireland B, Corbett JJ, Wallace RB. The search for causes of idiopathic intracranial hypertension. A preliminary case-control study. Arch Neurol. (1990) 47:315–20. doi: 10.1001/archneur.1990.00530030091021
52. Wall M, George D. Idiopathic intracranial hypertension. A prospective study of 50 patients. Brain. (1991) 114:155–80. doi: 10.1016/S0733-8619(18)30304-9
53. Daniels AB, Liu GT, Volpe NJ, Galetta SL, Moster ML, Newman NJ, et al. Profiles of obesity, weight gain, and quality of life in idiopathic intracranial hypertension (Pseudotumor cerebri). Am J Ophthalmol. (2007) 143:635–41. doi: 10.1016/j.ajo.2006.12.040
54. Fontana A, Taibi R, Timpanaro T, Barbagallo M, Marino L, Smilari P, et al. Pseudotumor cerebri syndrome (PTCS): clinical analysis on a cohort of 37 pediatric subjects. World Cancer Res J. (2020) 7:e1754. doi: 10.32113/wcrj_202011_1754
55. Moulin DE, Hagen N, Feasby TE, Amireh R, Hahn A. Pain in Guillain-Barre syndrome. Neurology. (1997) 48:328–31. doi: 10.1212/WNL.48.2.328
56. Chen A, Kim J, Henderson G, Berkowitz A. Posterior reversible encephalopathy syndrome in Guillain-Barre syndrome. J Clin Neurosci. (2015) 22:914–6. doi: 10.1016/j.jocn.2014.11.004
57. Soler D, Cox T, Bullock P, Calver DM, Robinson RO. Diagnosis and management of benign intracranial hypertension. Arch Dis Child. (1998) 78:89–94. doi: 10.1136/adc.78.1.89
58. Thurtell MJ, Wall M. Idiopathic intracranial hypertension (Pseudotumor cerebri): recognition, treatment, and ongoing management. Curr Treat Options Neurol. (2013) 15:1–12. doi: 10.1007/s11940-012-0207-4
59. Hughes RA, Brassington R, Gunn AA, Van Doorn PA. Corticosteroids for Guillain-Barre syndrome. Cochrane Database Syst Rev. (2016) 10:CD001446. doi: 10.1002/14651858.CD001446.pub5
Keywords: intracranial hypertension, Guillain–Barre syndrome, hydrocephalus, papilledema, headache
Citation: Doxaki C, Papadopoulou E, Maniadaki I, Tsakalis NG, Palikaras K and Vorgia P (2021) Case Report: Intracranial Hypertension Secondary to Guillain-Barre Syndrome. Front. Pediatr. 8:608695. doi: 10.3389/fped.2020.608695
Received: 21 September 2020; Accepted: 18 December 2020;
Published: 20 January 2021.
Edited by:
Piero Pavone, University of Catania, ItalyReviewed by:
Carlotta Spagnoli, Santa Maria Nuova Hospital, ItalyMaria Gogou, Aristotle University of Thessaloniki, Greece
Copyright © 2021 Doxaki, Papadopoulou, Maniadaki, Tsakalis, Palikaras and Vorgia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christina Doxaki, Y2RveGFraUBtZWQudW9jLmdy; Pelagia Vorgia, dm9yZ2lhLnBAdW9jLmdy
 Christina Doxaki
Christina Doxaki Eleftheria Papadopoulou
Eleftheria Papadopoulou Iliana Maniadaki1
Iliana Maniadaki1 Nikolaos G. Tsakalis
Nikolaos G. Tsakalis Konstantinos Palikaras
Konstantinos Palikaras Pelagia Vorgia
Pelagia Vorgia