
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pediatr. , 20 November 2020
Sec. Pediatric Rheumatology
Volume 8 - 2020 | https://doi.org/10.3389/fped.2020.593435
This article is part of the Research Topic Pathogenesis, Clinical Findings and Treatment Advances in Kawasaki Disease View all 12 articles
Background: In the latest 2017 American Heart Association guidelines for Kawasaki disease (KD), there are no recommendations regarding the early administration of intravenous immunoglobulin (IVIG). Therefore, the purpose of this systematic review and meta-analysis was to investigate the effects of early IVIG therapy on KD.
Methods: We searched databases including the PubMed, Medline, the Cochrane Library, and the Clinicaltrials.gov website until July 2019.
Results: Fourteen studies involving a total of 70,396 patients were included. Early treatment with IVIG can lead to an increased risk of IVIG unresponsiveness [OR 2.24; 95% CI (1.76, 2.84); P = 0.000]. In contrast to the studies performed in Japan [OR 1.27; 95% CI (0.98, 1.64); P = 0.074] that found no significant difference in coronary artery lesions (CAL) development, studies conducted in China [OR 0.73; 95% CI (0.66, 0.80); P = 0.000] and the United States [OR 0.50; 95% CI (0.38, 0.66); P = 0.000] showed a reduced risk in the occurrence of CAL with early IVIG treatment.
Conclusions: At present, the evidence does not support the treatment with IVIG in the early stage of the onset of KD. But, early IVIG treatment could be a protective factor against the development of CAL, which needs to be further clarified.
Kawasaki disease (KD) is an acute self-limiting disease leading to vasculitis that predominantly affects infants and young children. Coronary artery lesion (CAL) is the most common complication. In severe cases, giant coronary artery aneurysms or coronary artery ectasia can develop, which is the leading cause of acquired heart disease (1, 2). Intravenous immunoglobulin (IVIG) therapy is the first-line treatment of KD with well-established therapeutic effects in preventing coronary artery abnormalities (3). Coronary artery aneurysms (CAAs) develop in ~25% of untreated patients (4); however, in patients receiving a timely high dose of IVIG, it is reported that only about 5% of patients (5–7).
The mechanism as to why IVIG is effective in the treatment of KD is unknown. IVIG is considered to reduce the prevalence of CAL by regulating the immune system, including the modulation of cytokine production, neutralization of bacterial superantigens or other etiologic agents, and suppression of endothelial cell activation. The 2004 American Heart Association (AHA) guidelines of KD stated that IVIG was recommended to be used within 10 days after the onset of the disease and, if possible, within seven days. And, according to a study by Muta and Fong, early administration of IVIG (within 5 days) seemed to show no significant improvement in the cardiovascular outcomes, but there was an increased need for IVIG retreatment (4, 8, 9). In the latest AHA guidelines for KD in 2017, it is suggested that for experienced clinical experts, the diagnosis of KD can be made as early as 3 days after the onset of disease if typical symptoms are present; however, there is no guideline on the timing of IVIG administration and if it can be given earlier (10).
Therefore, in our research, studies meeting the inclusion criteria were included to compare the outcomes of KD children treated with IVIG at early and routine time, including coronary artery outcome and non-response to IVIG, so as to understand the effects of early IVIG therapy in KD and to provide evidence on the timing of IVIG administration.
This systematic review and meta-analysis was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement (11) (Supplementary Checklist 1).
We searched databases including the PubMed, Medline, the Cochrane Library, and the Clinicaltrials. gov website until July 2019 without language restrictions. The keywords and subject terms were (“immunoglobulin” OR “IVIG” OR “immune globulin”) and (“Kawasaki disease” OR “Mucocutaneous Lymph Node Syndrome” OR “Kawasaki Syndrome”).
The population, intervention, comparison, and outcome approach was used for the study inclusion. The population of interest were children diagnosed with KD. The intervention of interest was the treatment using IVIG as the initial therapy and studies had to include a comparison between the early treatment with IVIG (IVIG administration <5 days of onset of disease) and ≥5 days of KD onset. Outcome measurements included the incidence of CAL, IVIG unresponsiveness, and CAA.
Meanwhile, the studies included fulfilled the following criteria: (1) sufficient data to extract the number of cases and controls in each group or provision of odds ratios (ORs) with 95 % confidence intervals (CIs) and (2) the types of studies should be randomized controlled or non-randomized controlled clinical trials, case-control, cross-sectional, or cohort studies.
For each eligible study, the following information was independently extracted by two researchers (FY and HZ): last name of first author, study design, duration of follow-up, study duration, study location, age of participants, proportion of males, sample size in each group (subgroup by time of IVIG treatment), diagnostic criteria of CAL, definition of IVIG unresponsiveness KD, outcomes, and quality criteria. The quality of the included studies was also independently assessed by two researchers (FY and HZ) using the Newcastle-Ottawa scale (12) (Supplementary Tables 1, 2). When disagreements arose, group discussions would be carried out to resolve it.
The primary outcomes were CAL and IVIG unresponsiveness. CAL included the coronary artery dilation, CAA, and coronary artery stenosis meeting the definition of the Japanese Ministry of Health criteria, coronary artery Z score system, or Chinese literature (13–15). IVIG unresponsiveness was defined as a persistent or recrudescent fever in a period after the completion of the first IVIG infusion. The secondary outcome was CAA.
We estimate the pooled odds ratio (OR) with a 95% confidence interval (CI) using study-specific ORs abstracted or calculated from raw data; results from a multivariate analysis or adjusted ORs were preferred to those of univariate analysis or crude results. Considering the within– and between–study variability, the random effects model was chosen. Heterogeneity among the studies was assessed using the Q test. It was quantified by I2 values and 25, 50, and 75% were considered as low, moderate, and high levels of heterogeneity, respectively. To test the publication bias, the Begg's and Egger's tests were used. P < 0.1 indicated that a publication bias existed. Sensitivity analysis was performed by evaluating the pooled estimate after omitting a study each time. Additionally, a subgroup analysis and meta-regression was conducted to detect the potential factors for heterogeneity. Statistical analysis was performed using the STATA version 15 (StataCorp, College Station, TX).
After searching for and removing the duplicates, a total of 3,351 titles and abstracts of relevant articles were checked. After the above screening, 59 articles were read in full and 15 articles met the inclusion criteria. Among them, the participants included in the study of Muta and Abrams overlapped (8, 16). Since the subjects included in Abrams had a larger time frame, the study of Muta was excluded. The final 14 studies were enrolled in this meta-analysis. The flow chart of the included studies is shown in Figure 1. The characteristics of the 14 enrolled studies involving a total of 70,396 patients are summarized in Table 1 (9, 16–28). The outcomes of the eight studies included the effects of early IVIG administration on the occurrence of CAL (17–21, 26–28), 10 studies involved IVIG unresponsiveness (9, 17–20, 22–25, 28), and CAA (9, 20) was investigated in two studies.
Meta-analysis for the pooled OR in the early treatment with IVIG on CAL is shown in Figure 2. With upper 95% CI = 1.0, there was no evidence supporting significant differences in the incidence of CAL between the early treatment with IVIG and 5 days after onset [OR 0.74; 95% CI (0.55, 1.00); P = 0.048]. However, significant heterogeneity was detected between the studies (P < 0.01; I2 = 84.3%). Begg's test (P = 0.266) and Egger's test (P = 0.263) showed no marked asymmetry.
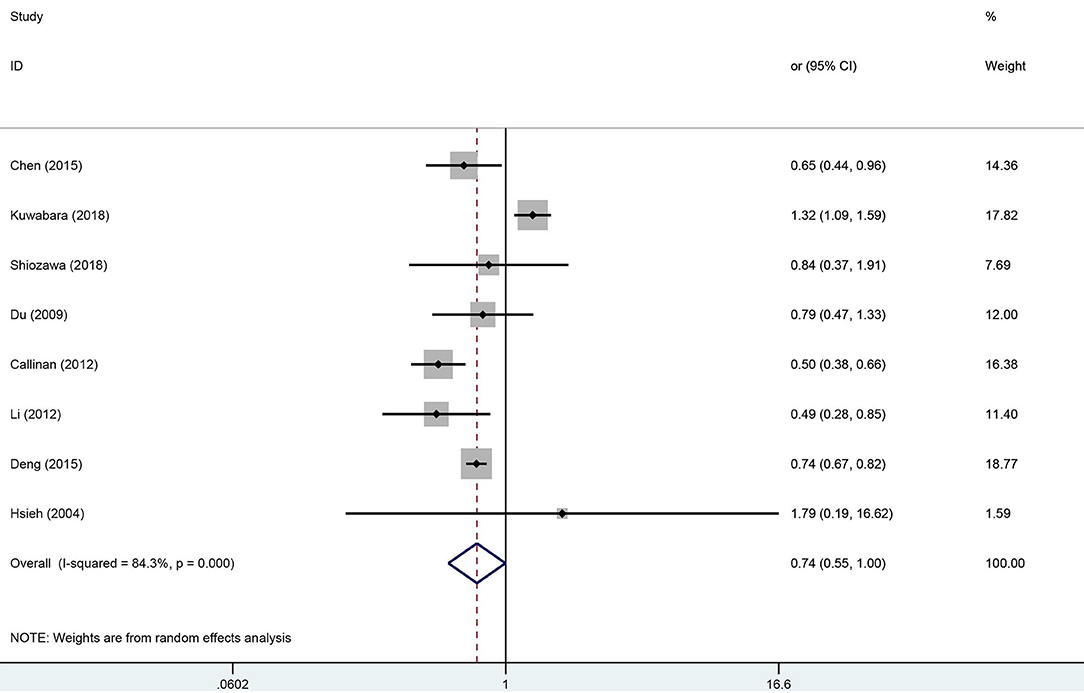
Figure 2. Pooled odds ratio for CAL development by the timing of IVIG therapy in KD (<5 days of disease onset vs. ≥5 days).
For sensitivity analysis, after omitting a study each time, the overall result was still stable (Supplementary Figure 1). To find the potential sources of heterogeneity, we used the study location, CAL diagnostic criteria, and duration of follow-up as covariates for the meta-regression analysis (Supplementary Table 3). The meta-regression results showed that the study location influenced the pooled estimate (China, P = 0.003, America, P = 0.003). A subgroup analysis was performed based on the study locations (Figure 3), we observed a significant decrease in the heterogeneity. From the overall results by each region, the Chinese studies supported that early treatment with IVIG was a protective factor against the developing CAL [OR 0.73; 95% CI (0.66, 0.80); P < 0.001] with a low heterogeneity (P = 0.543; I2 = 0.0%); however, the Japanese studies failed to show significant differences in the incidence of CAL regardless of the timing of IVIG treatment [OR 1.27; 95% CI (0.98, 1.64); P = 0.074]. Callinan's study was the only American study enrolled in this meta-analysis [OR 0.50; 95% CI (0.38, 0.66); P < 0.001].
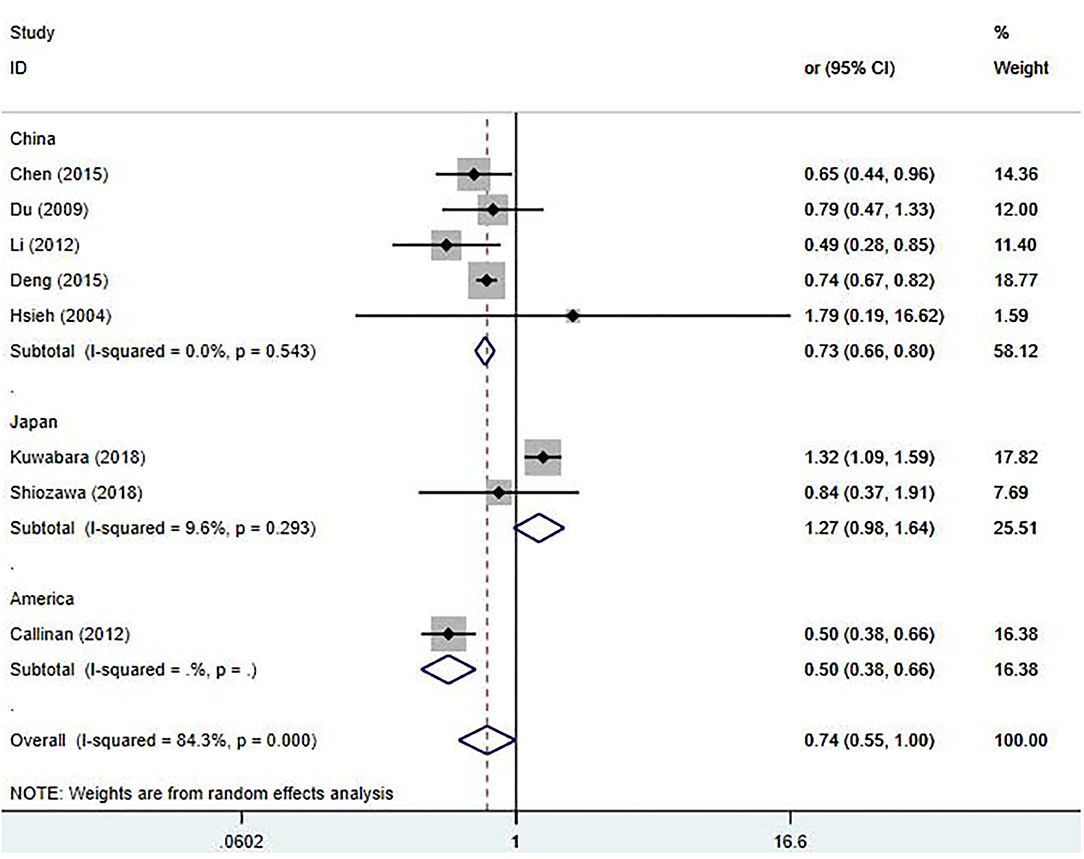
Figure 3. Pooled odds ratio for CAL development by the timing of IVIG therapy in KD (subgroup analysis basing on the study location).
Due to having a small sample size, Hsieh's study was excluded (28). Nine studies (9, 17–20, 22–25) involving IVIG unresponsiveness were included in this meta-analysis; results are shown in Figure 4. Early treatment with IVIG was a significant risk factor for IVIG unresponsiveness [OR 2.24; 95% CI (1.76, 2.84); P < 0.001]. However, a significant heterogeneity was observed (P = 0.028; I2 = 53.4%). Begg's test (P = 0.917) and Egger's test (P = 0.877) showed no marked asymmetry.
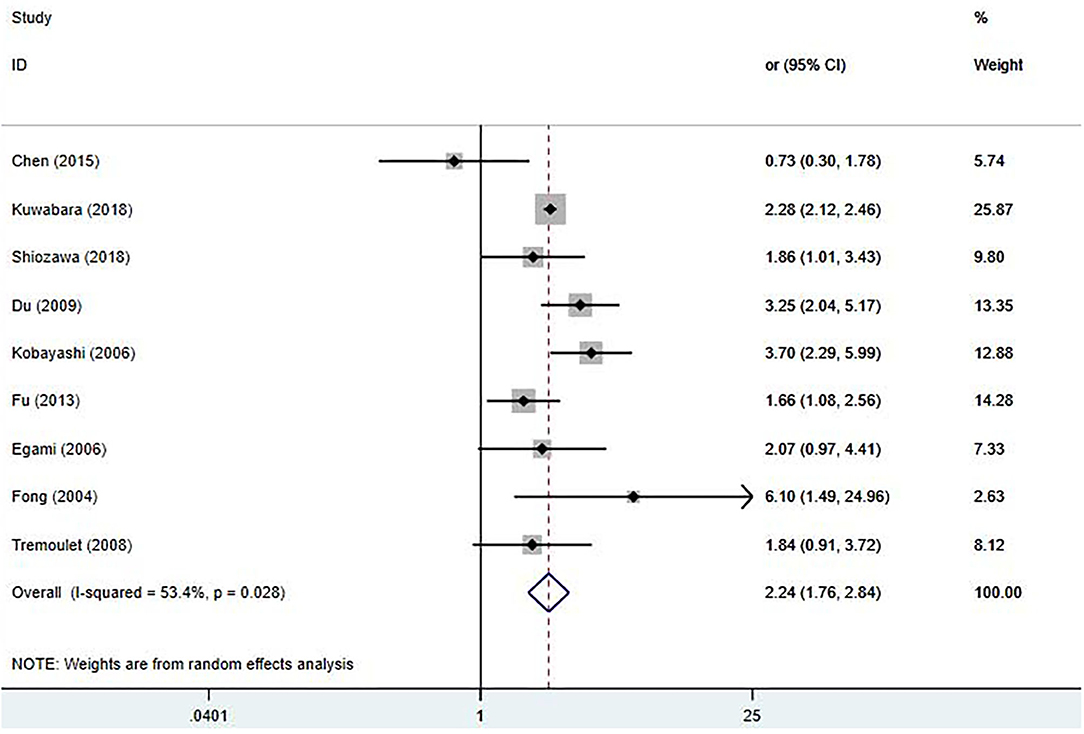
Figure 4. Pooled odds ratio for IVIG unresponsiveness by the timing of IVIG therapy in KD (<5 days of disease onset vs. ≥5 days).
In the sensitivity analysis, the total findings did not change significantly (Supplementary Figure 2). It was noticed that Chen's study (17) reported an extremely low incidence of IVIG unresponsiveness (4.9%), which was far lower than the other studies (12.3–38.3%). After omitting this study, the heterogeneity significantly decreased [OR 2.37; 95% CI (1.95, 2.90); P < 0.001; I2 = 35.5%, P = 0.145]. A meta-regression analysis, including the definition of IVIG unresponsiveness and study location, was also performed. However, these factors had no significant effect on the homogeneity of the included studies (Supplementary Table 4).
Only two studies were included in the meta-analysis of the relationship between the early IVIG treatment and the development of CAA (9, 20). We found no significant differences in the incidence and a significant heterogeneity between the studies [OR 1.16; 95% CI (0.22, 6.06); P = 0.858; I2 = 56.2%, P = 0.131] (Figure 5).
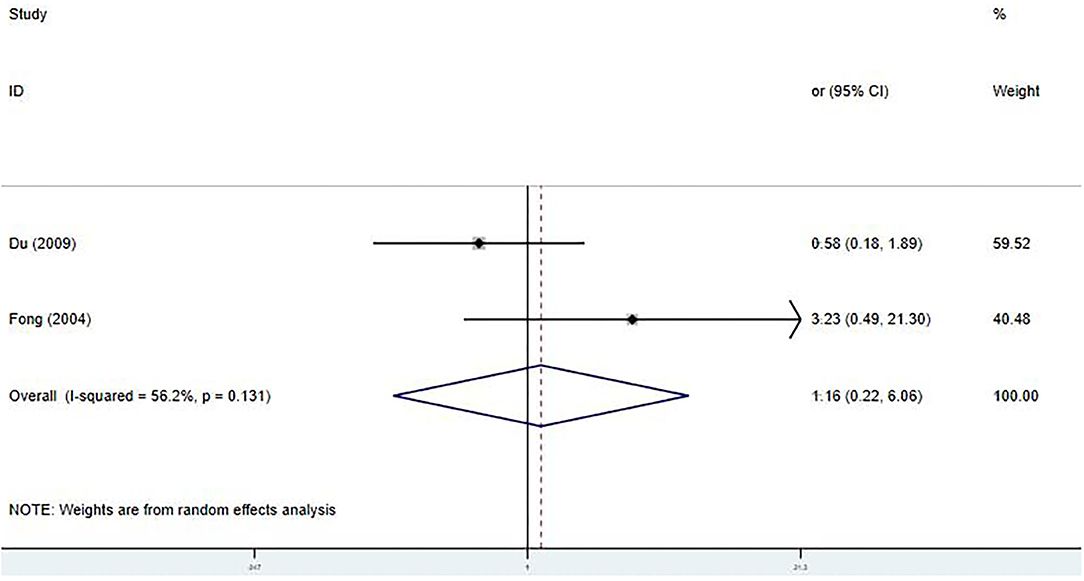
Figure 5. Pooled odds ratio for CAA development by the timing of IVIG therapy in KD (<5 days of disease onset vs. ≥5 days).
KD is characterized by a persistent fever in addition to five typical clinical manifestations (29) and can lead to systemic vasculitis, especially damaging the coronary arteries (30). The most effective treatment is known to be IVIG, which is recommended as a 2 g/kg single infusion 5–10 days after the onset of KD (4). Various studies have shown that the patients with a delayed IVIG treatment have a higher incidence of CAL and IVIG resistance (20, 31–33). However, current researches on the early use of IVIG is somewhat controversial.
It was reported that after the acute phase of the KD, 1,356 patients were followed-up for up to 15 years, the coronary artery events increased significantly in the patients with severe coronary dilatation. In patients with both a Z score ≥10 and an absolute dimension ≥8 mm, the incidence was as high as 48% (34). Therefore, CAL has been the focus for clinicians and researchers for a long time. Earlier literature reported that the incidence of CAL dropped from 25 to 4% after the conventional IVIG and aspirin treatment (35, 36). However, the incidence rates reported in recent years have risen significantly. In a nationwide survey on the epidemiology of KD in Japan (2011–2012), 9.3% of patients had one or more cardiac lesions (37). In 2012, Callinan reported a higher CAL incidence of 19% in California (21). Several studies have been conducted in order to reduce the occurrence of CAL, for example involving adjunctive corticosteroid therapy, different doses of aspirin, and tumor necrosis factor inhibitors in immunoglobulin-resistant KD (3, 38, 39). Unfortunately, they all failed to find convincing positive results.
This meta-analysis was performed to investigate the effects of IVIG therapy timing on the outcomes of KD. Overall, the early use of IVIG did not significantly reduce the incidence of CAL and CAA. However, the meta-regression analysis found that the study location had a significant influence on the outcomes. In the subgroup analysis, compared to the studies done in Japan, the studies conducted in China and the United States supported early IVIG treatment as a protective factor against the development of CAL. Considering the worldwide epidemiology, the incidence of KD and CAL in East Asia is significantly higher than that in Europe and the United States, it was reported that the KD incidence per 100,000 children were reported as 264.8 cases in Japan, 134.4 cases in Korea, 82.7 in Taiwan, 51.4 in Singapore, 21.9 in Canada, 18.1 in the United States, 15.2 in Ireland, and 9 in France (40, 41). In addition, a few scoring systems used to predict the IVIG resistance are highly sensitive and specific in the Japanese population but they have not been as useful in other areas (42, 43). Thus, the characteristics of KD are thought to vary between ethnicities, which may be the reason for the difference. However, considering the study design, the results must be interpreted with caution. Part of the studies we included were a comparison of the IVIG intervention within 5 days and after 5 days, including 10 days after the KD onset. When we meta-analyzed four studies comparing IVIG treatment within 5 days and within 5–10 days, there were no significant differences in the CAL outcomes (Supplementary Figure 3). In addition, the different definitions of CAL and the length of follow-up after KD onset may also influence the results.
Moreover, patient-related factors are speculated to be an important risk factor for CAL development, too. Patients undergoing the early IVIG treatment may be inclined to have more typical clinical manifestations and more intense inflammatory responses than patients with the conventional or delayed IVIG treatment, leading to an early introduction of IVIG treatment that could lead to CAL development. Thus, more well-designed randomized controlled study is important for a rigorous conclusion regarding the impact of IVIG timing on the development of CAL.
The 2003–2004, 2007–2008, and 2011–2012 Nationwide Surveys in Japan reported the incidence of IVIG unresponsiveness to be 26.4, 22.1, and 17%, respectively (8, 37, 44, 45). Tremoulet reported an incidence of IVIG unresponsiveness of up to 38.3% in San Diego in 2006 (25). In a previous meta-analysis, it was found that the patients who were IVIG-unresponsive had a 3.43 times higher risk of developing CAA (46). Therefore, by the early identification of patients with IVIG unresponsiveness and active administration of additional IVIG supplemented by other treatments, the incidence of CAL may be further reduced.
Several IVIG unresponsiveness scoring systems have been published. The main factors for IVIG unresponsiveness are the days of illness at initial treatment, neutrophil percentage and count, C-reactive protein (CRP), and age (23). We also found that early infusion of IVIG is a risk factor for IVIG unresponsiveness. However, patient–related factors have been speculated to be a cause of IVIG unresponsiveness in patients who received early IVIG treatment. In the Kobayashi scoring system established in Japan, Kobayashi found that the patients in the early IVIG treatment group had higher scores regardless of the IVIG treatment time. Therefore, it is speculated that the patients receiving IVIG at an early stage may have had a more severe form of KD (22). In 2018, Shiozawa et al. conducted a study with a better design to control the impact of patients' baseline characteristics. They divided the study subjects into two groups: one group was given IVIG within 5 days after the onset of KD while the other group who were diagnosed early were not treated with IVIG until day five. They, then, adjusted for known confounders using the propensity scores. A higher rate of treatment resistance still appeared in the early treatment group (19). It was suggested that the timing of IVIG treatment influenced the reactivity to treatment regardless of the severity of KD. Besides, It was reported that in patients with KD, inflammatory mediators, including plasma white blood cell count, absolute neutrophil count, and CRP peak within 5–10 days of KD onset, thus, the inappropriate timing of IVIG treatment may lead to an increased incidence of IVIG unresponsiveness (47). However, owing to the potential confounders between groups, subsequent studies are still needed to focus on controlling for differences in patients' baseline characteristics in order to obtain reliable results.
Our study has several limitations. First, all included studies were non-randomized retrospective studies. Second, there were slight differences in the CAL diagnostic criteria, IVIG unresponsiveness criteria, and the time of follow-up. Third, the grouping of IVIG infusion time points in the different literatures have some differences, which limits the number of included studies. Moreover, a few studies had slightly smaller sample sizes, such as studies of Hsieh et al. and Fong et al. Thus, more randomized controlled studies with careful controlling of the confounders between groups following the standard treatment and follow-up procedures are needed to identify better time points for IVIG therapy.
In conclusion, early treatment with IVIG can lead to an increased risk of IVIG unresponsiveness. In contrast to the studies performed in Japan that found no significant difference in coronary outcomes, the studies conducted in China and the United States showed a reduced risk in the occurrence of CAL with an early IVIG treatment. At present, the evidence does not support the treatment with IVIG in the early stage of the onset of KD. But, early IVIG treatment could be a protective factor against the development of CAL, which needs to be further clarified.
All datasets generated for this study are included in the article/Supplementary Material.
This systematic review and meta-analysis is based on a collection of data retrieved from studies that have already been published. We did not collect individual patient data and did not have direct contact with any of the included patients.
FY, HZ, and FZ designed and conceived the experiments. FY and HZ performed the experiments. FY, RX, and XC analyzed the data. XC and YC contributed the reagents, materials, and analysis tools. FY, RX, and FZ wrote the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2020.593435/full#supplementary-material
Supplementary Figure 1. Sensitivity analysis of the included studies for the incidence analysis of CAL.
Supplementary Figure 2. Sensitivity analysis of the included studies for the incidence analysis of IVIG unresponsiveness.
Supplementary Figure 3. Pooled odds ratio for CAL development by the timing of IVIG therapy in KD (<5 days of disease onset vs. 5–10 days).
Supplementary Table 1. The NEWCASTLE-OTTAWA SCALE for case control studies.
Supplementary Table 2. The NEWCASTLE-OTTAWA SCALE for cohort studies.
Supplementary Table 3. Meta-regression analysis of the included studies for the primary outcome (CAL development).
Supplementary Table 4. Meta-regression analysis of the included studies for the primary outcome (IVIG unresponsiveness).
Supplementary Checklist 1. PRISMA checklist.
1. Sánchez-Manubens, Judith, Bou R, Anton J. Diagnosis and classification of Kawasaki disease. J Autoimmunity. (2014) 48–9:113–7. doi: 10.1016/j.jaut.2014.01.010
2. Taubert, KA, Rowley, AH, Shulman, ST. Nationwide survey of Kawasaki disease and acute rheumatic fever. J Pediatrics. (1991) 119:279–82. doi: 10.1016/S0022-3476(05)80742-5
3. Xue LJ, Wu R, Du GL, Xu Y, Yuan KY, Feng ZC, et al. Effect and safety of TNF inhibitors in immunoglobulin-resistant Kawasaki Disease: a meta-analysis. Clin Rev Aller Immunol. (2017) 52:389–400. doi: 10.1007/s12016-016-8581-4
4. Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Pediatrics. (2004) 114:1708–33. doi: 10.1542/peds.2004-2182
5. Dajani AS, Taubert KA, Takahashi M, Bierman FZ, Freed MD, Ferrieri P, et al. Guidelines for long-term management of patients with Kawasaki disease. Report from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. (1994) 89:916–22. doi: 10.1161/01.CIR.89.2.916
6. Durongpisitkul K, Gururaj VJ, Park JM, Martin CF. The prevention of coronary artery aneurysm in Kawasaki disease: a meta-analysis on the efficacy of aspirin and immunoglobulin treatment. Pediatrics. (1995) 96:1057–61.
7. Terai M, Shulman ST. Prevalence of coronary artery abnormalities in Kawasaki disease is highly dependent on gamma globulin dose but independent of salicylate dose. J Pediatr. (1997) 131:888–93. doi: 10.1016/S0022-3476(97)70038-6
8. Muta H, Ishii M, Egami K, Furui J, Sugahara Y, Akagi T, et al. Early intravenous gamma-globulin treatment for Kawasaki disease: The nationwide surveys in Japan. J Pediatr. (2004) 144:496–9. doi: 10.1016/j.jpeds.2003.12.033
9. Fong NC, Hui YW, Li CK, Chiu MC. Evaluation of the efficacy of treatment of Kawasaki Disease before day 5 of illness. Pediatr Cardiol. (2004) 25:31–4. doi: 10.1007/s00246-003-0558-4
10. McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, et al. Diagnosis, treatment, and long-term management of Kawasaki Disease: a scientific statement for health professionals from the American Heart Association. Circulation. (2017) 135:e927. doi: 10.1161/CIR.0000000000000484
11. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP A, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Int Med. (2009) 151:65–94. doi: 10.7326/0003-4819-151-4-200908180-00136
12. Wells G. The Newcastle-Ottawa Scale (NOS) for assessing the quality of non-randomized studies in meta-analyses. In: Symposium on Systematic Reviews: Beyond the Basics. Oxford (2014).
13. Research Committee on Kawasaki Disease. Report of Subcommittee on Standardization of Diagnostic Criteria and Reporting of Coronary Artery Lesions in Kawasaki Disease. Tokyo: Ministry of Health and Welfare (1984).
14. de Zorzi A, Colan SD, Gauvreau K, Baker AL, Sundel RP, Newburger JW. Coronary artery dimensions may be misclassified as normal in Kawasaki disease. J Pediatr. (1998) 133:254–8. doi: 10.1016/S0022-3476(98)70229-X
15. Fu S, Gong F, Xie C, Zhu W, Wang W, Shen H, et al. S100A12 on circulating endothelial cells surface in children with Kawasaki Disease. Pediatr Res. (2010) 68:165. doi: 10.1203/PDR.0b013e3181e67ce8
16. Abrams JY, Belay ED, Uehara R, Maddox RA, Schonberger LB, Nakamura Y. Cardiac complications, earlier treatment, and initial disease severity in Kawasaki Disease. J Pediatrics. (2017) 188:64–9. doi: 10.1016/j.jpeds.2017.05.034
17. Chen JJ, Ma XJ, Liu F, Yan WL, Huang MR, Huang M, et al. Epidemiologic features of Kawasaki Disease in Shanghai from 2008 through 2012. Pediatr Infect Dis J. (2015) 35:7–12. doi: 10.1097/INF.0000000000000914
18. Masanari K, Mayumi Y, Ryusuke A, Hiroshi Y, Yosikazu N. The effects of early intravenous immunoglobulin therapy for Kawasaki disease: The 22nd nationwide survey in Japan. Int J Cardiol. (2018) 269:334–8. doi: 10.1016/j.ijcard.2018.07.092
19. Shiozawa Y, Inuzuka R, Shindo T, Mafune R, Hayashi T, Hirata Y, et al. Effect of intravenous immunoglobulin within day 4 of illness in Kawasaki Disease. Pediatr Int. (2018) 60:334–41. doi: 10.1111/ped.13512
20. Du ZD, Di Z, Du JB, Lu S, Yi JM, Hou AC, et al. Comparison of efficacy among early, conventional and late intravenous gamma globulin treatment of Kawasaki disease. Zhonghua Yi Xue Za Zhi. (2009) 89:1841−3. doi: 10.3760/cma.j.issn.0376-2491.2009.26.014
21. Callinan LS, Tabnak F, Holman RC, Maddox RA, Kim JJ, Schonberger LB, et al. Kawasaki syndrome and factors associated with coronary artery abnormalities in California. Pediatr Infect Dis J. (2012) 31:894–8. doi: 10.1097/INF.0b013e31825c4d7c
22. Kobayashi, T. Prediction of intravenous immunoglobulin unresponsiveness in patients with Kawasaki Disease. Circulation. (2006) 113:2606–12. doi: 10.1161/CIRCULATIONAHA.105.592865
23. Fu PP, Du ZD, Pan SY. Novel predictors of intravenous immunoglobulin resistance in Chinese children with Kawasaki disease. Pediatr Infect Dis J. (2013) 32:319–23. doi: 10.1097/INF.0b013e31828e887f
24. Egami K, Muta H, Ishii M, Suda K, Sugahara Y, Iemura M, et al. Prediction of resistance to intravenous immunoglobulin treatment in patients with Kawasaki disease. J Pediatr. (2006) 149:237–40. doi: 10.1016/j.jpeds.2006.03.050
25. Tremoulet AH, Best BM, Song S, Wang S, Corinaldesi E, Eichenfield JR, et al. Resistance to intravenous immunoglobulin in children with Kawasaki disease. J Pediatr. (2008) 153:117–21.e3. doi: 10.1016/j.jpeds.2007.12.021
26. Li Y, Wang XM, Liu YL, Shi K, Yang YF, Guo YH. Risk factors for coronary artery lesions in children with Kawasaki disease. Zhongguo Dang Dai Er Ke Za Zhi. (2012) 14:938–41. doi: 10.1007/s11783-011-0280-z
27. Yong-Chao D, Xun W, Xi-Chun T, Cai-Zhi H, Li-Ya M. Risk factors for coronary artery lesions secondary to Kawasaki disease in children. Chin J Contemp Pediatr. (2015) 17:927–31. doi: 10.7499/j.issn.1008-8830.2015.09.008
28. Hsieh KS, Weng KP, Lin CC, Huang TC, Lee CL, Huang SM. Treatment of acute Kawasaki Disease: aspirin's role in the febrile stage revisited. Pediatrics. (2004) 114:e689. doi: 10.1542/peds.2004-1037
29. Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children. Arerugi. (1967) 16:178–222.
30. Eleftheriou D, Levin M, Shingadia D, Tulloh R, Klein NJ, Brogan PA. Management of Kawasaki disease. Arch Dis Childh. (2014) 99:74–83. doi: 10.1136/archdischild-2012-302841
31. Qin L, Saumu MT, Wang H, Shi H, Cheng P. Reevaluation of the efficacy of intravenous gammaglobulin in the prevention and treatment of coronary artery lesion in Kawasaki disease. J Huazhong Univ Sci Technol. (2005) 25:348. doi: 10.1007/BF02828164
32. Lucron H, Bosser G, Lethor JP, Sommelet D, Feillet F, Burger G, et al. Kawasaki disease in newborns and infants: refractory forms to immunoglobulin therapy. Arch Maladies Coeur Et Des Vaisseaux. (2004) 97:522–8. doi: 10.1007/s10016-004-0020-y
33. Tulloh RMR, Mayon-White R, Harnden A, Ramanan AV, Tizard EJ, Shingadia D, et al. Kawasaki disease: a prospective population survey in the UK and Ireland from 2013 to 2015. Arch Dis Childh. (2018) 640–6. doi: 10.1136/archdischild-2018-315087
34. Manlhiot C, Millar K, Golding F, McCrindle BW. Improved classification of coronary artery abnormalities based only on coronary artery z-scores after Kawasaki disease. Pediatr Cardiol. (2010) 31:242–49. doi: 10.1007/s00246-009-9599-7
35. Amano S, Hazama F, Hamashima Y. Pathology of Kawasaki disease: ii. distribution and incidence of the vascular lesions. Japan Circ J. (1979) 43:741–8. doi: 10.1253/jcj.43.741
36. Newburger JW, Takahashi M, Burns JC, Beiser AS, Chung KJ, Duffy CE. The treatment of Kawasaki syndrome with intravenous gamma globulin. New Engl J Med. (1986) 315:341–7. doi: 10.1056/NEJM198608073150601
37. Makino N, Nakamura Y, Yashiro M, Ae R, Tsuboi S, Aoyama Y, et al. Descriptive epidemiology of Kawasaki Disease in Japan, 2011–2012: from the results of the 22nd Nationwide survey. J Epidemiol. (2015) 25:239–45. doi: 10.2188/jea.JE20140089
38. Kobayashi T, Kobayashi T, Morikawa A, Ikeda K, Seki M, Shimoyama S, et al. Efficacy of Intravenous immunoglobulin combined with prednisolone following resistance to initial intravenous immunoglobulin treatment of acute Kawasaki Disease. J Pediatr. (2013) 163:521–6.e1. doi: 10.1016/j.jpeds.2013.01.022
39. Zheng X, Yue P, Liu L, Tang C, Ma F, Zhang Y, et al. Efficacy between low and high dose aspirin for the initial treatment of Kawasaki disease: current evidence based on a meta-analysis. PLoS ONE. (2019) 14:e0217274. doi: 10.1371/journal.pone.0217274
40. Singh S, Vignesh P, Burgner D. The epidemiology of Kawasaki disease: a global update. Arch Dis Childh. (2015) 100:1084–8. doi: 10.1136/archdischild-2014-307536
41. Barut K, Sahin S, Kasapcopur O. Pediatric vasculitis. Curr Opin Rheumatol. (2016) 28:29–38. doi: 10.1097/BOR.0000000000000236
42. Sleeper LA, Minich LL, McCrindle BM, Li JS, Mason W, Colan SD, et al. Evaluation of Kawasaki Disease risk-scoring systems for intravenous immunoglobulin resistance. J Pediatr. (2011) 158:831–5.e3. doi: 10.1016/j.jpeds.2010.10.031
43. Davies S, Sutton N, Blackstock S, Gormley S, Hoggart CJ, Levin M, et al. Predicting IVIG resistance in UK Kawasaki disease. Arch Dis Childh. (2015) 100:366–8. doi: 10.1136/archdischild-2014-307397
44. Nakamura Y, Yashiro M, Uehara R, Oki I, Kayaba K, Yanagawa H. Increasing incidence of Kawasaki disease in Japan: nationwide survey. Pediatr Int. (2010) 50:287–90. doi: 10.1111/j.1442-200X.2008.02572.x
45. Nakamura Y, Yashiro M, Uehara R, Sadakane A, Chihara I, Aoyama Y, et al. Epidemiologic features of Kawasaki disease in Japan: results of the 2007-2008 nationwide survey. J Epidemiol. (2010) 20:302–7. doi: 10.2188/jea.JE20090180
46. Yan F, Pan B, Sun H, Tian J, Li M. Risk factors of coronary artery abnormality in children with Kawasaki Disease: a systematic review and meta-analysis. Front Pediatr. (2019) 7:374. doi: 10.3389/fped.2019.00374
47. Samadli S, Liu FF, Mammadov G, Wang JJ, Liu HH, Wu YF, et al. The time option of IVIG treatment is associated with therapeutic responsiveness and coronary artery abnormalities but not with clinical classification in the acute episode of Kawasaki disease. Pediatr Rheumatol. (2019) 17:53. doi: 10.1186/s12969-019-0352-3
Keywords: Kawasaki disease, intravenous immunoglobulin therapy, coronary artery lesion, coronary artery aneurysm, IVIG unresponsiveness
Citation: Yan F, Zhang H, Xiong R, Cheng X, Chen Y and Zhang F (2020) Effect of Early Intravenous Immunoglobulin Therapy in Kawasaki Disease: A Systematic Review and Meta-Analysis. Front. Pediatr. 8:593435. doi: 10.3389/fped.2020.593435
Received: 10 August 2020; Accepted: 29 September 2020;
Published: 20 November 2020.
Edited by:
Teresa Giani, University of Florence, ItalyReviewed by:
Ozgur Kasapcopur, Istanbul University-Cerrahpasa, TurkeyCopyright © 2020 Yan, Zhang, Xiong, Cheng, Chen and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Furong Zhang, NzkyNTIzNDk2QHFxLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.