
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Pediatr., 12 January 2021
Sec. Pediatric Immunology
Volume 8 - 2020 | https://doi.org/10.3389/fped.2020.577413
This article is part of the Research TopicDietary Interventions and Nutritional Factors in the Prevention of Allergic Diseases in InfantsView all 16 articles
Atopic dermatitis is one of the most frequent chronic skin diseases worldwide and often develops within the first few years of life. Recent advancements in our knowledge of its pathophysiology have brought to light the role of genetic predisposition and environmental triggers. With the increasing prevalence of allergic diseases, there is a strong need for a better understanding of the various modifiable eliciting factors of such conditions. The concomitant rise in food allergy and insights into the skin barrier function has highlighted the role of nutrition and diet in the prevention and modification of allergic disorders. Furthermore, the identification of the skin as an important route of sensitization, and the risk of progression to asthma later in life, stress the significance of optimizing our management of skin inflammation in the prevention of allergies. Many nutritional factors, including the type of maternal diet during pregnancy, the duration of breastfeeding, the epicutaneous exposure of allergenic food proteins in the first few years of life, the timing of the introduction of complementary foods, the supplementation of vitamins and probiotics/prebiotics during prenatal and early life, have been assessed as potential targets for the prevention of atopy and eczema. Here, we review the latest data addressing prenatal and perinatal nutritional and dietary interventions in the primary prevention of atopic dermatitis. Also, we define knowledge gaps and targets for future research in the prevention of atopic dermatitis.
Atopic dermatitis (AD) is a common inflammatory skin disease, which affects as many as one-fifth of all individuals (1) and is associated with a high financial and psychosocial burden for patients and their families (2, 3). The prevalence differs greatly in many parts of the world but has been found to have increased significantly in industrialized and developing countries in the last few decades (1, 4, 5). Changes in the exposome, due to urban expansion and socioeconomic growth, have led to greater energy consumption and waste production. The industrial revolution in the nineteenth century has led to increased exposure to air pollutants and chemical hazards, which has had an impact on the integrity of the physical epidermal barrier (6).
Recent findings in the pathogenesis of AD have revealed a complex interplay between impairment of the skin barrier function, environmental and nutritional factors, and immune dysregulation (6–9), which begins in early life. Some evidence suggests that AD is primarily a skin barrier defect (10, 11), which influences the development of sensitization and atopy (9, 12), and early AD may be a causative factor in developing food allergy (13). Indeed, the condition is often regarded as the first step of the “allergic march,” which leads to a progressive course of atopic illness, including food allergy, asthma, and allergic rhinitis.
As a result of the recent rise in allergic diseases worldwide, there has been a growing interest in the exploration of risk factors involved in the development of AD and epidermal barrier dysfunction (14, 15). Recent research has focused on the role of dietary and nutritional intervention in early life for the prevention of allergic diseases, as these factors are modifiable and can influence the immune system maturation in a crucial phase of its development (16).
In this review, we focus on currently available evidence on the nutritional and dietary factors that could be involved in the occurrence of AD and therefore could be targeted for the prevention of this disease (Figure 1).
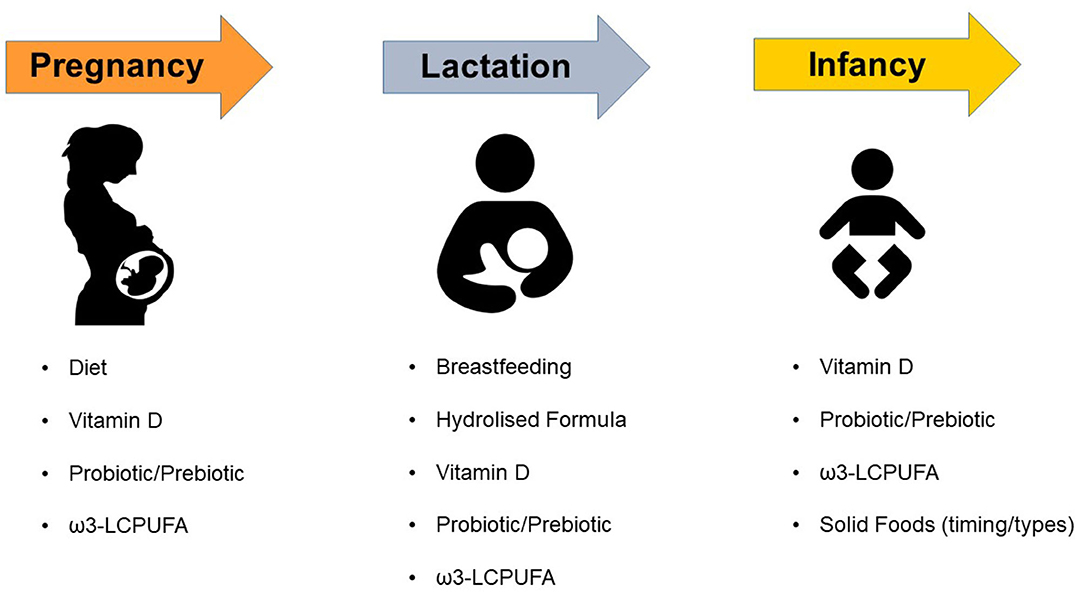
Figure 1. Dietary and Nutritional factors that may affect the risk of atopic dermatitis in children. ω3-LCPUFA, omega-3 long-chain polyunsaturated fatty acids.
Maternal prenatal nutrition and dietary diversity are crucial factors in a child's development. Some of the known health risks associated with intake at this time include obesity, hypertension, and diabetes (17). Several studies have assessed the role of the first 1,000 days after conception and their impact on the pathogenesis of allergic diseases (18). Current literature supports the hypothesis that the process of colonization by a healthy microbiome in the gut, airways, and skin in early life, can affect immune development and maturation, and the susceptibility to immune-mediated disorders later in life, including allergies (19, 20). The prenatal and early infancy period is a critical period for the type of microbiome colonization as well as for the maturation of immune responses, and exposure at this stage can promote immune tolerance (21, 22). The evidence for the prenatal maternal consumption of allergenic foods and their impact on allergic diseases is conflicting, and different for various foods. In addition, there have been many studies assessing the effect of prenatal nutritional exposures on early-life wheezing and asthma, and a paucity of data on AD. An earlier 2007 UK birth cohort found a beneficial effect on maternal oily fish consumption, with eczema at 5 years, but no association was found with other allergenic food groups investigated (23). This was consistent with a previous study by Dunstan et al. (24), which examined the effect of fish oil supplementation during pregnancy on early developing immune responses and outcomes in infants with atopic predisposition. A 2019 review of four longitudinal birth cohort studies found no significant effect of diverse Mediterranean diet patents in pregnancy on atopic outcomes in the offspring (25). Guidelines from Australasia, Germany, and the UK recommend eating fatty fish regularly during pregnancy (26). In 2015 Beckhaus et al. (27) showed that maternal consumption of various supplements (including vitamins C, D, E) had a protective effect on early life wheezing, but this did not extend to other atopic conditions. A recent cohort study found that the higher maternal intake of meat is associated with an increased risk of allergic rhinitis, wheezing, and AD in children (28). Overall, there is conflicting evidence on the effect of prenatal maternal consumption of certain food on the risk of allergy outcomes in childhood (29). Further studies are needed to assess the relationship between maternal dietary intake during pregnancy and long-term allergy outcomes in offspring. Furthermore, dietary diversity needs to be clearly defined to harmonize research into the effect of specific foods, considering geographic and cultural differences.
The worldwide rise in allergic diseases has paralleled a vitamin D (VD) deficiency epidemic in Westernized countries (30), which supports the hypothesis that VD might influence the development of allergies (30, 31). VD levels are mainly influenced by sun exposure but also by diet, which makes it an important modifiable factor in allergy prevention. It has been suggested that children born to mothers with low VD intake during pregnancy have an increased prevalence of AD (32). Cross-sectional studies also showed that children born during autumn and winter have a higher prevalence of AD compared with children born in spring and summer (33). In 2015 Beckhaus et al. (27) found that maternal intake of VD, vitamin E, and zinc during pregnancy was associated with a reduced risk of early life wheezing illnesses, but not of childhood-onset asthma or other atopic conditions in offspring. A recent meta-analysis from four prospective cohort studies showed that lower maternal VD serum level during pregnancy was associated with an increased risk of AD in offspring (34). Another recent meta-analysis of observational studies found no significant association between prenatal VD status (i.e., circulating 25-hydroxyvitamin D levels in maternal blood during pregnancy or cord blood at birth) and the risk of AD in offspring from age 1 to 9 years (35). However, a correlation between prenatal VD levels and the risk of AD was found at higher latitude, highlighting the effect of regional and geographic changes (35). More research is needed to analyze the influence of VD maternal status on the occurrence of AD in childhood.
The is conflicting evidence on the relationship between breastfeeding and allergy risk, with some studies reporting protective effect against AD development, while others showing no effect or even an increased risk for AD occurrence (36). Still, international scientific societies recommend exclusive breastfeeding for at least 4–6 months for primary prevention of allergic disease (37, 38). Breastmilk supports diverse microbial colonization and drives the immune system maturation of the newborn (39–41). Breastfeeding has been associated with decreased morbidity and mortality in infants and lower incidence of allergic diseases (42). A 2005 birth cohort study enrolling 4,089 children showed that exclusive breastfeeding for ≥4 months reduced the risk for developing AD at 4 years of age, irrespective of the concomitant presence of either family history of atopy, allergic sensitization, or asthma (43). A systematic review of 18 prospective studies demonstrated that exclusive breastfeeding for the first 3 months after birth is associated with a lower incidence of AD in childhood, even in the presence of a family history of atopy (44). In contrast, a subsequent systematic review of prospective cohort studies comparing breastfeeding with conventional infant formula feeding or partial breast-feeding in developed countries, revealed that exclusive breast-feeding for at least 3 months was not significantly protective against the development of AD (45). Finally, a recent meta-analysis found that exclusive breastfeeding for 3–4 months was associated with a reduced risk of early life AD (<2 years of age), but the quality of evidence was low (46). In summary, the effect of breastfeeding on the risk of AD remains controversial (36), possibly due to different study populations and designs, and requires more randomized controlled trials (RCTs).
Elemental cow's milk formula and hydrolyzed cow's milk or soy formulas are often prescribed to infants with the intention to prevent allergic diseases. However, their role in allergy risk reduction is still unclear (47). Partially (pHF) and extensively (eHF) hydrolyzed protein formulas have been widely investigated for their role in allergy and AD prevention. Two earlier RCTs reported no significant difference between pHF and eHF for the prevention of allergic diseases and AD in children (48, 49). This finding was in contrast to an earlier study by Oldaeus et al. (50), who found a lower incidence of AD in at-risk infants fed with a casein-based eHF, compared with those receiving a whey-based pHF or standard cow's milk formula (CMF). The GINI study, a prospective, randomized, double-blind trial, conducted among at-risk children, found a lower risk of AD at 3 and 6 years of life among those children who received a whey-based pHF or a casein-based eHF in their first 4 months, compared to those receiving CMF (51). Interestingly, this finding was exclusive to eczema as hydrolysate nutrition did not have a preventive effect on asthma or childhood wheezing (51). In the nationwide ELFE birth cohort study on infant feeding (comparing breast milk only, pHF with hypoallergenic label [pHF-HA] or without a hypoallergenic label [pHF-non-HA], and CMF), pHF-HA use was not associated with a lower risk of AD (52). The difference in these outcomes could be explained by the fact that the GINI trial was based only on whey-based pHF, whereas the ELFE cohort considered all types on pHF-HA formulas (51, 52). Finally, a recent Cochrane review found that nutrition with hydrolyzed formula, particularly pHF compared to CMF, in the early days of infancy, does not prevent atopic diseases among non-exclusively breastfed infants (47).
VD is a pleiotropic hormone and its insufficiency represents a growing global health concern. The VD receptor has been found in numerous immune and non-immune cells, including keratinocytes, and current evidence demonstrates that it modulates the expression of more than 200 genes (53–55). In recent years, the relationship between VD serum levels and the prevalence and severity of AD has been widely studied. Peroni et al. (56) showed that the serum levels of the circulating form of VD, the 25-hydroxyvitamin D, were inversely related to AD severity, although this finding was not confirmed in other studies (57, 58). In a Norwegian study, Byremo et al. (59) randomly selected 30 children from 4 to 13 years of age with severe AD to settle in a tropical zone for 4 weeks, and 26 children with AD to remain in Norway. A significant improvement was observed in disease activity as well as in the quality of life in the group who moved in a tropical zone after 4 weeks and 3 months (59). In a double-blind RCT, in which 60 AD patients aged ≥14 years were randomized to receive either 1,600 IU/day of VD or placebo, authors showed a significant improvement in the active group after 60 days, regardless of the initial severity of AD, which suggests that VD supplementation may improve AD (53). In contrast, Back et al. (60) showed that greater intake of VD during childhood correlated with an increased risk of AD at 6 years of age. VD supplementation in infancy has also been associated with a reduced risk of sensitization to house dust mites at age 18 months, which is an important trigger for the occurrence and severity of AD (61). Even though there are promising results regarding the role and therapeutic use of VD in AD, currently available data are conflicting. RCTs are needed to establish the optimal dose, desired levels, duration of treatment, and effect of VD supplementation in both prevention and treatment of AD.
It has been hypothesized that an imbalance in the intestinal microbiota composition and metabolic function due to dietary and lifestyle changes may be involved in the pathogenesis of atopic disease (22). The activation of the IL-4/IL-13 axis in AD promotes the skin barrier breakdown and is associated with changes in the gut microbiota (62). Several studies examining the role of oral administration of prebiotics and probiotics in atopy have shown that alterations in gut microbiota composition can precede the occurrence of AD (22, 62). In an earlier case-control study, individuals with AD had lower intestinal concentrations of Bifidobacterium compared to healthy controls (63). The Bifidobacterium levels were also inversely correlated with AD disease severity, suggesting that intestinal flora might play a role in AD onset and severity (63). The KOALA birth cohort revealed that the presence of Clostridium difficile was associated with a higher risk of developing AD and other allergic diseases, while a stronger association was found with Escherichia coli, which conferred an exponential risk to AD only (22). Prenatal and postnatal treatment with Lactobacillus and Bifidobacterium strains have been shown to reduce the risk of AD in infants (62). In a recent double-blind RCT, that included 50 children (aged 4–17 years), Navarro-López et al. (64) reported that a mixture of Bifidobacterium strains was effective in reducing AD severity as measured by the Scoring AD (SCORAD) index. A meta-analysis by Huang et al. (65) confirmed this result with improved SCORAD scores in 568 children treated with different strains. In a 2 year follow-up RCT including 132 at atopy risk infants, authors found that the cumulative incidence for AD was lower in the group fed with a formula that contained a mixture of prebiotic oligosaccharides (13.6%) compared to the placebo group (27.9%) (66). A recent Cochrane review, which evaluated the effect of oral prebiotics for the prevention of allergy in infants, reported a significant reduction in AD (67). In summary, supplementation with specific probiotic strains may modulate gut bacteria, which may influence skin inflammation, protect some children against AD development, and be considered a useful therapy in the future (68). However, the strain-specific effects of probiotics make it difficult to make recommendations (Table 1). Future studies comparing strains and adopting a common method of outcome measurement (such as SCORAD) will greatly improve our data and clinical recommendations.
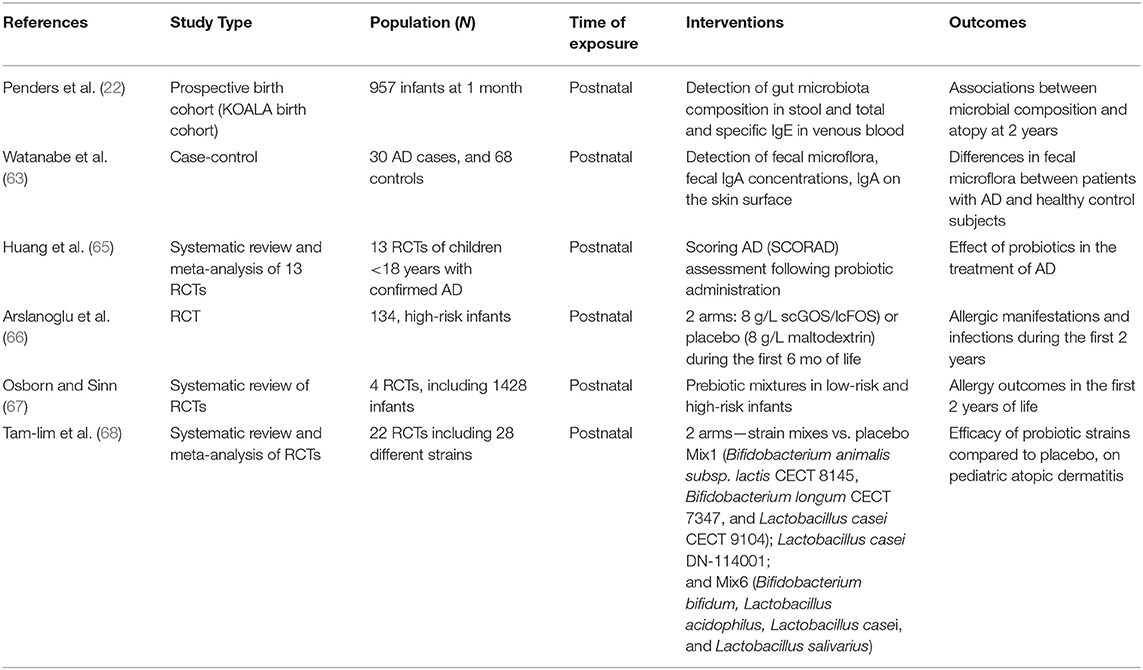
Table 1. Randomized and non-randomized studies on the use of pre and probiotics for the prevention of atopic dermatitis in children.
Several birth-cohort studies have reported that increased omega-3 long-chain polyunsaturated fatty acids (LCPUFA) intake during pregnancy may reduce the risk of AD, asthma, and sensitization to house dust mite (69). The supplementation of LCPUFA, through the administration of fish oil during pregnancy and early life, has been proposed for the prevention of allergic sensitization and atopic diseases (70, 71). LCPUFA influence cell membrane structure and function, and potentially modulate inflammatory responses by increasing cell membrane docosahexaenoic acid (DHA; 22:6 ω-3) and eicosapentaenoic acid (EPA; 20:5 ω-3), thus competing with the synthesis of inflammatory arachidonic acid (AA, 20:4, ω-6). This results in a reduction in prostaglandin E synthesis and inhibition of cytokine and immunoglobulin E (IgE) production (72). While some studies showed that maternal fish oil supplementation during pregnancy is associated with a lower incidence of AD in offspring (73, 74), other authors reported no differences in the incidence of allergic diseases (75, 76). Best et al. (77) recently published the long-term follow-up of the DOMInO trial (78), where pregnant women were randomized to receive either fish oil capsules (900 mg of ω-3 LCPUFA) or vegetable oil capsules without ω-3 LCPUFA (control group) daily from the 2nd trimester of gestation until birth. The longitudinal analysis of 706 at-risk offspring from the DOMIno trial showed no significant difference in the progression of allergic diseases between the active and control groups assessed at 1, 3, and 6 years (77, 78). Conversely, a different RCT reported protective effects of prenatal supplementation with ω-3 LCPUFA on the risk of IgE-mediated AD at 1 year, and on follow up at 2 years (73, 74). Finally, a 2015 Cochrane review found that ω-3 LCPUFA supplementation in pregnant or breastfeeding mothers was associated with a reduction in AD in high-atopy risk children aged 12 to 36 months (but not at any other time point) but concluded that there was “limited evidence” to support supplementation with LCPUFA during pregnancy and/or lactation for the prevention of allergic diseases in children (79). In summary, despite the presence of RCTs suggesting protective effects, the data are still inconsistent, and long term follow-ups are crucial to determine whether prenatal and early postnatal ω-3 LCPUFA supplementation may be of benefit as a primary prevention strategy for AD.
Contrary to previous belief, the delayed introduction of solids in an infant's diet does not reduce the risk of allergic sensitization and atopic diseases (80–83). In a 2011 birth cohort of more than 18,000 newborns and 1,000 AD cases, Chuang et al. (84) found no evidence of a protective effect of delayed introduction of solid foods to infant's diet on the risk of AD at 18 months of age, while a longer duration of breastfeeding increased this risk. A recent case-control study conducted by the HYGIENE Study Group demonstrated that early introduction of solids was inversely related to the risk of AD. Children weaned at 4 months had a lower AD risk (OR = 0.41, 95% CI, 0.20–0.87) compared to those exclusively breastfed, and weaning started at 5 months of age revealed similar results (OR = 0.39, 95% CI, 0.18–0.83) (85, 86). Moreover, findings from the ISAAC Phase II Study found no evidence that prolonged exclusive breastfeeding protected against eczema (87).
The early introduction of fish has been associated with a reduced risk of allergic sensitization in some reports (88, 89), probably due to its high content of LCPUFA (70–72). However, not all the studies confirmed this protective role of fish introduction on the development of allergic diseases (89, 90). Despite the discrepancies in findings, observational studies find that delaying the introduction of solids increases the risk of AD. The difficulty in accepting early weaning to prevent AD is strongly linked with the emphasis given to the nutritional and health benefits of exclusive breastfeeding. Whilst more robust evidence is being sought to specify food types, quantities, and timing, recommendations should be aimed at gradually integrating a more diversified diet from 4 months of age, in addition to breastfeeding.
Globally, robust recommendations on dietary intake during pregnancy for the prevention of allergic diseases are sparse. Some guidelines recommend eating fatty fish or taking LCPUFA supplements during pregnancy to reduce AD in the offspring. From our review of common dietary interventional strategies, there is conflicting evidence to support such recommendations. A most recent systematic review of 17 RCTs and 78 observational studies found no consistent evidence of a clear benefit of nutritional factors in the alteration of the risk of AD in children (91). Long-term follow-up studies are essential to determine the true benefit of prenatal and early life dietary and nutritional interventions as a primary prevention strategy for AD.
TT and PC made substantial contributions to the conception, design, and acquisition of data. TT and PC drafted the initial manuscript. ED'A, DP, and GZ critically reviewed the manuscript for important intellectual content. All authors approved the final version of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
AD, Atopic dermatitis; eHF, Extensively hydrolyzed milk formulas; LCPUFA, Long-chain polyunsaturated fatty acids; pHF, Partially hydrolyzed milk formulas; RCT, Randomized controlled trial; SCORAD, Scoring Atopic Dermatitis; CMF, Standard cow's milk formula; VD, Vitamin D.
1. Asher MI, Montefort S, Björkstén B, Lai CK, Strachan DP, Weiland SK, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC phases one and three repeat multicountry cross-sectional surveys. Lancet. (2006) 368:733–43. doi: 10.1016/S0140-6736(06)69283-0
2. McKenna SP, Doward LC. Quality of life of children with atopic dermatitis and their families. Curr Opin Allergy Clin Immunol. (2008) 8:228–31. doi: 10.1097/ACI.0b013e3282ffd6cc
3. Kapur S, Watson W, Carr S. Atopic dermatitis. Allergy Asthma Clin Immunol. (2018) 14(Suppl. 2):52. doi: 10.1186/s13223-018-0281-6
4. Akinbami LJ, Simon AE, Rossen LM. Changing trends in asthma prevalence among children. Pediatrics. (2016) 137:e20152354. doi: 10.1542/peds.2015-2354
5. Dharmage SC, Perret JL, Custovic A. Epidemiology of asthma in children and adults. Front Pediatr. (2019) 7:246. doi: 10.3389/fped.2019.00246
6. Celebi Sözener Z, Cevhertas L, Nadeau K, Akdis M, Akdis CA. Environmental factors in epithelial barrier dysfunction. J Allergy Clin Immunol. (2020) 145:1517–28. doi: 10.1016/j.jaci.2020.04.024
7. Egawa G, Kabashima K. Multifactorial skin barrier deficiency and atopic dermatitis: essential topics to prevent the atopic march. J Allergy Clin Immunol. (2016) 138:350–8. doi: 10.1016/j.jaci.2016.06.002
8. Loxham M, Davies DE. Phenotypic and genetic aspects of epithelial barrier function in asthmatic patients. J Allergy Clin Immunol. (2017) 139:1736–51. doi: 10.1016/j.jaci.2017.04.005
9. Moens E, Veldhoen M. Epithelial barrier biology: good fences make good neighbours. Immunology. (2012) 135:1–8. doi: 10.1111/j.1365-2567.2011.03506.x
10. Egawa G, Kabashima K. Barrier dysfunction in the skin allergy. Allergol Int. (2018) 67:3–11. doi: 10.1016/j.alit.2017.10.002
11. Menon GK, Lee SE, Lee SH. An overview of epidermal lamellar bodies: novel roles in biological adaptations and secondary barriers. J Dermatol Sci. (2018) 92:10–7. doi: 10.1016/j.jdermsci.2018.03.005
12. Nomura T, Kabashima K. Advances in atopic dermatitis in 2015. J Allergy Clin Immunol. (2016) 138:1548–55. doi: 10.1016/j.jaci.2016.10.004
13. Tsakok T, Marrs T, Mohsin M, Baron S, du Toit G, Till S, et al. Does atopic dermatitis cause food allergy? A systematic review. J Allergy Clin Immunol. (2016) 137:1071–8. doi: 10.1016/j.jaci.2015.10.049
14. Polk BI, Bacharier LB. Potential strategies and targets for the prevention of pediatric asthma. Immunol Allergy Clin North Am. (2019) 39:151–62. doi: 10.1016/j.iac.2018.12.010
15. Peroni DG, Bonomo B, Casarotto S, Boner AL, Piacentini GL. How changes in nutrition have influenced the development of allergic diseases in childhood. Ital J Pediatr. (2012) 38:22. doi: 10.1186/1824-7288-38-22
16. Holt PG, Jones CA. The development of the immune system during pregnancy and early life. Allergy. (2000) 55:688–97. doi: 10.1034/j.1398-9995.2000.00118.x
17. Schwarzenberg SJ, Georgieff MK, Committee on Nutrition. Advocacy for improving nutrition in the first 1000 days to support childhood development and adult health. Pediatrics. (2018) 141:e20173716. doi: 10.1542/peds.2017-3716
18. Prescott SL. Early origins of allergic disease: a review of processes and influences during early immune development. Curr Opin Allergy Clin Immunol. (2003) 3:125–32. doi: 10.1097/00130832-200304000-00006
19. Peroni DG, Nuzzi G, Trambusti I, Di Cicco ME, Comberiati P. Microbiome composition and its impact on the development of allergic diseases. Front Immunol. (2020) 11:700. doi: 10.3389/fimmu.2020.00700
20. Marsland BJ, Trompette A, Gollwitzer ES. The gut-lung axis in respiratory disease. Ann Am Thorac Soc. (2015) 12(Suppl. 2):S150–6. doi: 10.1513/AnnalsATS.201503-133AW
21. Lunjani N, Satitsuksanoa P, Lukasik Z, Sokolowska M, Eiwegger T. Recent developments and highlights in mechanisms of allergic diseases: microbiome. Allergy. (2018) 73:2314–27. doi: 10.1111/all.13634
22. Penders J, Thijs C, van den Brandt PA, Kummeling I, Snijders B, Stelma F, et al. Gut microbiota composition and development of atopic manifestations in infancy: the KOALA birth cohort study. Gut. (2007) 56:661–7. doi: 10.1136/gut.2006.100164
23. Willers SM, Devereux G, Craig LC, McNeill G, Wijga AH, Abou El-Magd W, et al. Maternal food consumption during pregnancy and asthma, respiratory and atopic symptoms in 5-year-old children. Thorax. (2007) 62:773–9. doi: 10.1136/thx.2006.074187
24. Dunstan JA, Mori TA, Barden A, Beilin LJ, Taylor AL, Holt PG, et al. Fish oil supplementation in pregnancy modifies neonatal allergen-specific immune responses and clinical outcomes in infants at high risk of atopy: a randomized, controlled trial. J Allergy Clin Immunol. (2003) 112:1178–84. doi: 10.1016/j.jaci.2003.09.009
25. Venter C, Greenhawt M, Meyer RW, Agostoni C, Reese I, du Toit G, et al. EAACI position paper on diet diversity in pregnancy, infancy and childhood: novel concepts and implications for studies in allergy and asthma. Allergy. (2019) 75:497–523. doi: 10.1111/all.14051
26. Schäfer T, Bauer CP, Beyer K, Bufe A, Friedrichs F, Gieler U, et al. S3-Guideline on allergy prevention: 2014 update: guideline of the German Society for Allergology and Clinical Immunology (DGAKI) and the German Society for Pediatric and Adolescent Medicine (DGKJ). Allergo J Int. (2014) 23:186–99. doi: 10.1007/s40629-014-0022-4
27. Beckhaus AA, Garcia-Marcos L, Forno E, Pacheco-Gonzalez RM, Celedon JC, Castro-Rodriguez JA. Maternal nutrition during pregnancy and risk of asthma, wheeze, and atopic diseases during childhood: a systematic review and meta-analysis. Allergy. (2015) 70:1588–604. doi: 10.1111/all.12729
28. Baïz N, Just J, Chastang J, Forhan A, de Lauzon-Guillain B, Magnier AM, et al. Maternal diet before and during pregnancy and risk of asthma and allergic rhinitis in children. Allergy Asthma Clin Immunol. (2019) 15:40. doi: 10.1186/s13223-019-0353-2
29. Netting MJ, Middleton PF, Makrides M. Does maternal diet during pregnancy and lactation affect outcomes in offspring? A systematic review of food-based approaches. Nutrition. (2014) 30:1225–41. doi: 10.1016/j.nut.2014.02.015
30. Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med. (1995) 332:133–8. doi: 10.1056/NEJM199501193320301
31. Devereux G, Litonjua AA, Turner SW, Craig LC, McNeill G, Martindale S, et al. Maternal vitamin D intake during pregnancy and early childhood wheezing. Am J Clin Nutr. (2007) 85:853–9. doi: 10.1093/ajcn/85.3.853
32. Miyake Y, Sasaki S, Tanaka K, Hirota Y. Dairy food, calcium and vitamin D intake in pregnancy, and wheeze and eczema in infants. Eur Respir J. (2010) 35:1228–34. doi: 10.1183/09031936.00100609
33. Kuzume K, Kusu M. Before-birth climatologic data may play a role in the development of allergies in infants. Pediatr Allergy Immunol. (2007) 18:281–7. doi: 10.1111/j.1399-3038.2006.00526.x
34. Wei Z, Zhang J, Yu X. Maternal vitamin D status and childhood asthma, wheeze, and eczema: a systematic review and meta-analysis. Pediatr Allergy Immunol. (2016) 27:612–9. doi: 10.1111/pai.12593
35. Pacheco-Gonzalez RM, Garcia-Marcos L, Morales E. Prenatal vitamin D status and respiratory and allergic outcomes in childhood: a meta-analysis of observational studies. Pediatr Allergy Immunol. (2018) 29:243–53. doi: 10.1111/pai.12876
36. Kim JH. Role of breast-feeding in the development of atopic dermatitis in early childhood. Allergy Asthma Immunol Res. (2017) 9:285–7. doi: 10.4168/aair.2017.9.4.285
37. Muraro A, Halken S, Arshad SH, Beyer K, Dubois AE, Du Toit G, et al. EAACI food allergy and anaphylaxis guidelines. Primary prevention of food allergy. Allergy. (2014) 69:590–601. doi: 10.1111/all.12398
38. Fleischer DM, Spergel JM, Assa'ad AH, Pongracic JA. Primary prevention of allergic disease through nutritional interventions. J Allergy Clin Immunol Pract. (2013) 1:29–36. doi: 10.1016/j.jaip.2012.09.003
39. Neville MC, Anderson SM, McManaman JL, Badger TM, Bunik M, Contractor N, et al. Lactation and neonatal nutrition: defining and refining the critical questions. J Mamm Gland Biol Neoplasia. (2012) 17:167–88. doi: 10.1007/s10911-012-9261-5
40. Munblit D, Treneva M, Peroni DG, Colicino S, Chow LY, Dissanayeke S, et al. Immune components in human milk are associated with early infant immunological health outcomes: a prospective three-country analysis. Nutrients. (2017) 9:532. doi: 10.3390/nu9060532
41. Munblit D, Treneva M, Peroni DG, Colicino S, Chow L, Dissanayeke S, et al. Colostrum and mature human milk of women from London, Moscow, and Verona: determinants of immune composition. Nutrients. (2016) 8:695. doi: 10.3390/nu8110695
42. Minniti F, Comberiati P, Munblit D, Piacentini GL, Antoniazzi E, Zanoni L, et al. Breast-milk characteristics protecting against allergy. Endocr Metab Immune Disord Drug Targets. (2014) 14:9–15. doi: 10.2174/1871530314666140121145045
43. Kull I, Böhme M, Wahlgren CF, Nordvall L, Pershagen G, Wickman M. Breast-feeding reduces the risk for childhood eczema. J Allergy Clin Immunol. (2005) 116:657–61. doi: 10.1016/j.jaci.2005.04.028
44. Gdalevich M, Mimouni D, David M, Mimouni M. Breast-feeding and the onset of atopic dermatitis in childhood: a systematic review and meta-analysis of prospective studies. J Am Acad Dermatol. (2001) 45:520–27. doi: 10.1067/mjd.2001.114741
45. Yang YW, Tsai CL, Lu CY. Exclusive breastfeeding and incident atopic dermatitis in childhood: a systematic review and meta-analysis of prospective cohort studies. Br J Dermatol. (2009) 161:373–83. doi: 10.1111/j.1365-2133.2009.09049.x
46. Lodge CJ, Tan DJ, Lau MX, Dai X, Tham R, Lowe AJ, et al. Breastfeeding and asthma and allergies: a systematic review and meta-analysis. Acta Paediatr. (2015) 104:38–53. doi: 10.1111/apa.13132
47. Osborn DA, Sinn JK, Jones LJ. Infant formulas containing hydrolysed protein for prevention of allergic disease. Cochrane Database Syst Rev. (2018) 10:CD003664. doi: 10.1002/14651858.CD003664.pub6
48. Halken S, Hansen KS, Jacobsen HP, Estmann A, Faelling AE, Hansen LG, et al. Comparison of a partially hydrolyzed infant formula with two extensively hydrolyzed formulas for allergy prevention: a prospective, randomized study. Pediatr Allergy Immunol. (2000) 11:149–61. doi: 10.1034/j.1399-3038.2000.00081.x
49. Nentwich I, Michkova E, Nevoral J, Urbanek R, Szepfalusi Z. Cow's milk-specific cellular and humoral immune responses and atopy skin symptoms in infants from atopic families fed a partially (pHF) or extensively (eHF) hydrolyzed infant formula. Allergy. (2001) 56:1144–56. doi: 10.1111/j.1398-9995.2001x.00926.x
50. Oldaeus G, Anjou K, Björkstén B, Moran JR, Kjellman NI. Extensively and partially hydrolyzed infant formulas for allergy prophylaxis. Arch Dis Child. (1997) 77:4–10. doi: 10.1136/adc.77.1.4
51. von Berg A, Filipiak-Pittroff B, Krämer U, Link E, Heinrich J, Koletzko S, et al. The German infant nutritional intervention study (GINI) for the preventive effect of hydrolyzed infant formulas in infants at high risk for allergic diseases. Design and selected results. Allergol Select. (2017) 1:28–38. doi: 10.5414/ALX01462E
52. Davisse-Paturet C, Raherison C, Adel-Patient K. Use of partially hydrolysed formula in infancy and incidence of eczema, respiratory symptoms or food allergies in toddlers from the ELFE cohort. Pediatr Allergy Immunol. (2019) 30:614–23. doi: 10.1111/pai.13094
53. Amestejani M, Salehi BS, Vasigh M, Sobhkhiz A, Karami M, Alinia H, et al. Vitamin D supplementation in the treatment of atopic dermatitis: a clinical trial study. J Drugs Dermatol. (2012) 11:327–30.
54. Russell M. Assessing the relationship between vitamin D3 and stratum corneum hydration for the treatment of xerotic skin. Nutrients. (2012) 4:1213–8. doi: 10.3390/nu4091213
55. Reinholz M, Schauber J. Vitamin D and innate immunity of the skin. Dtsch Med Wochenschr. (2012) 137:2385–9. doi: 10.1055/s-0032-1327277
56. Peroni DG, Piacentini GL, Cametti E, Chinellato I, Boner AL. Correlation between serum 25-hydroxyvitamin D levels and severity of atopic dermatitis in children. Br J Dermatol. (2011) 164:1078–82. doi: 10.1111/j.1365-2133.2010.10147.x
57. Chiu YE, Havens PL, Siegel DH, Ali O, Wang T, Holland KE, et al. Serum 25-hydroxyvitamin D concentration does not correlate with atopic dermatitis severity. J Am Acad Dermatol. (2013) 69:40–6. doi: 10.1016/j.jaad.2013.01.010
58. D'Auria E, Barberi S, Cerri A, Boccardi D, Turati F, Sortino S, et al. Vitamin D status and body mass index in children with atopic dermatitis: a pilot study in Italian children. Immunol Lett. (2017) 181:31–35. doi: 10.1016/j.imlet.2016.11.004
59. Byremo G, Rød G, Carlsen KH. Effect of climatic change in children with atopic eczema. Allergy. (2006) 61:1403–10. doi: 10.1111/j.1398-9995.2006.01209.x
60. Bäck O, Blomquist HK, Hernell O, Stenberg B. Does vitamin D intake during infancy promote the development of atopic allergy. Acta Derm Venereol. (2009) 89:28–32. doi: 10.2340/00015555-0541
61. Grant CC, Crane J, Mitchell EA, Sinclair J, Stewart A, Milne T, et al. Vitamin D supplementation during pregnancy and infancy reduces aeroallergen sensitization: a randomized controlled trial. Allergy. (2016) 71:1325–34. doi: 10.1111/all.12909
62. Zhu TH, Zhu TR, Tran KA, Sivamani RK, Shi VY. Epithelial barrier dysfunctions in atopic dermatitis: a skin-gut-lung model linking microbiome alteration and immune dysregulation. Br J Dermatol. (2018) 179:570–81. doi: 10.1111/bjd.16734
63. Watanabe S, Narisawa Y, Arase S, Okamatsu H, Ikenaga T, Tajiri Y, et al. Differences in fecal microflora between patients with atopic dermatitis and healthy control subjects. J Allergy Clin Immunol. (2003) 111:587–91. doi: 10.1067/mai.2003.105
64. Navarro-López V, Ramírez-Boscá A, Ramón-Vidal D, Ruzafa-Costas B, Genovés-Martínez S, Chenoll-Cuadros E, et al. Effect of oral administration of a mixture of probiotic strains on SCORAD index and use of topical steroids in young patients with moderate atopic dermatitis: a randomized clinical trial. JAMA Dermatol. (2018) 154:37–43. doi: 10.1001/jamadermatol.2017.3647
65. Huang R, Ning H, Shen M, Li J, Zhang J, Chen X. Probiotics for the treatment of atopic dermatitis in children: a systematic review and meta-analysis of randomized controlled trials. Front Cell Infect Microbiol. (2017) 7:392. doi: 10.3389/fcimb.2017.00392
66. Arslanoglu S, Moro GE, Schmitt J, Tandoi L, Rizzardi S, Boehm G. Early dietary intervention with a mixture of prebiotic oligosaccharides reduces the incidence of allergic manifestations and infections during the first two years of life. J Nutr. (2008) 138:1091–5. doi: 10.1093/jn/138.6.1091
67. Osborn DA, Sinn JK. Prebiotics in infants for prevention of allergy. Cochrane Database Syst Rev. (2013) 28:CD006474. doi: 10.1002/14651858.CD006474.pub3
68. Tan-Lim CSC, Esteban-Ipac NAR, Mantaring JBV 3rd, Chan Shih Yen E, Recto MST, Sison OT, et al. Comparative effectiveness of probiotic strains for the treatment of pediatric atopic dermatitis: a systematic review and network meta-analysis. Pediatr Allergy Immunol. (2020). doi: 10.1111/pai.13305. [Epub ahead of print].
69. Best KP, Gold M, Kennedy D, Martin J, Makrides M. Omega-3 long-chain PUFA intake during pregnancy and allergic disease outcomes in the offspring: a systematic review and meta-analysis of observational studies and randomized controlled trials. Am J Clin Nutr. (2016) 103:128–43. doi: 10.3945/ajcn.115.111104
70. Calder PC, Kremmyda LS, Vlachava M, Noakes PS, Miles EA. Is there a role for fatty acids in early life programming of the immune system? Proc Nutr Soc. (2010) 69:373–80. doi: 10.1017/S0029665110001552
71. D'Auria E, Miraglia Del Giudice M, Barberi S, Mandelli M, Verduci E, Leonardi S, et al. Omega-3 fatty acids and asthma in children. Allergy Asthma Proc. (2014) 35:233–40. doi: 10.2500/aap.2014.35.3736
72. Miyake Y, Sasaki S, Tanaka K, Ohya Y, Miyamoto S, Matsunaga I, et al. Fish and fat intake and prevalence of allergic rhinitis in Japanese females: the Osaka maternal and child health study. J Am Coll Nutr. (2007) 26:279–87. doi: 10.1080/07315724.2007.10719612
73. Furuhjelm C, Warstedt K, Larsson J, Fredriksson M, Böttcher MF, Fälth-Magnusson K, et al. Fish oil supplementation in pregnancy and lactation may decrease the risk of infant allergy. Acta Paediatr. (2009) 98:1461–67. doi: 10.1111/j.1651-2227.2009.01355.x
74. Furuhjelm C, Warstedt K, Fagerås M, Fälth-Magnusson K, Larsson J, et al. Allergic disease in infants up to 2 years of age in relation to plasma omega-3 fatty acids and maternal fish oil supplementation in pregnancy and lactation. Pediatr Allergy Immunol. (2011) 22:505–14. doi: 10.1111/j.1399-3038.2010.01096.x
75. Best KP, Sullivan T, Palmer D, Gold M, Kennedy DJ, Martin J, et al. Prenatal fish oil supplementation and allergy: 6-year follow-up of a randomized controlled trial. Pediatrics. (2016) 137:e20154443. doi: 10.1542/peds.2015-4443
76. Palmer DJ, Sullivan T, Gold MS, Prescott SL, Heddle R, Gibson RA, et al. Randomized controlled trial of fish oil supplementation in pregnancy on childhood allergies. Allergy. (2013) 68:1370–6. doi: 10.1111/all.12233
77. Best KP, Sullivan TR, Palmer DJ, Gold M, Martin J, Kennedy D, et al. Prenatal omega-3 LCPUFA and symptoms of allergic disease and sensitization throughout early childhood - a longitudinal analysis of long-term follow-up of a randomized controlled trial. World Allergy Organ J. (2018) 11:10. doi: 10.1186/s40413-018-0190-7
78. Makrides M, Gibson RA, McPhee AJ, Yelland L, Quinlivan J, Ryan P, et al. Effect of DHA supplementation during pregnancy on maternal depression and neurodevelopment of young children: a randomized controlled trial. JAMA. (2010) 304:1675–83. doi: 10.1001/jama.2010.1507
79. Gunaratne AW, Makrides M, Collins CT. Maternal prenatal and/or postnatal n-3 long chain polyunsaturated fatty acids (LCPUFA) supplementation for preventing allergies in early childhood. Cochrane Database Syst Rev. (2015) 22:CD010085. doi: 10.1002/14651858.CD010085.pub2
80. Zutavern A, Brockow I, Schaaf B, von Berg A, Diez U, Borte M, et al. Timing of solid food introduction in relation to eczema, asthma, allergic rhinitis, and food and inhalant sensitization at the age of 6 years: results from the prospective birth cohort study LISA. Pediatrics. (2008) 121:e44–52. doi: 10.1542/peds.2006-3553
81. Zutavern A, Brockow I, Schaaf B, Bolte G, von Berg A, Diez U, et al. Timing of solid food introduction in relation to atopic dermatitis and atopic sensitization: results from a prospective birth cohort study. Pediatrics. (2006) 117:401–11. doi: 10.1542/peds.2004-2521
82. Nwaru BI, Erkkola M, Ahonen S, Kaila M, Haapala AM, Kronberg-Kippilä C, et al. Age at the introduction of solid foods during the first year and allergic sensitization at age 5 years. Pediatrics. (2010) 125:50–9. doi: 10.1542/peds.2009-0813
83. Greer FR, Sicherer SH, Burks AW. The effects of early nutritional interventions on the development of atopic disease in infants and children: the role of maternal dietary restriction, breastfeeding, hydrolyzed formulas, and timing of introduction of allergenic complementary foods. Pediatrics. (2019) 143:e20190281. doi: 10.1542/peds.2019-0281
84. Chuang CH, Hsieh WS, Chen YC, Chang PJ, Hurng BS, Lin SJ, et al. Infant feeding practices and physician diagnosed atopic dermatitis: a prospective cohort study in Taiwan. Pediatr Allergy Immunol. (2011) 22:43–9. doi: 10.1111/j.1399-3038.2010.01007.x
85. Turati F, Bertuccio P, Galeone C, Pelucchi C, Naldi L, Bach JF, et al. Early weaning is beneficial to prevent atopic dermatitis occurrence in young children. Allergy. (2016) 71:878–88. doi: 10.1111/all.12864
86. Chatenoud L, Bertuccio P, Turati F, Galeone C, Naldi L, Chatenoud L, et al. Markers of microbial exposure lower the incidence of atopic dermatitis. Allergy. (2020) 75:104–15. doi: 10.1111/all.13990
87. Flohr C, Nagel G, Weinmayr G, Kleiner A, Strachan DP, Williams HC, et al. Lack of evidence for a protective effect of prolonged breastfeeding on childhood eczema: lessons from the international study of asthma and allergies in childhood (ISAAC) phase two. Br J Dermatol. (2011) 165:1280–9. doi: 10.1111/j.1365-2133.2011.10588.x
88. Kull I, Bergstrom A, Lilja G, Pershagen G, Wickman M. Fish consumption during the first year of life and development of allergic diseases during childhood. Allergy. (2006) 61:1009–15. doi: 10.1111/j.1398-9995.2006.01115.x
89. Ierodiakonou D, Garcia-Larsen V, Logan A, Groome A, Cunha S, Chivinge J, et al. Timing of allergenic food introduction to the infant diet and risk of allergic or autoimmune disease: a systematic review and meta-analysis. JAMA. (2016) 316:1181–92. doi: 10.1001/jama.2016.12623
90. Hesselmar B, Saalman R, Rudin A, Adlerberth I, Wold A. Early fish introduction is associated with less eczema, but not sensitization, in infants. Acta Paediatr. (2010) 99:1861–67. doi: 10.1111/j.1651-2227.2010.01939.x
91. Venter C, Agostoni C, Arshad SH, Ben-Abdallah M, Du Toit G, Fleischer DM, et al. Dietary factors during pregnancy and atopic outcomes in childhood: a systematic review from the European Academy of Allergy and Clinical Immunology. Pediatr Allergy Immunol. (2020) 31:889–912. doi: 10.1111/pai.13303
Keywords: atopic dermatitis, breastfeeding, children, complementary foods, omega-3 long-chain polyunsaturated fatty acids, prevention, probiotics, vitamin D
Citation: Trikamjee T, Comberiati P, D'Auria E, Peroni D and Zuccotti GV (2021) Nutritional Factors in the Prevention of Atopic Dermatitis in Children. Front. Pediatr. 8:577413. doi: 10.3389/fped.2020.577413
Received: 29 June 2020; Accepted: 04 December 2020;
Published: 12 January 2021.
Edited by:
Claudio Pignata, University of Naples Federico II, ItalyReviewed by:
Rashmi Ranjan Das, All India Institute of Medical Sciences, IndiaCopyright © 2021 Trikamjee, Comberiati, D'Auria, Peroni and Zuccotti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Diego Peroni, ZGllZ28ucGVyb25pQHVuaXBpLml0
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.