
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pediatr., 27 October 2020
Sec. Pediatric Oncology
Volume 8 - 2020 | https://doi.org/10.3389/fped.2020.556802
Purpose: Increasing evidence suggests that circulating biomarkers may serve diagnostic and longitudinal monitoring purposes in pediatric neuro-oncology. Mutant tumor DNA is detectable in the cerebrospinal fluid (CSF) of pediatric diffuse midline glioma (DMG) patients and quantity can reflect disease burden. CSF sampling (“liquid biopsy”) via a CSF access device could therefore play a role in DMG management. Therefore, we set to evaluate the incidence of hydrocephalus (HCP) in DMG patients, and to characterize ventricular reservoir placement and access practices.
Methods: A single institution retrospective review of DMG patients ≤21-years-old was performed (1984–2019). MEDLINE searches for reports of ventricular reservoir or shunt placement in DMG, and reservoir access for intraventricular chemotherapy (IVC) were performed.
Results: At our institution, 62.6% of DMG patients (67/108) required intervention for HCP: 19.4% provided transient CSF access (ETV alone n = 3, EVD n = 8, unspecified n = 2), and 80.6% permanent CSF access (ETV + reservoir n = 13, shunt n = 41). Further, 22/34 patients with initially transient CSF devices required conversion to a permanent device. Five devices were revised for malfunction, one for infection. Seventeen articles cited HCP in 22 to 100% of DMG patients. IVC administration was described in 632 patients (seven articles), with 42 infectious and 63 non-infectious complications.
Conclusions: Management of HCP is often necessary in children with DMG. Given the low rate of clinical risk associated with VAD placement and access, and the potential utility of longitudinal disease monitoring via CSF analysis, VAD placement could be considered in future clinical trials to guide DMG treatment.
Pediatric diffuse midline gliomas (DMGs), including thalamic and Diffuse Intrinsic Pontine Gliomas (DIPGs), and are highly morbid brain tumors. Children with DMG have a median survival of only 9 months, with a near 100% mortality despite treatment (1). Importantly, recurrent somatic missense mutations in histone H3 isoforms, resulting in H3K27M mutant proteins, are found in 80% of pediatric DMGs, and portend poorer prognosis and response to therapy (2–5). As a result, all gliomas harboring the H3K27M mutation are now considered high-grade, irrespective of histological features (6). In turn, clinical detection of this mutation is now critical to patient diagnosis and management, but tissue sampling from these deep seated tumors is not without risk, and is not feasible for longitudinal monitoring.
Importantly, tumor mutant DNA and proteins are detectable in cerebrospinal fluid (CSF) of children with DMG, with levels correlating with both CSF proximity to the tumor and changes in tumor volume in response to treatment (7–9). Circulating tumor DNA (ctDNA) levels in CSF from adult patients with brain tumors are also prognostic, demonstrating the potential clinical power of a “liquid biopsy” approach for clinical diagnosis and monitoring effects of therapy, including differentiating tumor recurrence from treatment response (10–16). Given the desperate need for more effective treatment for DMG, and the potential clinical utility of liquid biopsy for diagnosis and management, serial CSF sampling via lumbar puncture (LP) is now included in one pediatric clinical trial to identify clinical correlates of treatment response (17). However, since the concentration of ctDNA and proteins is greater in CSF collected from closer proximity to the tumor, serial ventricular CSF sampling may be of greater utility than LP. Therefore, in order to explore the incidence and feasibility of ventricular CSF sampling from children with diffuse midline glioma, we sought to determine the incidence and clinical outcomes of ventricular catheter placement and access in these patients at our institution, and as reported in the literature.
The studies involving human participants were reviewed and approved by the Ann & Robert H. Lurie Children's Hospital of Chicago Institutional Review Board (IRB# 2005-12252). A retrospective review was conducted of the medical records of pediatric patients (≤21 years old) diagnosed with thalamic and brainstem glioma who received all or a portion of their neuro-oncologic treatment at the Ann & Robert H. Lurie Children's Hospital of Chicago from December 1984 to February 2019. Diagnosis, presenting symptoms, and neurosurgical interventions were evaluated, including the presence of hydrocephalus (HCP) with the need for CSF access or diversion during the course of treatment [lumbar puncture (LP), endoscopic third ventriculostomy (ETV), ventricular access device or reservoir (VAD), external ventricular drain (EVD), CSF or ventriculoperitoneal shunt (VPS)]. CSF access was categorized as transient if the patient underwent LP, ETV or EVD placement, or permanent if the patient received placement of a VAD or shunt.
A systematic MEDLINE search was also conducted using key words “pediatric” and “hydrocephalus,” “ommaya reservoir,” “ventriculoperitoneal shunt,” or “third ventriculostomy,” and “diffuse midline glioma,” “diffuse intrinsic pontine glioma,” “brainstem glioma,” or “thalamic glioma” (Supplementary Figure 1). A second systematic MEDLINE search was conducted using key words “pediatric ommaya reservoir” or “pediatric ventricular reservoir” and “intraventricular chemotherapy” (Supplementary Figure 2). Reports of tumors of the posterior fossa without brainstem involvement, tumors isolated to the pineal region or tectum, patients > 21 years of age, or lacking perioperative outcomes of cerebrospinal fluid access device placement were excluded, as well as those without full-text available for review in English. Reports were evaluated for VAD placement and access practices, interventions for hydrocephalus (HCP) management, and device-related complications.
A total of 3,521 pediatric brain and spine tumor patient records were available for retrospective review at our institution, with 108 diagnosed with DMG (Table 1). One of these patients' records was excluded because their case was reviewed for pathology purposes only (midline supratentorial thalamus, EVD). The remaining 107 patients received either all of their care at our institution, were seen in consultation for a second opinion (n = 17) or underwent biopsy and/or management of HCP at our institution prior to continuing care elsewhere (n = 2). Of these, 67 (62.6%) required a ventricular access procedure during their course of treatment (Table 2). A total of 13/67 children with DMG underwent transient CSF access procedures (19.4%, ETV alone n = 3, EVD n = 8, or unspecified n = 2), while 54/67 had permanent CSF access devices placed (80.6%, ETV + VAD n = 13, or CSF shunt n = 41). VADs were placed in these children for emergent ventricular access in case of ventriculostomy failure in combination with ETV (n = 13). An additional four DMG patients had clinical documentation of HCP but did not undergo intervention; three for unspecified reasons, and one because the family declined intervention. Of note, none of our DMG patients underwent VAD or shunt placement for intraventricular chemotherapy (IVC) administration, and none of our DMG patients underwent serial access of their VAD for any other indication.
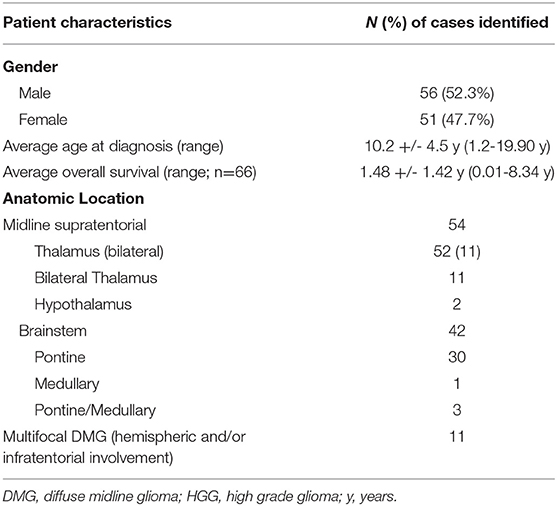
Table 1. Case characteristics of 108 pediatric diffuse midline glioma (DMG) cases treated at our institution (1984–2019).
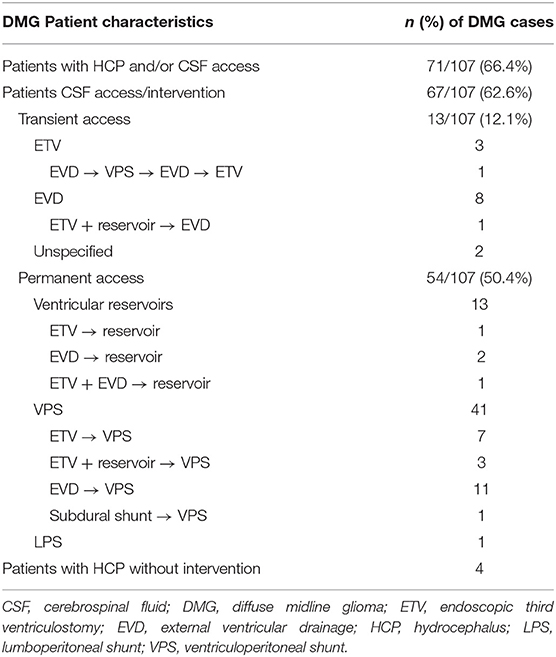
Table 2. Rate of hydrocephalus (HCP) and/or cerebrospinal fluid (CSF) access in Diffuse Midline Glioma (DMG) patients at our institution.
Amongst the 67 DMG patients that required intervention for HCP, 25 (37.3%) were treated for HCP perioperatively, within 7 days of initial tumor biopsy or resection, and 13 (19.4%) underwent HCP treatment in a delayed fashion. Other patients did not undergo tumor resection or biopsy and were treated surgically for hydrocephalus alone. For most patients, CSF diversion was initially attempted without foreign body implantation, but ultimately conversion to an indwelling device was required due to persistent hydrocephalus. For example, ETV and/or EVD was attempted as the primary intervention in 50.7% of patients (n = 34/67; ETV alone n = 10, ETV with EVD n = 1, EVD alone n = 21, unspecified n = 2). Of the ten DMG patients who underwent initial ETV alone, eight eventually required an indwelling CSF access device (VAD n = 1, VPS n = 7). One ETV patient underwent a second ETV attempt, which subsequently failed and required VPS placement. Another patient with initial concurrent EVD and ETV ultimately required a VPS. 61.9% of patients with EVDs (n = 13/21) also required conversion to either a VAD (n = 2) or VPS (n = 11).
A total of eight DMG patients with HCP experienced multiple device-related complications (11.9%, Table 2). Five (7.5%) of these patients had non-infectious shunt malfunctions and device revisions: one subdural shunt was revised to a VPS, one patient required a single VPS revision, and three patients required two or more VPS revisions. Two patients with a VPS required a second ventricular catheter for entrapped ventricles. Only one infectious complication was identified (1.5%): this child had an EVD placed at the time of tumor resection that was converted to a VPS, and complicated by serratia odorifae infection two weeks later requiring externalization and intravenous antibiotics before reinternalization. This patient also suffered multiple non-infectious complications, including shunt malfunction requiring revision, undergoing shunt removal and ETV. Due to quality of and access to outside medical records, meaningful analysis of effects of different shunt hardware on outcomes was not feasible.
Review of the literature yielded 18 articles describing 383 patients with glioma of the thalamus or brainstem, citing rates of HCP from 22 to 100% (overall 55.6%, n = 213/383), and intervention in 22–63% (overall 53.3%, n = 204/383) (Table 3) (18–35). Of these cases, there were 132 ventricular shunts (including 2 Torkildsen shunts) (22), 70 ETVs, and two EVDs that were ultimately weaned (31). Nine patients with evidence of HCP were untreated, with reports citing anatomic distortion from thalamic gliomas (n = 2 ETV deferred) (23), reasons unspecified, (36), patient refusal, or poor overall clinical condition (n = 6) (18, 22). Infectious complications were reported in association with placement of one Torkildsen shunt (0.01% of all shunts placed) (22). Non-infectious complications secondary to shunt placement included shunt malfunction [n = 4, one Torkildsen shunt converted to a VPS (22), one confirmed VPS malfunction requiring revision (18) and two suspected VPS malfunctions (25)], and contralateral subdural hematoma that resolved after placement of temporary subduroperitoneal shunts (n = 3) (31). Of note, there were four reported cases of extraneural metastases via CSF seeding through shunt systems (19, 28, 32, 34). These occurred most often after other evidence of tumor recurrence or progression, particularly with leptomeningeal dissemination. Reported ETV complications (n = 6, 8.5%) consisted of failure to effectively treat HCP requiring conversion to VPS (18, 23–25).
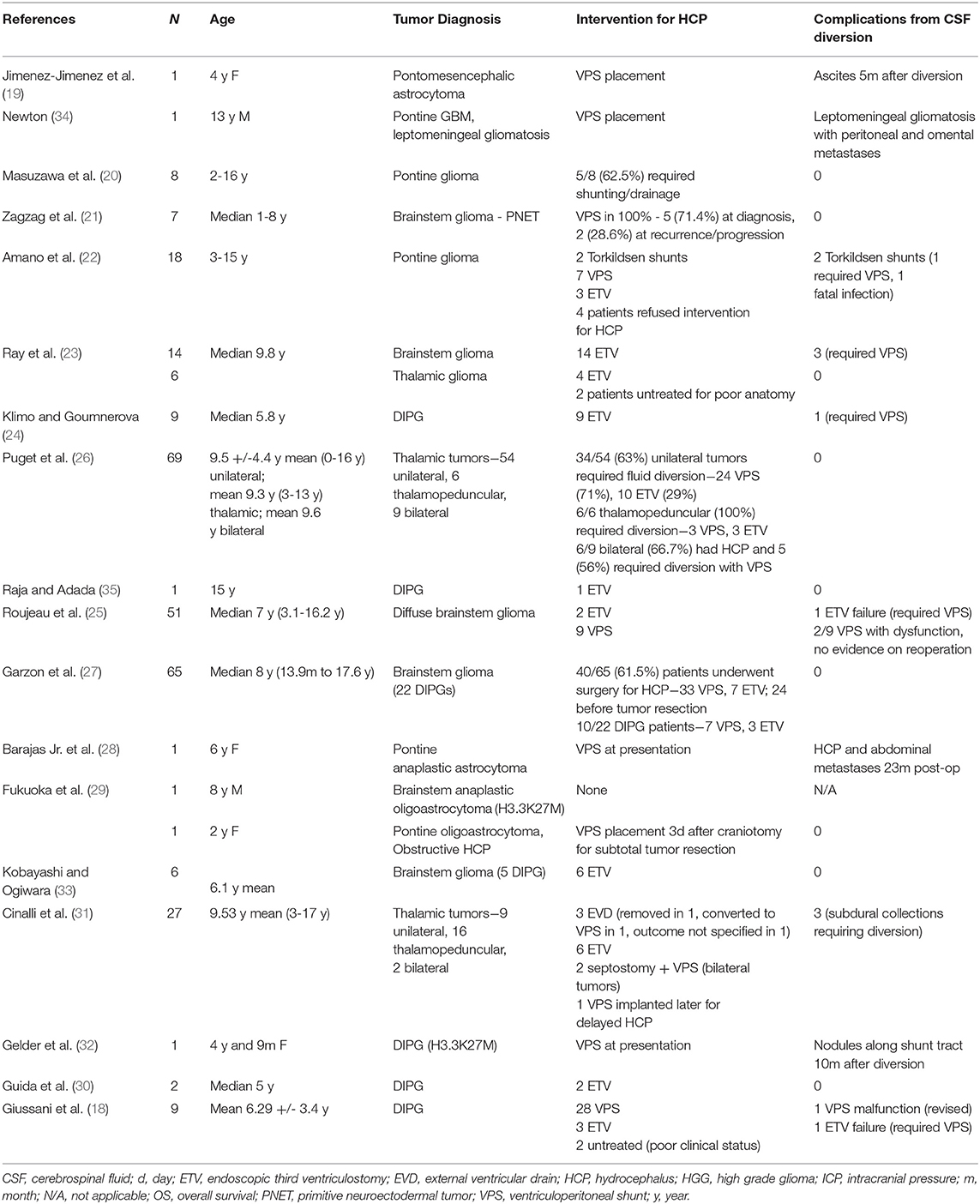
Table 3. Results of literature review for Cerebrospinal fluid (CSF) diversion procedures in children with diffuse midline gliomas (DMG) and hydrocephalus (HCP).
Review of the literature also yielded six articles describing placement of 492 VADs for IVC delivery in 483 patients with brain tumors. There were no reports of VAD placement for IVC administration in DMG patients. CSF reservoirs were in place for up to 70.3 months in some cases, with reports of up to 280 IVC instillations per device (Table 4). 42 infectious (8.5%) and 46 (9.3%) non-infectious reservoir related complications were reported, some occurring in the same patient (Table 4) (37–42). The most common non-infectious reservoir-related complications included catheter migration and/or malfunction (n = 14), and CSF leak and/or wound dehiscence (n = 18). Nine ventricular reservoirs were eventually converted to VPS for treatment of HCP.
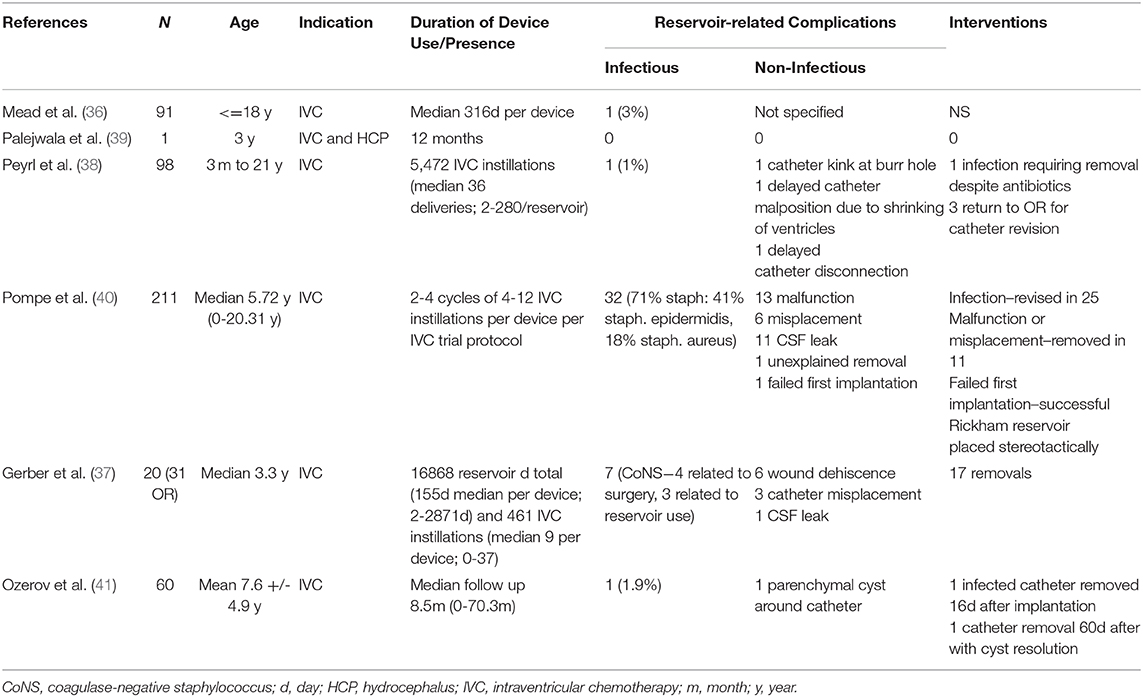
Table 4. Results of literature review for clinical outcomes of serial access of Cerebrospinal fluid (CSF) access devices in pediatric brain tumor patients.
Of note, among all VAD placement procedures reported in this review, intra-operative catheter misplacement or failure of reservoir placement occurred in seven instances (41, 42). In addition, there was one instance of a catheter-associated cyst, which resolved after removal of the catheter (42).
Brain tumors are the most common solid tumor of childhood and leading cause of cancer death in children. Of these, DMGs have the highest mortality, with no improvement in clinical outcomes despite over 40 years of clinical trials (1). Recent advances in stereotactic surgical technology now enable surgeons to perform tumor biopsy, providing tissue for histologic and molecular analysis. Importantly, the recent discovery of a high rate of histone H3 mutation in DMG, and oncogenic downstream effects, has led to new therapeutic strategies and clinical trials (1, 2). As a result, tissue analysis for histone H3 mutations is now routinely performed for molecular diagnosis (5). In turn, multiple studies have evaluated biomarkers of various central nervous system tumors and their utility in diagnosis or longitudinal monitoring of response to treatment, including proteins and nucleic acids in the blood and CSF (43–45). While repeat tumor biopsy is often not clinically feasible, sampling of these liquid specimens to monitor Histone H3 mutant ctDNA or other potential disease biomarkers could prove valuable in guiding management. Importantly, sufficient tumor DNA and protein can be extracted from CSF retrieved from the lateral ventricle of a patient with thalamic or brainstem glioma, and ctDNA levels have been shown to correlate with changes in tumor size in response to targeted therapy (7–9). While children with brain tumors often undergo LP to assess disease dissemination or as part of chemotherapeutic protocols for intrathecal drug delivery, LP can be painful and dangerous in patients with an intracranial mass lesion. In addition, multiple studies have demonstrated that ventricular CSF is superior to lumbar for ctDNA sequencing and quantification, due to a higher concentration of ctDNA with closer proximity to tumor (7–9). Therefore, lateral or fourth ventricular device placement for CSF sampling may serve as a safer and more effective method for liquid biopsy compared to LP.
While ventricular device placement requires a neurosurgical intervention with foreign body implantation, retrospective review of our institutional cohort of DMG patients and the literature shows that over half already undergo intervention for HCP during their clinical course (62.6%, n = 67/107 and 53.2%, n = 204/383, respectively). While HCP may be treated in select cases with favorable anatomy and CSF dynamics via ETV to avoid foreign body implantation, 81.8% (n = 9/11) of DMG patients in our institutional cohort treated with ETV ultimately required permanent device placement. In addition, of the 21 patients in our cohort with an EVD, 62% (n = 13) required conversion to permanent devices. These data further demonstrate that ventricular access devices (VAD or shunt) are often necessary in this patient population: a clinical trial testing ventricular placement vs. ETV in DMG patients with hydrocephalus incorporating the ETV success score would therefore be prudent (46). Further study to determine measurable patient or tumor characteristics that can predict which patients will require permanent CSF diversion or suffer a complication, is also warranted. For now, current rates of ventricular device placement (VAD or shunt) could facilitate both CSF diversion and CSF sampling in the majority of these patients.
Importantly, the morbidity associated with ventricular catheter placement in this patient population is also very low. The most common non-infectious complications reported in the literature are device malfunction, misplacement of the catheter, and wound dehiscence with associated CSF leak. Importantly, there were no cases of DMG patients with wound dehiscence and CSF leak after VPS, ETV, or EVD placement in our patient cohort. Device malfunction was reported in only 2.8% of VADs (n = 14/492) and 2.9% of shunts (n = 3/104) in the literature, while we observed shunt malfunction in 12.1% of our DMG patients (n = 5/41). Complications related to ventricular catheter placement itself (2%; n = 10/492 in the literature) may be minimized with use of endoscopy, stereotactic neuronavigation, and intra-operative confirmation of CSF access through the device (39, 42).
Indeed, ventricular catheter placement is not without significant clinical risk. We found that only 64% of our DMG patients required ventricular access for HCP management, and do not recommend children undergo surgeries that are not clinically indicated: whether a liquid biopsy will provide information that will benefit patients in the form of decreased need for surgery or increased survival remains to be determined in clinical trials. However, given the potential for CSF studies to guide therapy in the future, we sought to evaluate the potential risks of VAD placement and access in DMG patients without HCP for the sole purpose of CSF sampling. We therefore looked to a patient population without HCP that undergoes ventricular catheter placement and serial device access for interventricular chemotherapy (IVC). Our review revealed a slightly higher rate of infectious (8.5%) and non-infectious (9.3%) complications associated with serial VAD access for IVC administration, relative to device placement alone. Indeed, it is likely that repeated mechanical access of these devices, in the setting of local and systemic effects of the chemotherapy, increases complication risk in these patients, underscoring the need for a stringent protocol for sterile device access. Martinez et al. found a high degree of variability in techniques used by neurosurgeons to access ventricular reservoirs or shunts, including skin cleansing solutions, and use of sterile draping (47). Importantly, multiple studies note that standardized perioperative antibiotics and local antiseptics decrease rates of infectious complications of serial VAD access (47, 48), suggesting the relative safety of VAD access in DMG patients for liquid biopsy purposes. In line with this, we, and others, have found that implementing a standardized protocol for VAD access, and increasing practitioner education and awareness of this protocols at all levels of the patient care team, can significantly reduce the risks of VAD associated infection (49–51). For example, a retrospective review of a cohort of 37 neonates with post-hemorrhagic HCP who underwent serial device taps at our institution using a stringent sterile access protocol revealed no infections after a total of 630 cumulative taps (average 17 taps per reservoir; range 0–83) across 10,420 collective indwelling reservoir days (average 282 per patient; range 6–3,700). Although reservoir access is usually performed by neurosurgeons, aspiration is also routinely performed by advanced practice nurses at our institution, and studies have documented low rates of complications when aspiration was performed either neurosurgery or neonatology teams (50, 52).
Of note, we could not identify any cases of VAD placement for IVC for DMG patients, either at our institution or in the literature, so should use caution when extrapolating these findings. Indeed, it is important to note that none of our DMG patients underwent serial access of their VAD, neither for IVC administration or other indications, so we cannot comment on whether this would have resulted in increased infection rates. Interestingly, there are reports that VAD placement for IVC administration into the fourth ventricle is feasible, with a low rate of complications (53, 54). Since the concentration of mutant ctDNA in CSF is greater with closer proximity to tumor, placement of a fourth ventricular catheter for longitudinal monitoring of liquid biomarkers may be of particular consideration for DIPG patients. As mentioned above, however, adequate ctDNA can be isolated from a lateral ventricular catheter (8, 9), so a neurosurgeon's best judgement should always be exercised when considering catheter placement.
Due to the retrospective nature of this study, limitations include inconsistent or incomplete documentation of patient clinical data. Some patients were treated before the implementation of the queried electronic medical record, and some received portions of their care at multiple institutions. The decision to include patients who were seen only in consultation for a second opinion was made in order to better examine the incidence of HCP in a large sample size. Exclusion of these patients eliminates the one documented infectious complication in our cohort, as well as two patients who required revisions for shunt malfunction. It is possible that the two patients who continued their care at outside institutions did have revisions or infectious complications that were not captured in our retrospective review. Best efforts were made to reconcile available data with any additional details available on review of patient electronic medical records. Despite these limitations, the authors believe that this literature review and retrospective cohort analysis accurately demonstrates indications, incidence, and outcomes of HCP management and ventricular reservoir placement in the examined patient populations.
A review of the literature and our own institutional practice reveals that over half of pediatric DMG patients require a CSF diversion device for management of HCP. Additionally, ventricular catheter placement is technically feasible in children with DMG, with a low rate of associated complications when a standardized protocol for device access is utilized. Liquid biopsy from these patients could potentially aid in stratification to targeted therapies and disease monitoring. As a result, CSF sampling could be considered in future clinical trials as part of a liquid biopsy approach to guide clinical management and measure treatment response, with the goal to ultimately improve clinical outcomes for these devastating pediatric brain tumors.
All datasets presented in this study are included in the article/ Supplementary Material.
The studies involving human participants were reviewed and approved by the Ann & Robert H. Lurie Children's Hospital of Chicago Institutional Review Board (IRB# 2005-12252).
DL is the first author having performed the majority data analysis, systematic review and writing the manuscript. WS is a major contributor in data collection. KR, MK, and SS participated in the review of the manuscript. AS is the principal investigator having contributed to the majority of the conception and review of the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors would like to acknowledge the generosity of all patients and their families. In addition, the authors would like to acknowledge Dr. Sandi Lam for contributing her insightful perspectives on the clinical importance of the topic addressed in this manuscript.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2020.556802/full#supplementary-material
1. Jansen MHA, van Vuurden DG, Vandertop WP, Kaspers GJL. Diffuse intrinsic pontine gliomas: a systematic update on clinical trials and biology. Cancer Treat Rev. (2012) 38:27–35. doi: 10.1016/j.ctrv.2011.06.007
2. Bartels U, Hawkins C, Vézina G, Kun L, Souweidane M, Bouffet E. Proceedings of the diffuse intrinsic pontine glioma (DIPG) toronto think tank: advancing basic and translational research and cooperation in DIPG. J Neurooncol. (2011) 105:119–25. doi: 10.1007/s11060-011-0704-4
3. Gielen GH, Gessi M, Hammes J, Kramm CM, Waha A, Pietsch T. H3F3A k27M mutation in pediatric CNS tumors: a marker for diffuse high-grade astrocytomas. Am J Clin Pathol. (2013) 139:345. doi: 10.1309/AJCPABOHBC33FVMO
4. Schwartzentruber J, Korshunov A, Liu X, Jones DTW, Pfaff E, Jacob K, et al. Driver mutations in histone h3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature. (2012) 482:226. doi: 10.1038/nature10833
5. Wu G, Diaz AK, Paugh BS, Rankin SL, Ju B, Li Y, et al. The genomic landscape of diffuse intrinsic pontine glioma and pediatric non-brainstem high-grade glioma. Nat Genet. (2014) 46:444–50. doi: 10.1038/ng.2938
6. Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. (2016) 131:803–20. doi: 10.1007/s00401-016-1545-1
7. Stallard S, Savelieff MG, Wierzbicki K, Mullan B, Miklja Z, Bruzek A, et al. CSF h3F3A k27M circulating tumor dNA copy number quantifies tumor growth and in vitro treatment response. Acta Neuropathol Commun. (2018) 6:80. doi: 10.1186/s40478-018-0580-7
8. Huang TY, Piunti A, Lulla RR, Qi J, Horbinski CM, Tomita T, et al. Detection of histone h3 mutations in cerebrospinal fluid-derived tumor dNA from children with diffuse midline glioma. Acta Neuropathol Commun. (2017) 5:28–12. doi: 10.1186/s40478-017-0436-6
9. Panditharatna E, Kilburn LB, Aboian MS, Kambhampati M, Gordish-Dressman H, Magge SN, et al. Clinically relevant and minimally invasive tumor surveillance of pediatric diffuse midline gliomas using patient-Derived liquid biopsy. Clin Cancer Res. (2018) 24:5850–9. doi: 10.1158/1078-0432.CCR-18-1345
10. De Mattos-arruda L, Mayor R, Ng CKY, Weigelt B, Martínez-ricarte F, Torrejon D, et al. Cerebrospinal fluid-derived circulating tumour DNA better represents the genomic alterations of brain tumours than plasma. Nat Commun. (2015) 6:8839. doi: 10.1038/ncomms9839
11. Li Y, Pan W, Connolly I, Reddy S, Nagpal S, Quake S, et al. Tumor dNA in cerebral spinal fluid reflects clinical course in a patient with melanoma leptomeningeal brain metastases. J Neurooncol. (2016) 128:93–100. doi: 10.1007/s11060-016-2081-5
12. Momtaz P, Pentsova E, Abdel-Wahab O, Diamond E, Hyman D, Merghoub T, et al. Quantification of tumor-derived cell free dNA(cfDNA) by digital pCR (DigPCR) in cerebrospinal fluid of patients with bRAFV600 mutated malignancies. Oncotarget. (2016) 7:85430–6. doi: 10.18632/oncotarget.13397
13. Pan W, Gu W, Nagpal S, Gephart MH, Quake SR. Brain tumor mutations detected in cerebral spinal fluid. Clin Chem. (2015) 61:514–22. doi: 10.1373/clinchem.2014.235457
14. Siravegna G, Geuna E, Mussolin B, Crisafulli G, Bartolini A, Galizia D, et al. Genotyping tumour dNA in cerebrospinal fluid and plasma of a hER2-positive breast cancer patient with brain metastases. ESMO Open. (2017) 2:e000253. doi: 10.1136/esmoopen-2017-000253
15. Wang Y, Springer S, Zhang M, Mcmahon KW, Kinde I, Dobbyn L, et al. Detection of tumor-derived DNA in cerebrospinal fluid of patients with primary tumors of the brain and spinal cord. Proc Natl Acad Sci USA. (2015). doi: 10.1073/pnas.1511694112
16. Zhao J, Ye X, Xu Y, Chen M, Zhong W, Sun Y, et al. EGFR mutation status of paired cerebrospinal fluid and plasma samples in EGFR mutant non-small cell lung cancer with leptomeningeal metastases. Cancer Chemother Pharmacol. (2016) 78:1305–10. doi: 10.1007/s00280-016-3155-y
17. ONC201 in Pediatric H3 K27M Gliomas. (2018). Available online at: https://clinicaltrials.gov/ct2/show/NCT03416530?term=ONC-201&age=0&draw=2&rank=1.
18. Giussani C, Guida L, Biassoni V, Schiavello E, Carrabba G, Trezza A, et al. Retrospective analysis of the clinical and radiological features of 94 consecutive DIPGs patients to investigate the factors determining the development of hydrocephalus and its impact on clinical status and survival. Childs Nerv Syst. (2020). doi: 10.1007/s00381-020-04589-4. [Epub ahead of print].
19. Jimenez-Jimenez FJ, Garzo-Fernández C, De Inovencio-Arocena J, Pérez-Sotelo M, Castro-De Castro P, Salinero-Paniagua E. Extraneural metastases from brainstem astrocytoma through ventriculoperitoneal shunt. J Neurol Neurosurg Psychiatry. (1991) 54:281–2.
20. Masuzawa H, Sato J, Kamitani H, Kamikura T, Aoki N. Pontine gliomas causing locked-in syndrome. Child's Nerv Syst. (1993) 9:256–9. doi: 10.1007/BF00306266
21. Zagzag D, Miller DC, Knopp E, Farmer J, Lee M, Biria S, et al. Primitive neuroectodermal tumors of the brainstem: investigation of seven cases. Pediatrics. (2000) 106:1045–53. doi: 10.1542/peds.106.5.1045
22. Amano T, Inamura T, Nakamizo A, Inoha S, Wu C, Ikezaki K. Case management of hydrocephalus associated with the progression of childhood brain stem gliomas. Childs Nerv Syst. (2002) 18:599–604. doi: 10.1007/s00381-002-0637-5
23. Ray P, Jallo GI, Kim RYH, Kim B, Wilson S, Kothbauer K, et al. Endoscopic third ventriculostomy for tumor-related hydrocephalus in a pediatric population. Neurosurg Focus. (2005) 19:E8. doi: 10.3171/foc.2005.19.6.9
24. Klimo P, Goumnerova LC. Endoscopic third ventriculocisternostomy for brainstem tumors. J Neurosurg Pediatr. (2006) 105:271–4. doi: 10.3171/ped.2006.105.4.271
25. Roujeau T, Di Rocco F, Dufour C, Bourdeaut F, Puget S, Rose C, et al. Shall we treat hydrocephalus associated to brain stem glioma in children? Childs Nerv Syst. (2011) 27:1735–9. doi: 10.1007/s00381-011-1538-2
26. Puget S, Crimmins DW, Garnett MR, Grill J, Oliveira R, Boddaert N, et al. Thalamic tumors in children: a reappraisal. J Neurosurg. (2007) 106:354–62. doi: 10.3171/ped.2007.106.5.354
27. Garzón M, García-Fructuoso G, Guillén A, Suñol M, Mora J, Cruz O. Brain stem tumors in children and adolescents: single institutional experience. Childs Nerv Syst. (2013) 29:1321–31. doi: 10.1007/s00381-013-2137-1
28. Barajas R, Phelps A, Foster H, Courtier J, Buelow B, Gupta N, et al. Metastatic diffuse intrinsic pontine glioma to the peritoneal cavity via ventriculoperitoneal shunt: case report and literature review. J Neurol Surg Rep. (2015) 76:e91–6. doi: 10.1055/s-0035-1547365
29. Fukuoka K, Yanagisawa T, Watanabe Y, Suzuki T, Shirahata M, Adachi J, et al. Brainstem oligodendroglial tumors in children: two case reports and review of literatures. Childs Nerv Syst. (2015) 31:449–55. doi: 10.1007/s00381-014-2563-8
30. Guida L, Roux F, Massimino M, Marras CE, Sganzerla E, Giussani C. Safety and efficacy of endoscopic third ventriculostomy in diffuse intrinsic pontine glioma related hydrocephalus: a Systematic review. World Neurosurg. (2019) 124:29–35. doi: 10.1016/j.wneu.2018.12.096
31. Cinalli G, Aguirre DT, Mirone G, Ruggiero C, Cascone D, Quaglietta L, et al. Surgical treatment of thalamic tumors in children. J Neurosurg Pediatr. (2018) 21:247–57. doi: 10.3171/2017.7.PEDS16463
32. Gelder CL, Hawkins C, Zapotocky M, Dirks P, Bartels U, Bouffet E. Diffuse intrinsic pontine glioma ventricular peritoneal shunt metastasis: a case report and literature review. Childs Nerv Syst. (2019) 35:861–4. doi: 10.1007/s00381-019-04069-4
33. Kobayashi N, Ogiwara H. Endoscopic third ventriculostomy for hydrocephalus in brainstem glioma: a case series. Childs Nerv Syst. (2016) 32:1251–5. doi: 10.1007/s00381-016-3065-7
34. Newton HB, Rosenblum MK, Walker RW. Extraneural metastases of infratentorial glioblastoma multiforme to the peritoneal cavity. Cancer. (1992) 69:2149–53. doi: 10.1002/1097-0142(19920415)69:8<2149::aid-cncr2820690822>3.0.co;2-g
35. Raja AI, Adada B. Immediate resolution of tonsillar herniation and severe cervicothoracic syringomyelia after third ventriculostomy for hydrocephalus caused by a brainstem tumor. J Neurosurg. (2007) 106:44–7. doi: 10.3171/ped.2007.106.1.44
36. Puget S, Beccaria K, Blauwblomme T, Roujeau T, James S, Grill J, et al. Biopsy in a series of 130 pediatric diffuse intrinsic pontine gliomas. Childs Nerv Syst. (2015) 31:1773–80. doi: 10.1007/s00381-015-2832-1
37. Mead PA, Safdieh JE, Nizza P, Tuma S, Sepkowitz KA. Ommaya reservoir infections: a 16-year retrospective analysis. J Infect. (2013) 68:225–30. doi: 10.1016/j.jinf.2013.11.014
38. Gerber NU, Müller A, Bellut D, Bozinov O, Berger C, Grotzer MA. Ventricular catheter systems with subcutaneous reservoirs (Ommaya reservoirs) in pediatric patients with brain tumors: infections and other complications. Neuropediatrics. (2015) 46:401. doi: 10.1055/s-0035-1565271
39. Peyrl A, Chocholous M, Azizi A, Czech T, Dorfer C, Mitteregger D, et al. Safety of ommaya reservoirs in children with brain tumors: a 20-year experience with 5472 intraventricular drug administrations in 98 patients. J Neurooncol. (2014) 120:139–45. doi: 10.1007/s11060-014-1531-1
40. Palejwala SK, Stidd DA, Skoch JM, Gupta P, Lemole J, G Michael, et al. Use of a stop-flow programmable shunt valve to maximize cNS chemotherapy delivery in a pediatric patient with acute lymphoblastic leukemia. Surg Neurol Int. (2014) 5:S273–7. doi: 10.4103/2152-7806.139381
41. Pompe RS, von Bueren AO, Mynarek M, von Hoff K, Friedrich C, Kwiecien R, et al. Intraventricular methotrexate as part of primary therapy for children with infant and/or metastatic medulloblastoma: feasibility, acute toxicity and evidence for efficacy. Eur J Cancer. (2015) 51:2634–42. doi: 10.1016/j.ejca.2015.08.009
42. Ozerov S, Thomale U, Schulz M, Schaumann A, Samarin A, Kumirova E. The use of a smartphone-assisted ventricle catheter guide for ommaya reservoir placement—experience of a retrospective bi-center study. Childs Nerv Syst. (2018) 34:853–9. doi: 10.1007/s00381-017-3713-6
43. Lu VM, Power EA, Zhang L, Daniels DJ. Liquid biopsy for diffuse intrinsic pontine glioma: an update. J Neurosurg. (2019) 24:593–600. doi: 10.3171/2019.6.PEDS19259
44. Russell MD, Young AMH, Karri SK. Biomarkers of pediatric brain tumors. Front Pediatr. (2013) 1:7. doi: 10.3389/fped.2013.00007
45. Pan C, Diplas BH, Chen X, Wu Y, Xiao X, Jiang L, et al. Molecular profiling of tumors of the brainstem by sequencing of CSF-derived circulating tumor DNA. Acta Neuropathol. (2019) 137:297–306. doi: 10.1007/s00401-018-1936-6
46. Kulkarni AV, Drake JM, Kestle JRW, Mallucci CL, Sgouros S, Constantini S. Predicting who will benefit from endoscopic third ventriculostomy compared with shunt insertion in childhood hydrocephalus using the eTV success score. Pediatrics. (2010) 6:310–5. doi: 10.3171/2010.8.PEDS103
47. Martinez CI, Fletcher S, Shah MN, Kerr ML, Sandberg DI. Survey of techniques utilized to access ventricular shunts and reservoirs. Pediatr Neurosurg. (2017) 52:250–6. doi: 10.1159/000474948
48. Cohen-Pfeffer JL, Gururangan S, Lester T, Lim DA, Shaywitz AJ, Westphal M, et al. Intracerebroventricular delivery as a safe, long-Term route of drug administration. Pediatr Neurol. (2017) 67:23–35. doi: 10.1016/j.pediatrneurol.2016.10.022
49. Brouwer AJ, Groenendaal F, van den Hoogen A, Verboon-Maciolek M, Hanlo P, Rademaker KJ, et al. Incidence of infections of ventricular reservoirs in the treatment of post-haemorrhagic ventricular dilatation: a retrospective study (1992–2003). Arch Dis Childhood - Fetal Neonatal. (2007) 92:F41–3. doi: 10.1136/adc.2006.096339
50. Flanders TM, Kimmel AC, Lang SS, Bellah R, Chuo J, Wellons JC 3rd, et al. Standardizing treatment of preterm infants with post-hemorrhagic hydrocephalus at a single institution with a multidisciplinary team. Childs Nerv Syst. (2020) 36:1737–44. doi: 10.1007/s00381-020-04508-7
51. Spader HS, Hertzler DA, Kestle JRW, Riva-Cambrin J. Risk factors for infection and the effect of an institutional shunt protocol on the incidence of ventricular access device infections in preterm infants. Pediatrics. (2015) 15:156–60. doi: 10.3171/2014.9.PEDS14215
52. Kormanik K, Praca J, Garton HJL, Sarkar S. Repeated tapping of ventricular reservoir in preterm infants with post-hemorrhagic ventricular dilatation does not increase the risk of reservoir infection. J Perinatol. (2010) 30:218-21. doi: 10.1038/jp.2009.154
53. Sandberg D, Yu B, Patel R, Hagan J, Miesner E, Sabin J, et al. Infusion of 5-Azacytidine (5-AZA) into the fourth ventricle or resection cavity in children with recurrent posterior fossa ependymoma: a pilot clinical trial. J Neurooncol. (2019) 141:449–57. doi: 10.1007/s11060-018-03055-1
Keywords: pediatric diffuse midline glioma, hydrocephalus, ventricular access device, liquid biopsy, circulating tumor DNA
Citation: Li D, Stellpflug W, Romanski K, Kilgallon M, Speck S and Saratsis AM (2020) Ventricular Cerebrospinal Fluid Sampling in Pediatric Diffuse Midline Glioma Patients: Institutional Experience and Review of the Literature. Front. Pediatr. 8:556802. doi: 10.3389/fped.2020.556802
Received: 28 April 2020; Accepted: 16 September 2020;
Published: 27 October 2020.
Edited by:
Zoltan Patay, St. Jude Children's Research Hospital, United StatesReviewed by:
Joseph Louis Lasky, Cure 4 the Kids, United StatesCopyright © 2020 Li, Stellpflug, Romanski, Kilgallon, Speck and Saratsis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amanda M. Saratsis, YXNhcmF0c2lzQGx1cmllY2hpbGRyZW5zLm9yZw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.