
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 30 November 2020
Sec. Pediatric Nephrology
Volume 8 - 2020 | https://doi.org/10.3389/fped.2020.591520
 Nicholas Farris1
Nicholas Farris1 Rupesh Raina2,3
Rupesh Raina2,3 Abhishek Tibrewal3
Abhishek Tibrewal3 Miraides Brown4
Miraides Brown4 Maria Colvis5
Maria Colvis5 Andrew Schwaderer6
Andrew Schwaderer6 Kirsten Kusumi2,7*
Kirsten Kusumi2,7*Background: Acute kidney injury (AKI) due to urinary stone disease (USD) is rare in adults; AKI rates in children with USD may be higher, and emerging data links stones to chronic kidney disease (CKD) development in adults.
Methods: This study is a retrospective analysis of USD patients at a single pediatric hospital system's emergency department (ED). Patients were initially identified by USD ICD codes; USD was then confirmed by imaging or physician documentation; patients had to have baseline creatinine (Cr) and Cr in the ED for comparison to be included. AKI was defined by Kidney Disease: Improving Global Outcomes (KDIGO), Acute Kidney Injury Network (AKIN), and Pediatric Risk, Injury, Failure, Loss, End Stage (pRIFLE).
Results: Of the 589 total visits, 264/589 (45%) had data to evaluate for AKI, 23% were AKI(+) and 77% were AKI(–). pRIFLE was most common (82%) and 18% were only positive by AKIN/KDIGO. AKI(+) were more likely to be younger (16.7 vs. 17.4 years, p = 0.046) and more likely to present with vomiting {odds ratio [OR] [95% confidence interval (CI)]: 2.4 [1.4–4.3], p = 0.002}; also, the proportion of AKI(+) was significantly higher in <18 vs. ≥18 years [26.9 vs. 15.5%, p = 0.032, OR (95% CI): 2.0 (1.1–3.9)]. Urinary tract infection (UTI) and obstruction rates were similar between groups. AKI(+) patients had a significant OR <1 suggesting less risk of receiving non-steroidal anti-inflammatory drugs (NSAIDs); however, 51% of them did receive NSAIDs during their ED encounter. AKI(+) patients were more likely to require admission to the hospital (53 vs. 32%, p = 0.001).
Conclusion: We have demonstrated a novel association between USD-induced renal colic and AKI in a group of young adults and children. AKI(+) patients were younger and were more likely to present with vomiting. AKI(+) patients did not have higher rates of obstruction or UTI, and 51% of AKI(+) received NSAIDs.
Urinary stone disease (USD) is common in the United States, affecting 1 in 10 adults, and is becoming more common in the pediatric population (1). The incidence of pediatric USD has increased by 4–6% annually, and the number of stone-forming adolescents has doubled in the last 20 years (1, 2). While urinary stones are notorious for causing severe pain and renal colic, morbidity has traditionally been linked to urinary tract obstruction and/or infection (3). Acute kidney injury (AKI) is defined by an abrupt decrease in renal function and has been strongly linked to increases in morbidity and mortality (4–7). USD-associated AKI is most often attributed to the obstruction of a solitary kidney or both kidneys simultaneously and occurs in 1–2% of adult stone formers (8–11). AKI in pediatric USD formers has not been well-described, but there is some evidence that children may be more susceptible with rates as high as 30% (12).
In adults, increases in creatinine (Cr) as small as 0.3 mg/dl have been linked to increased mortality; furthermore, AKI can increase hospital care costs and length of stay (5, 6, 13, 14). While AKI is uncommon in adult USD formers, there is evidence that they are at increased risk for development of chronic kidney disease (CKD) independent of other comorbidities such as diabetes and hypertension (1, 15–18). If pediatric USD formers develop AKI more frequently, they may be at greater risk for long-term renal sequelae as children have higher stone recurrence rates compared to adults due to their greater prevalence of stone metabolic risk factors (19). Thus, younger patients cared for in a children's hospital may constitute a previously unrecognized high-risk group for AKI with potential for long-term CKD development. Therefore, the objective of this study was to evaluate acute renal colic patients in a pediatric emergency department (ED) for AKI.
This study was a retrospective review with approval by the Akron Children's Institutional Review Board (ID 1205631-7). Electronic medical records were reviewed for all patients under 25 years at a single freestanding pediatric hospital system identified by ICD coding from January 2008 to December 2017. The ICD 10 codes included the following: N20, N20.1, and N20.9 and ICD9: 592, 592.1, and 592.9 (calculus of kidney, ureter, and lower urinary tract, as well as urinary calculus, unspecified). All charts were reviewed to confirm diagnosis of urinary stones, and patients were only included if they either had (1) radiographic imaging positive for stones [renal ultrasound or computed tomography (CT)] or (2) documentation by an attending nephrologist or urologist of kidney stone disease. Patient charts were then reviewed for ED visits due to stone-associated renal colic. Visits were included if they either had (1) positive imaging (renal ultrasound or CT) of stones at that visit or (2) documentation by an attending physician that presenting symptoms were due to urinary stones.
The data collected included anthropometrics at the time of ED visit (age, sex, weight, height, BMI, and corresponding Z-scores), presenting clinical symptoms (flank pain, abdominal pain, nausea, vomiting, hematuria, and/or dysuria), serum chemistry, urinalysis, cultured urine organisms, and imaging (renal ultrasound and CT). Abdominal X-rays of the kidney, ureter, and bladder (KUB) were not included as local practice patterns do not routinely utilize KUB as a screening tool in the ED, without also obtaining other imaging, due to the inability of KUB to evaluate patients for obstruction. Any urine culture with an organism growing >100,000 colony forming units was considered positive, even if the organism is not considered a traditional urinary infectious organism. Treatment in the ED including medications [intravenous fluids (IVF), pain medications, anti-emetics, alpha-agonists, and antibiotics] as well as discharge medications were identified. Disposition (hospital admission or discharge) were also reported.
AKI was defined utilizing three well-established pediatric criteria: the Kidney Disease Improving Global Outcomes (KDIGO), the Acute Kidney Injury Network (AKIN), and the Pediatric Risk, Injury, Failure, Loss, End Stage Renal Disease (pRIFLE) criteria (Table 1). The temporal and urine output criteria for AKI were not used due to the retrospective analysis and short-term nature of ED care. ED visits were designated as either able or unable to evaluate for AKI based on availability of data including height measurement and serum Cr levels. Baseline Cr and height measurements were included from outpatient visits, if available, from within the following time ranges dependent on the patients' age at the time of their ED visit: infants within 3 months, 2–6 years old within 6 months, 6–11 years within 8 months, 11–14 years within a year, and 14+ within 2 years. If multiple Cr measurements were available, baseline Cr was defined as the lowest value within the above age-defined time range. The glomerular filtration rate (GFR) was calculated using the bedside Schwartz formula (20). Any patient without available baseline serum Cr level was categorized as unable to evaluate for AKI and excluded from subsequent analysis.
Patient data were analyzed by ED visit and the visits were then compared: AKI by the criteria of pRIFLE, KDIGO, or AKIN [AKI(+)] vs. those without AKI [AKI(–)]. Comparisons of the AKI(+) vs. AKI(–) groups were made with Chi-square or Fisher's exact test for categorical variables and Student t-tests or Wilcoxon Rank-Sum tests were performed for continuous variables. The Hodges–Lehmann Estimator and odds ratio confidence intervals were calculated where appropriate. Statistical analyses were completed using SAS (version 9.4; SAS Institute Inc., Cary, NC, USA). All statistical tests were two-tailed with significance defined as p <0.05. The Venn diagram was generated by using Venny 2.1 online software (21).
A total of 1,226 patients, aged 1–25 years old, had ICD codes for kidney stones while 670 patients were excluded due to insufficient data to confirm urinary stone disease. Another 156 patients were excluded due to lack of ED visit for acute renal colic. Overall, there were 400 unique patients with 589 total visits that met the inclusion criteria for analysis (Figure 1).
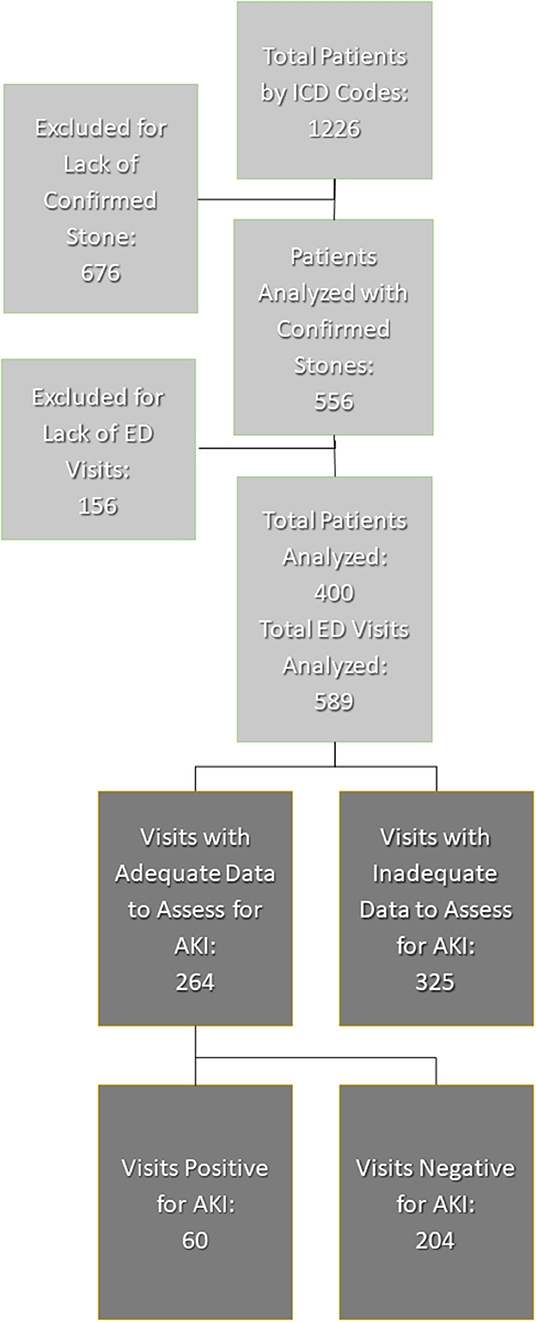
Figure 1. Inclusion and exclusion of patient visits. AKI, acute kidney injury; ED, emergency department.
Of the 589 total visits, 264/589 (45%) had adequate data to evaluate for AKI corresponding to 146 unique patients. All these patients had both baseline creatinine as well as creatinine at ED. Among the included patients, 86/146 (59%) had one visit, 44/146 (30%) had 2–3 visits, and 16/146 had more than three visits. A total of 60/264 (23%) visits met criteria for AKI while 204/264 (77%) did not.
Of all visits with AKI, all three criteria (pRIFLE, AKIN, and KDIGO) were met with 28/60 (47%), and pRIFLE was the most frequently met AKI criterion with 49/60 (82%) of visits compared to 38/60 (63%) of visits for AKIN and KDIGO. However, only 21/60 (35%) met pRIFLE criterion and only 11/60 (18%) visits met the AKIN/KDIGO criteria due to the former's inclusion of an increase in serum Cr > 0.3 mg/dl from baseline. The distribution of AKI diagnoses by each criterion is shown in Figure 2.
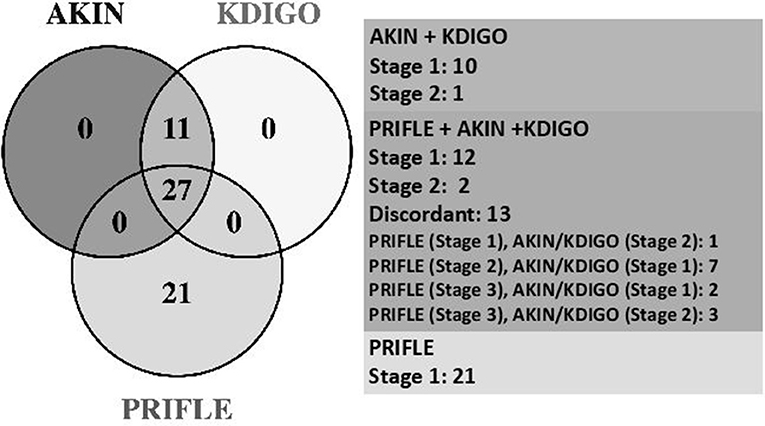
Figure 2. Comparative stages of AKI. AKIN, Acute Kidney Injury Network; KDIGO, Kidney Disease Improving Global Outcomes; PRIFLE, Pediatric Risk, Injury, Failure, Loss, End Stage Renal Disease.
Table 2 displays demographic and clinical characteristics for AKI(+) vs. AKI(–) groups. We included all patient visits for USD in the ED of our pediatric hospital system including young adults; a total 167 patients were <18 years with 45 (26.9%) AKI(+) vs. 122 (73.1%) AKI(–) vs. a total of 97 patients ≥18 years with 15 (15.5%) AKI(+) vs. 82 (84.5%) AKI(–). The oldest patient in our cohort was 24 years. The proportion of AKI(+) was observed to be significantly higher among those aged <18 years as compared to those aged ≥18 years {26.9 vs. 15.5%, p = 0.032, odds ratio [OR] [95% confidence interval (CI)]: 2.0 [1.1–3.9]} resulting in slightly younger age profile among those with AKI(+) vs. AKI(–) patients (median: 16.7 vs. 17.4 years, p = 0.045). All other anthropometric variables were similar in both groups. Also, AKI(+) patients were 2.5 times more likely to present with complaints of vomiting [OR (95% CI): 2.5 (1.4–4.4), p = 0.002] and 3.2 times more likely to complain of fever [OR (95% CI): 3.2 (1.1–9.3), p = 0.049]. Patients with AKI(+) had higher blood urea nitrogen (BUN) (13 vs. 12, p = 0.007) and Cr levels at ED (0.9 vs. 0.7, p < 0.001) vs. AKI(–) patients on serum testing. Also, patients with AKI(+) had lower GFR at ED (72.3 vs. 96.2, p < 0.001) and higher percentage decrease in GFR from baseline to ED (34 vs. 6%, p < 0.001) vs. AKI(–) patients. Although AKI(+) patients demonstrated slightly lower sodium levels {median [interquartile ranges (IQR)]: 138 [136.3, 139] vs. 138 [137–140], p = 0.043}, due to the retrospective nature of the study, it is not clear when these labs were obtained in relation to IVF therapy. Despite a higher incidence of complaints of fever on presentation among AKI(+) patients [AKI(+) 11.7% vs. AKI(–) 3.9%, p = 0.049), there was no significant difference between the groups' leukocyte esterase and nitrite testing on urinalysis. Furthermore, 65–70% of AKI(+) and AKI(–) groups had formal urine cultures with similar rates of positive urine culture growth. None of our AKI(+) patients had a solitary kidney, and we did not find a significant difference in rates of unilateral or bilateral obstruction between AKI(+) and AKI(–) groups (unilateral 66.7 vs. 57.5%, p = 0.19; bilateral 1.8 vs. 3.8%, p = 0.46).
Thirty seven percent (22/60) of AKI(+) visits had documented recognition of their abnormal renal function in the chart with no significant difference between criteria used for defining AKI (Table 3). Of these visits, 59% (13/22) included lab values in their physician's note without mention of Cr levels being abnormal, 23% (5/22) included notation of elevated Cr in the physician assessment, and 18% (4/22) included notation of AKI (Figure 3).
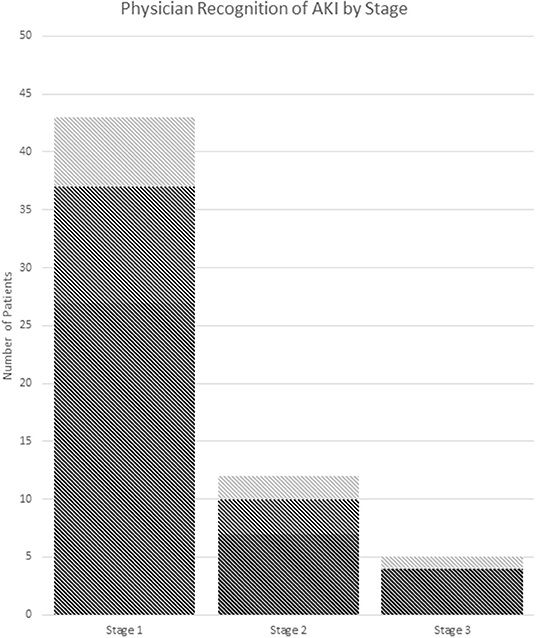
Figure 3. Recognition of AKI by stage. (A) Dark gray: no documentation. (B) Medium gray: documentation of abnormal labs. (C) Light gray: documentation of the presence of AKI or elevated Cr in assessment and/or plan. AKI stage is chosen by the highest stage met of any the pRIFLE, AKIN, or KDIGO criteria.
Of the treatments received in the ED, AKI(+) patients were slightly less likely to receive non-steroidal anti-inflammatory drugs (NSAIDS) [55 vs. 69%, p = 0.043; OR (95% CI): 0.55 (0.30–0.98)] as compared to AKI(–) patients (Table 2, Figure 4). However, AKI(+) patients were 2.6 times more likely to require admission to the hospital from ED [54 vs. 30%, p = 0.001, OR: 2.6 (1.4–4.9)]. There were no statistically significant differences in the treatments prescribed at discharge between AKI(+) and AKI(–) patients.
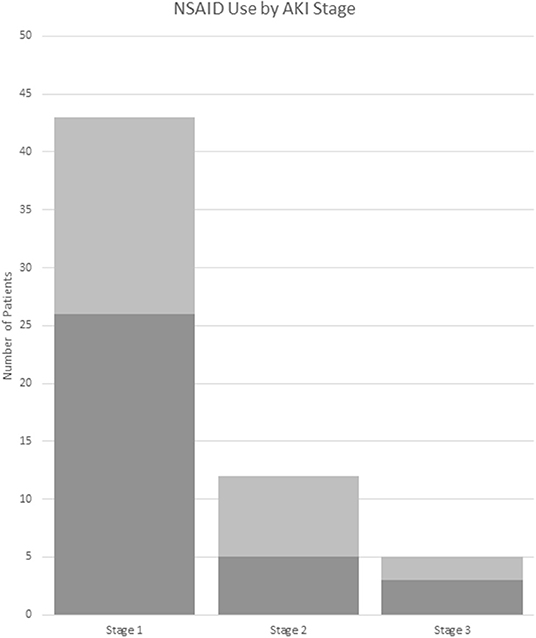
Figure 4. NSAID use by AKI stage. Dark gray: received NSAIDs. Light gray: did not receive NSAIDs. AKI stage is chosen by the highest stage met of any the pRIFLE, AKIN, or KDIGO criteria. NSAID, non-steroidal anti-inflammatory drug.
To our knowledge, this is the first study to assess a pediatric emergency department cohort for AKI associated with urinary stone-associated acute renal colic. Specifically, we have identified that AKI could only be assessed in less than half of ED visits due to insufficient data; of those visits with sufficient data, 23% of visits met the criteria for AKI. Most frequent missing data included serum Cr levels from the ED visit, height measurements, or baseline Cr for comparison. Pediatric AKI studies have typically focused on critically ill populations or those children requiring dialysis, thus making our analysis unique (8, 22–26). Additionally, large cross-sectional pediatric cohort studies have investigated inpatient populations and reported sepsis/shock, respiratory failure, liver or heart disease, bone marrow transplant, hemolytic-uremic syndrome, and nephrotoxic medications as the most common causes of pediatric AKI (25, 27–29). While urinary stones are noted in multiple studies, they are not considered a major cause of pediatric AKI; however, Jamal et al. found that urinary stones accounted for 30% of AKI in a single-center Pakistani cohort (12).
Notably, there is a paucity of data evaluating less severely ill patients for AKI in a children's hospital ED or outpatient setting. Furthermore, studies which did include ED patients are limited to those where patients being studied were subsequently admitted to the hospital (30). While the direct connection between AKI and development of CKD remains somewhat controversial, many recognize that milder episodes of AKI may contribute to long-term loss of GFR (4, 31). Given that adult urinary stone formers are at increased risk for CKD independent of established risk factors, this evidence of under-recognized AKI in urinary stone-forming children is particularly concerning (17, 18). Thus, while young adults and children with urinary stones have not previously been considered a high-risk population for AKI, we assert that urinary stone-associated AKI in this group is likely under-recognized and should be better addressed to prevent development of future CKD.
The high prevalence of AKI in our cohort was identified despite the fact that >50% of visits lacked the necessary data to assess for AKI. pRIFLE was the most frequently met definition for kidney injury; however, 18% of stone patients with AKI exclusively met the AKIN/KDIGO criteria. Thirty-five percent of patients would be missed by utilizing AKIN/KDIGO alone (Figure 2). The lack of concordance again highlights the need for greater consensus in the medical community regarding AKI criteria. The lack of an agreed-upon AKI definition limits researchers' ability to truly assess AKI prevalence and design of studies aimed at improving treatment and prevention (32). This is especially important as we begin to understand how common milder degrees of AKI are in pediatric populations and how AKI can impact long-term kidney function, including the development of CKD (31, 33, 34).
We demonstrated that younger urinary stone formers may be at greater risk for AKI based on a lower median age of AKI(+) vs. AKI(–) patients and that children have an odds ratio of 2 for suffering from AKI compared to those ≥18 years. This could potentially be due to the decreased probability of spontaneous stone passage in younger children leading to obstruction, which has traditionally been the main mechanism thought to drive stone-associated AKI (9, 11). However, we did not find a significant difference in rates of unilateral or bilateral obstruction between AKI(+) and AKI(–) groups (unilateral 66.7 vs. 57.5%, p = 0.22; bilateral 1.8 vs. 3.8%, p = 0.68), and none of our AKI(+) patients had a solitary kidney. Although, our assessment of obstruction was based on radiologist's official read including hydronephrosis or hydroureter, rather than the formal Mag-3 Renal Scan. Nonetheless, this preliminary data does raise the question of whether additional factors are diving AKI. In addition, we demonstrated that a significantly higher number of patients with AKI presented with complaints of vomiting (58.3 vs. 36.3%, p < 0.002). This prevalence of vomiting in the AKI(+) group is likely an indirect marker for pre-renal azotemia, a well-known cause of kidney injury in children. However, we did not find a significant difference between the specific gravity of urine samples in our AKI(+) vs. AKI(–) patients. This could be due to variability when urine samples were obtained in relation to IVF therapy, as patients who were significantly dehydrated may not have been able to provide a urine sample until they have already received IV hydration. AKI(+) patients were also more likely to complain of fever at presentation (11.7 vs. 3.9%, p = 0.049); however, there was not a significantly higher rate of positive urine culture in the AKI group, and thus, pyelonephritis is unlikely to be driving AKI in our population. Recently published evidence of a pro-inflammatory urinary tract environment in non-UTI USD adolescent patients could potentially account for complaints of fever and serve as a risk factor for AKI in this population (35).
ED management between groups was similar and most often included IVF, pain, and nausea control. Although AKI(+) patients had a significant OR <1, suggesting less risk of receiving NSAID compared to AKI(–) patients, 51% of them did receive NSAIDs during their ED encounter. This may be due to the low recognition of AKI in these patients by medical providers. Only 37% of AKI(+) patients had documentation in their chart of abnormal Cr levels, and 22% of these were counted due to the inclusion of labs in the physician's note. However, it is unclear if the abnormal nature of the Cr was recognized in these patients. More than 60% of AKI(+) patients did not have any documentation in their charts, and it is likely that their kidney injury was not recognized by the treating physician. Finally, we noted that children with AKI were more likely to require admission to the hospital. This higher admission rate is likely due to the presenting symptoms associated with AKI, such as vomiting, and the fact that these patients represent a more severely ill section of the pediatric stone cohort.
Limitations of this study include that data collection was from a single tertiary care pediatric hospital system in the Midwest; therefore, the practice patterns of this single care network may limit generalizability. Due to the retrospective nature of the study, data collection required reliance on electronic medical records for AKI assessment. Furthermore, estimating glomerular filtration rate (eGFR) from serum Cr levels utilizing the bedside Schwartz equation can result in differences in GFR estimates that are due to variations other than changes in kidney function in non-CKD children and adolescents (36). In non-CKD cohorts, changes in Cr may be representative of differences in growth, protein intake, or muscle mass rather than changes GFR. Thus, the changes in eGFR found in our cohort may be attributed, at least in part, to variation in creatinine-based GFR estimation. Implicit in this study design is our inability to accurately describe the timing of blood draws in relation to IVF administration, which has made assigning clinical meaning to laboratory findings such as small (but statistically significant) differences in serum sodium difficult and may have also influenced other laboratory findings such as urine specific gravity. While there are administration times documented by nurses in the medication administration record, we were unable to confirm how accurate this documentation was in relation to actual patient care. We were not able to utilize AKI criteria based on urine output due to the short nature of emergency room visits and could potentially miss patients who may have met criteria in a hospital setting. In addition, 55% of visits were lacking data for accurate AKI analysis, and approximately half of these did not have a serum Cr measurement at that ED visit. This could represent a systematic selection bias toward finding AKI in sicker patients as those patients which appeared more ill would be more likely to undergo laboratory evaluation. However, multiple patients could not have their GFR assessed due to the lack of height measurements, a limitation less likely to be affected by presenting illness severity. Even for patients with data to assess for AKI, the height was often obtained from outpatient visits within the defined age-based time range, again highlighting the difficulty in accurate recognition of AKI in the ED. Further complicating patient assessment was that the Cr obtained at that ED visit often represented the only available measurement for a given patient and thus limited physician's ability to compare multiple Cr measurements. AKI was recognized in a portion of the study population due to a lower post-visit Cr found at outpatient follow-up.
In this study, we demonstrated a novel association between urinary stone-induced renal coli and AKI in a group of young adults and children. This is of particular concern given the increasing number of children and adolescents affected by urinary stone disease and the known association of urinary stones with the development of CKD in adults (17, 18). Previous reports of urinary stone-associated AKI have been linked to urinary tract obstruction; however, our data suggests that dehydration may play a significant role. In addition, the poor recognition of milder stages of AKI by medical professionals and the reliance on NSAIDs for pain control need to be addressed. The lack of agreement on the definition of pediatric AKI and debate over whether AKI is truly causal for CKD limit our ability to draw a strong conclusion from retrospective data. However, this study does provide evidence that AKI in urinary stone-forming children and young adults is an area in need of greater research and attention. Thus, the potential for future CKD in a pediatric population who may develop recurrent mild episodes of AKI is concerning and should be addressed to improve their long-term care.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Akron Children's Hospital IRB. Written informed consent from the participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
NF and MC: study design and data analysis and acquisition. RR: data analysis/interpretation and mentorship. AT and MB: data analysis/interpretation and statistical analysis. AS: research idea, study design, and statistical analysis. KK: research idea, study design, data analysis and acquisition, supervision/mentorship, took responsibility that this study has been reported honestly, accurately, transparently, and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work were appropriately investigated and resolved. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Scales CD, Tasian GE, Schwaderer AL, Goldfarb DS, Star RA, Kirkali Z. Urinary stone disease: advancing knowledge, patient care, and population health. Clin J Am Soc Nephrol. (2016) 11:1305–12. doi: 10.2215/CJN.13251215
2. Tasian GE, Ross ME, Song L, Sas DJ, Keren R, Denburg MR, et al. Annual incidence of nephrolithiasis among children and adults in South Carolina from 1997 to 2012. Clin J Am Soc Nephrol. (2016) 11:488–96. doi: 10.2215/CJN.07610715
3. Moe OW. Kidney stones: pathophysiology and medical management. Lancet. (2006) 367:333–44. doi: 10.1016/S0140-6736(06)68071-9
4. Noble RA, Lucas BJ, Selby NM. Long-term outcomes in patients with acute kidney injury. Clin J Am Soc Nephrol. (2020) 15:423–9. doi: 10.2215/CJN.10410919
5. Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. (2005) 16:3365–70. doi: 10.1681/ASN.2004090740
6. Silver SA, Long J, Zheng Y, Chertow GM. Cost of acute kidney injury in hospitalized patients. J Hosp Med. (2017) 12:70–6. doi: 10.12788/jhm.2683
7. See EJ, Jayasinghe K, Glassford N, Bailey M, Johnson DW, Polkinghorne KR, et al. Long-term risk of adverse outcomes after acute kidney injury: a systematic review and meta-analysis of cohort studies using consensus definitions of exposure. Kidney Int. (2019) 95:160–72. doi: 10.1016/j.kint.2018.08.036
8. Sutherland SM, Byrnes JJ, Kothari M, Longhurst CA, Dutta S, Garcia P, et al. AKI in hospitalized children: comparing the pRIFLE, AKIN, and KDIGO definitions. Clin J Am Soc Nephrol. (2015) 10:554–61. doi: 10.2215/CJN.01900214
9. Tang X, Lieske JC. Acute and chronic kidney injury in nephrolithiasis. Curr Opin Nephrol Hypertens. (2014) 23:385–90. doi: 10.1097/01.mnh.0000447017.28852.52
10. Organ M, Norman RW. Acute reversible kidney injury secondary to bilateral ureteric obstruction. Can Urol Assoc J. (2011) 5:392–6. doi: 10.5489/cuaj.11058
11. Wang SJ, Mu XN, Zhang LY, Liu QY, Jin XB. The incidence and clinical features of acute kidney injury secondary to ureteral calculi. Urol Res. (2012) 40:345–8. doi: 10.1007/s00240-011-0414-6
12. Jamal A, Ramzan A. Renal and post-renal causes of acute renal failure in children. J Coll Physicians Surg Pak. (2004) 14:411–5.
13. Collister D, Pannu N, Ye F, James M, Hemmelgarn B, Chui B, et al. Health care costs associated with AKI. Clin J Am Soc Nephrol. (2017) 12:1733–43. doi: 10.2215/CJN.00950117
14. Lassnigg A, Schmid ER, Hiesmayr M, Falk C, Druml W, Bauer P, et al. Impact of minimal increases in serum creatinine on outcome in patients after cardiothoracic surgery: do we have to revise current definitions of acute renal failure? Crit Care Med. (2008) 36:1129–37. doi: 10.1097/CCM.0b013e318169181a
15. Rule AD, Krambeck AE, Lieske JC. Chronic kidney disease in kidney stone formers. Clin J Am Soc Nephrol. (2011) 6:2069–75. doi: 10.2215/CJN.10651110
16. Lieske JC, de la Vega LS, Gettman MT, Slezak JM, Bergstralh EJ, Melton LJ, et al. Diabetes mellitus and the risk of urinary tract stones: a population-based case-control study. Am J Kidney Dis. (2006) 48:897–904. doi: 10.1053/j.ajkd.2006.09.002
17. Rule AD, Bergstralh EJ, Melton LJ, Li X, Weaver AL, Lieske JC. Kidney stones and the risk for chronic kidney disease. Clin J Am Soc Nephrol. (2009) 4:804–11. doi: 10.2215/CJN.05811108
18. Saucier NA, Sinha MK, Liang KV, Krambeck AE, Weaver AL, Bergstralh EJ, et al. Risk factors for CKD in persons with kidney stones: a case-control study in Olmsted County, Minnesota. Am J Kidney Dis. (2010) 55:61–8. doi: 10.1053/j.ajkd.2009.08.008
19. Pietrow PK, Pope JC, Adams MC, Shyr Y, Brock JW. Clinical outcome of pediatric stone disease. J Urol. (2002) 167:670–3. doi: 10.1097/00005392-200202000-00060
20. Schwartz GJ, Haycock GB, Edelmann CM, Spitzer A. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics. (1976) 58:259–63.
21. Oliveros JC. An Interactive Tool for Comparing Lists With Venn's Diagrams. (2007). Available online at: https://bioinfogp.cnb.csic.es/tools/venny/index.html
22. Symons JM, Chua AN, Somers MJ, Baum MA, Bunchman TE, Benfield MR, et al. Demographic characteristics of pediatric continuous renal replacement therapy: a report of the prospective pediatric continuous renal replacement therapy registry. Clin J Am Soc Nephrol. (2007) 2:732–8. doi: 10.2215/CJN.03200906
23. Schneider J, Khemani R, Grushkin C, Bart R. Serum creatinine as stratified in the RIFLE score for acute kidney injury is associated with mortality and length of stay for children in the pediatric intensive care unit. Crit Care Med. (2010) 38:933–9. doi: 10.1097/CCM.0b013e3181cd12e1
24. Bailey D, Phan V, Litalien C, Ducruet T, Mérouani A, Lacroix J, et al. Risk factors of acute renal failure in critically ill children: a prospective descriptive epidemiological study. Pediatr Crit Care Med. (2007) 8:29–35. doi: 10.1097/01.pcc.0000256612.40265.67
25. Sutherland SM, Ji J, Sheikhi FH, Widen E, Tian L, Alexander SR, et al. AKI in hospitalized children: epidemiology and clinical associations in a national cohort. Clin J Am Soc Nephrol. (2013) 8:1661–9. doi: 10.2215/CJN.00270113
26. Selewski DT, Cornell TT, Heung M, Troost JP, Ehrmann BJ, Lombel RM, et al. Validation of the KDIGO acute kidney injury criteria in a pediatric critical care population. Intensive Care Med. (2014) 40:1481–8. doi: 10.1007/s00134-014-3391-8
27. Cleto-Yamane TL, Gomes CLR, Suassuna JHR, Nogueira PK. Acute kidney injury epidemiology in pediatrics. J Bras Nefrol. (2018) 41:275–83. doi: 10.1590/2175-8239-jbn-2018-0127
28. Li Z, Kang Z, Duan C, Wu T, Zhang L, Xun M, et al. Clinical and pathological features of acute kidney injury in children. Renal Failure. (2014) 36:1023–8. doi: 10.3109/0886022X.2014.917452
29. Zappitelli M. Epidemiology and diagnosis of acute kidney injury. Semin Nephrol. (2008) 28:436–46. doi: 10.1016/j.semnephrol.2008.05.003
30. Baddam S, Aban I, Hilliard L, Howard T, Askenazi D, Lebensburger JD. Acute kidney injury during a pediatric sickle cell vaso-occlusive pain crisis. Pediatr Nephrol. (2017) 32:1451–6. doi: 10.1007/s00467-017-3623-6
31. Chawla LS, Kimmel PL. Acute kidney injury and chronic kidney disease: an integrated clinical syndrome. Kidney Int. (2012) 82:516–24. doi: 10.1038/ki.2012.208
32. Goldstein SL. Pediatric acute kidney injury: it's time for real progress. Pediatr Nephrol. (2006) 21:891–5. doi: 10.1007/s00467-006-0173-8
33. Belayev LY, Palevsky PM. The link between acute kidney injury and chronic kidney disease. Curr Opin Nephrol Hypertens. (2014) 23:149–54. doi: 10.1097/01.mnh.0000441051.36783.f3
34. Stack AG, Li X, Kaballo M, Elsayed ME, Johnson H, Murray PT, et al. Temporal trends in acute kidney injury across health care settings in the Irish health system: a cohort study. Nephrol Dial Transplant. (2018) 35:447–57. doi: 10.1093/ndt/gfy226
35. Kusumi K, Ketz J, Saxena V, Spencer JD, Safadi F, Schwaderer A. Adolescents with urinary stones have elevated urine levels of inflammatory mediators. Urolithiasis. (2019) 47:461–6. doi: 10.1007/s00240-019-01133-1
Keywords: urolithiasis, kidney stones, AKI, pediatric, urinary stone disease (USD)
Citation: Farris N, Raina R, Tibrewal A, Brown M, Colvis M, Schwaderer A and Kusumi K (2020) Acute Kidney Injury Associated With Urinary Stone Disease in Children and Young Adults Presenting to a Pediatric Emergency Department. Front. Pediatr. 8:591520. doi: 10.3389/fped.2020.591520
Received: 04 August 2020; Accepted: 26 October 2020;
Published: 30 November 2020.
Edited by:
Robert P. Woroniecki, Stony Brook Children's Hospital, United StatesReviewed by:
Lyndsay Harshman, The University of Iowa, United StatesCopyright © 2020 Farris, Raina, Tibrewal, Brown, Colvis, Schwaderer and Kusumi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kirsten Kusumi, a2t1c3VtaUBha3JvbmNoaWxkcmVucy5vcmc=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.