- 1Pediatric Hematology and Oncology, Department of Pediatrics III, University Hospital Essen, University Duisburg-Essen, Essen, Germany
- 2Pediatric Endocrinology and Diabetology, Department of Pediatrics II, University Hospital Essen, University Duisburg-Essen, Essen, Germany
- 3Department of Endocrinology, Diabetes and Metabolism, University Hospital Essen, University Duisburg-Essen, Essen, Germany
- 4Division of Rare Diseases, Department of Pediatrics, St. Josef-Hospital, Ruhr-University Bochum, Bochum, Germany
Background: Impaired bone health is a late effect of childhood malignancies which can be difficult to detect in juvenile survivors. It may, however, lead to compromised quality of life, or even permanent disability later in life due to osteoporosis, pain or fractures if left untreated. Acute lymphoblastic leukemia (ALL) is the most frequent childhood malignancy with an over 85% five-year survival. ALL and its treatment cause bone alterations in adults, but little information on the bone health status in juvenile survivors is available.
Objective: To report data on skeletal late effects in juvenile survivors of childhood ALL based on a comprehensive assessment of bone health and to assess the influence of a vitamin D deficiency on bone health in this cohort.
Methods: In a single center cross sectional study 128 pediatric patients (11.9 ± 4.76 years) with a mean follow up of 5.88 ± 3.75 years after diagnosis of ALL were recruited. The bone health status of the survivors was assessed based on clinical examination, review of medical records, biochemical and radiographic analyses, by clinical experts. A score which utilized 8 different parameters was formed and used to assess the effect of a vitamin D deficiency on bone health.
Results: In this cohort, 18% of survivors displayed overt osteopathologies as defined by clinical expert assessment. Impaired bone health, defined by at least one pathological screening parameter, was detected in 77%. Despite recommendations for adequate vitamin D supplementation, 15% displayed a vitamin D deficiency associated with hyperparathyroidism. The applied score identified survivors with osteopathologies with high sensitivity and specificity. The median score did not differ between patients without and with severe vitamin D deficiency.
Conclusion: Our findings suggest that impaired bone health and osteopathologies are common skeletal late effects following treatment of childhood ALL. Major contributing factors are BMT, irradiation and older age at diagnosis. Vitamin D deficiency likely accounts for hyperparathyroidism in some patients but does not seem to further affect bone health in this cohort. Survivors of ALL need thorough surveillance to investigate bone health, since bone morbidity is common and still poorly understood. Early detection and appropriate intervention may improve bone health.
Introduction
Over the past decades, long-term survival rates in childhood malignancies and especially in acute lymphoblastic leukemia (ALL) have increased substantially with improved and adapted treatment regimens (1–3). In the growing group of survivors, late effects from cancer and its treatment are an increasing burden compromising health and quality of life. Endocrine late effects in general play a major role in survivorship morbidity (4–7). Bone health has been shown to be impaired in a significant number of children and adults surviving pediatric malignancies (8). Mostoufi-Moab and Halton review the subject for patients with childhood ALL (9). At time of diagnosis there is an incidence of vertebral fractures of up to 16% in the pediatric age group (10) and 72% develop osteonecroses, which often are asymptomatic (11). Patients undergoing treatment for ALL are particularly affected by low bone mineral content and low bone mineral density (BMD) (12, 13). Some studies report this to persist until adulthood (14, 15) while others report a normalization over time, especially in patients without cranial irradiation (16).
Beyond low bone mineral content and fractures many other factors may account for impaired bone health in patients with ALL, such as alterations in bone metabolism, chronic bone pain and stunted growth (17–20). In addition, the hormone vitamin D plays an important role in the maintenance of calcium homeostasis and thereby bone health in childhood and adolescence (21). Given its additional functions such as regulation immunity and cellular differentiation, an optimal vitamin D status may be important especially for patients with cancer (22). Therefore, the assessment of bone health in a population at risk for bone disease should account for all of the above. This is a difficult and complex task, in particular since the assessment of bone metabolism depends on age- and pubertal stage-appropriate interpretation of biochemical surrogate parameters.
In this manuscript, we describe skeletal late effects and vitamin D status in a cohort of juvenile survivors of childhood ALL based on a comprehensive assessment of bone health.
Methods
Patients
All patients who had received chemotherapy for acute lymphoblastic leukemia in the past and were either undergoing maintenance therapy or follow-up at the oncology outpatient clinic from August 2012 until August 2014 were invited to participate in this single-center cross-sectional study. Patients were recruited year-round to balance seasonal influences.
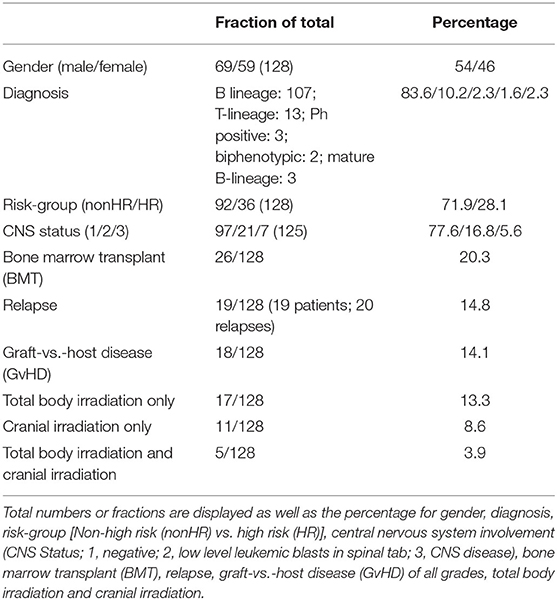
Data regarding ALL stratification and treatment of the patients is presented in Table 1. 134 patients were eligible to participate. Of those, 6 declined for personal reasons. Of the recruited 128 patients, 69 were male, and 59 were female. The patients had the following diagnoses: B-lineage ALL (n = 107), T-lineage ALL (n = 13), Philadelphia chromosome-positive ALL (n = 3), biphenotypic ALL (n = 2), mature B-lineage ALL (n = 3). The median initial blast count at diagnosis was around 4,000/μl with a wide range between 0 and 600,000/μl. All patients were treated as per the respective ALL-BFM protocols (23). Accordingly, 26 patients underwent allogeneic bone marrow transplantation (BMT). 11 patients underwent cranial irradiation only, 17 total body irradiation only and 5 patients received both treatments.
Clinical Parameters and Questionnaire
Clinical and anamnestic parameters were assessed as previously described in detail (24). Briefly, a physical examination, including determination of weight, height, and pubertal status was performed. Patients were also asked to complete a standardized questionnaire regarding vitamin D, calcium and nutritional supplement intake, screen hours and hours of physical activity per day (24). In addition, the patients were asked for presence of bone pain in the form of regularly occurring (on more than half of the days in the last month), spontaneous, back pain or exercise related knee pain. Pubertal development was assessed by a pediatric endocrinologist according to Tanner staging. Standard deviation scores (SDS) for pubertal development were calculated using “Puberty Plot Web Application” by Stef van Buuren (http://vps.stefvanbuuren.nl/puberty/) [accessed, May 12, 2020, (25)]. The program is based on a Dutch population study (26) and calculates pubertal SDS for breast development, pubic hair stage and testicular volume. While the calculation is based on the Tanner stages for breast development and pubic hair stage, the application allows only discrete measures for testicular volume (i.e., 2, 3, 4, 8, 12, 16, 20, and 25 ml). Therefore, volume measurements that fell in between these values, which were determined using an orchidometer, were rounded to the closest possible measurement (e.g., a measurement of 6 ml was rounded to 8 ml for this study). Skeletal age was determined by an experienced pediatric radiologist according to the method of Greulich and Pyle using X-ray images of the left hand (27).
Chart Review
The following parameters were obtained from the patients charts: The subtype of ALL including the CNS status, the risk stratification according to the individual patient's protocol (standard/medium/high risk), the cumulative dosage of chemotherapy as well as type and dosage of irradiation dosage patient “as treated” and the steroid dosage as prednisone equivalent dosage (28). In patients who underwent a BMT the occurrence of graft vs. host disease (GvHD) and its grade, length, and treatment was documented.
Upon chart review, fractures which had occurred after the diagnosis of ALL were recorded. Vertebral fractures or more than two subsequent fractures of long bones were categorized as “pathological fractures.”
Laboratory Tests
The following biochemical parameters of growth, pubertal status, bone turnover, and vitamin D metabolism were assessed in serum or plasma samples as part of the routine diagnostic laboratory workup in the central laboratory of the University Hospital Essen: 25-OH vitamin D (ng/ml); 1,25-(OH)2 vitamin D (pg/ml); serum phosphate (mmol/l), serum calcium (mmol/l), albumin (g/dl), total serum alkaline phosphatase, TSAP (U/l); bone-specific alkaline phosphatase, BAP (U/l); insulin-like growth factor-1, IGF-1 (ng/ml), PTH (pg/ml), and Osteocalcin (ng/ml).
Additionally, the urinary calcium to creatinine ratio (mg/mg) as well as markers of bone resorption including N-terminal telopeptide, NTX (nmol bone collagen equivalent (BCE)/mmol creatinine) and deoxypyridinoline, DPD (mg/g creatinine) were assessed in spot urine samples. Pediatric reference ranges were available and applied for all parameters. Serum IGF-1 levels were expressed as SDS values, according to age and sex, based on the data from Blum and Breier (29). To calculate the IGF-1 SDS, we used the software tool “SDSEasy,” (Mediagnost, Reutlingen, Germany).
Bone Densitometry
BMD was examined via dual-energy X-ray absorptiometry (DXA) (Lunar Prodigy, GE-Healthcare, Madison, WI, USA) in a subgroup of patients. BMD was assessed at the lumbar spine (L1–L4; anteroposterior view) and the left femoral neck. Z-scores (DXA Z) were calculated for the lumbar spine measurements based on age specific normal values (30, 31). A single investigator blinded to the clinical status of the patients was responsible for all BMD measurements. Height-adjusted Z-scores (HAZ) from DXA were calculated as described previously (32). The Bone Health Index (BHI) and its SDS (BHI-SDS) was calculated using the software BoneXpert from indices of three metacarpal bones as a parameter to approximate bone density from X-rays of the left hand (33).
Development of Score and Expert Opinion
Definition: The terms “osteopathology” and “impaired bone health” are being used in this manuscript to describe different levels of skeletal late effects. For the purpose of this manuscript the term “osteopathology” refers to overt bone disease and the term “impaired bone health” refers to a condition with at least one pathological reading of the many assessed parameters of bone health. Bone health was assessed jointly by two clinical experts (C.G., B.H.). Both of whom are experienced pediatric endocrinologists. For information on the assessment process see below and Supplementary Material 1.
The core dataset for each patient comprised of 121 variables of which 56 were used for the expert assessment of the bone health status (Supplementary Material 1). The experts reviewed history, clinical and laboratory data of every patient to assign the bone health status into one of five categories: healthy, most likely healthy, osteopathology, most likely osteopathology, unable to determine. See Supplementary Material 2 for schematic representation of the stratification algorithm.
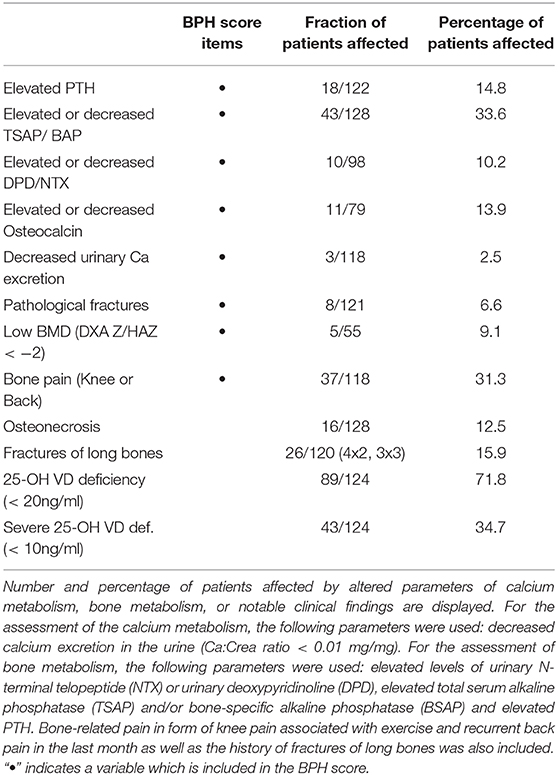
During the data analysis the challenge to define the status of bone health in individual patients due to a lack of a suitable scoring system became apparent. Therefore, for the purpose of this study, a subset of variables was used to assemble a score which we named Bone Pathology Harbinger—BPH. The variables were chosen based on the availability of age-specific reference ranges and/or evidence for osteopathology (e.g., pathological fracture). The following variables were included and scored if outside the normal range/pathological: (serum/plasma/urine) levels of (1) parathyroid hormone, (2) osteocalcin, (3) TSAP or BAP, (4) DPD, (5) urinary calcium to creatinine ratio, (6) pathological fractures after diagnosis of ALL, (7) knee pain on exercise or spontaneous back pain, and (8) bone mineral density reading (DXA Z < -2 in patients with normal height, or HAZ < -2 in patients with short stature). The sum of scored points divided by the number of possible points per patient represented the BPH score. The BPH therefore had a range from 0 (no pathology) to 1 (maximal pathology). It was calculated for 125 patients in whom at least 5 out of the 8 items were available.
Statistics
Statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC, USA) and PRISM for MAC 7.0 (GraphPad Software, Inc., La Jolla, CA, USA). Values are expressed as the mean +/- standard deviation (SD) and range unless stated otherwise. As in most of the variables normal distribution could not be assumed, associations between single variables were described by Spearman correlation coefficient. Differences in continuous variables between the groups were tested using the Mann-Whitney U-test for two-group comparisons, and the Kruskal-Wallis tests for more than two groups. Group differences in categorical variables were tested using the chi-square test statistics. The receiver operating curve (ROC) was calculated using the SAS macro as presented by Harris (34). For all tests, statistical significance was presumed at P < 0.05.
Results
Descriptive Statistics and Parameters of Bone Health
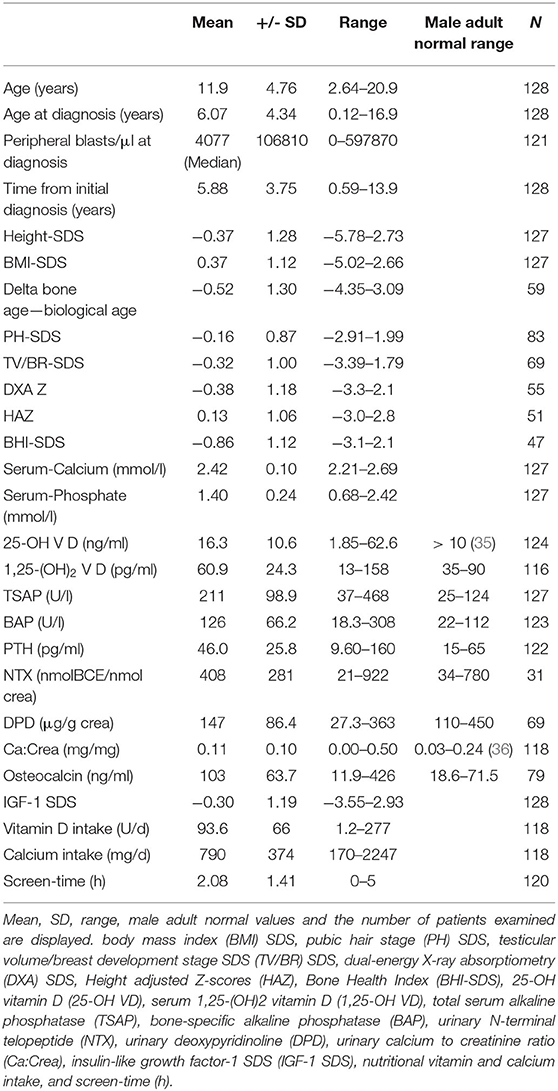
The mean age of the patients at follow up was 11.9 ± 4.76 years (2.6–20.9). Mean age at diagnosis was 6.07 ± 4.34 years (0.12–16.9), making for a mean of 5.88 ± 3.75 years after first diagnosis of ALL (range 0.59–13.9).
Eight patients of the cohort were in the second year of maintenance therapy. These patients were 1.33 years (±0.27) after initial diagnosis. The other 120 survivors were 4.59 years (±3.39 years) after the end of treatment (4.55 years ± 3.5 after end of chemotherapy; 4.72 ± 2.9 after BMT).
The mean height SDS was −0.37 ± 1.28 (-5.78–2.73), BMI SDS 0.37 ± 1.12 (-5.02–2.66). The Tanner stage SDS for pubic hair development and testicular volume/breast development was −0.16 ± 0.87 (−2.91–1.99) and −0.32 ± 1.0 (−3.39–1.79), respectively.
The questionnaire was completed by 120 of 128 survivors. Patients spent an average of 2.08 ± 1.41 h (0–5) of daily screen time. Of the patients three stated that they would spend most of the day non-ambulatory (sitting or lying down). Thirteen stated physical activity of up to three hours. One hundred four were physically active more than 3 h per day.
More detailed descriptive statistics of the 128 patients are displayed in Table 2. Of note, in Table 2 the male adult reference range is being displayed for the readers information. However, the applicable age-, sex-, and pubertal status adjusted reference ranges were used for the assessments of the individual data.
The frequency of aberrant biochemical and radiological findings on bone health are summarized in Table 3. In total in 77 % of patients any kind of bone health impairment was detected.
Bone Health According to Gender and Age
The mean DXA Z was significantly lower in female patients than in males (−1.06 vs. −0.08; SD 1.1 for both, P = 0.0035). This difference persisted when comparing the height adjusted Z-scores (HAZ, −0.44 ± 1.12 vs. 0.39 ± 0.94, P = 0.02). Osteonecrosis was present more often in female patients (12 vs. 4, P = 0.07). As expected, patients with osteonecrosis were older at diagnosis than patients without osteonecrosis 10.8 ± 4.39 years (3.25–16.9) vs. 5.39 ± 3.90 years (0.12–16.6). No further difference in bone health related parameters was detectable between male and female patients.
Quantification of Bone Health via Expert Opinion and Bone Pathology Harbinger (BPH)
An expert opinion on the status of bone health was obtained for all patients. Thirty nine patients were labeled as healthy or most likely healthy and 23 patients as affected with osteopathologies or most likely affected with osteopathologies. In 63 patients a definite classification based on the available parameters by the expert was not possible. Of these, 46 patients were not categorizable due to unconcise or conflicting findings and data, 12 patients had osteonecroses but did not display any sign for osteopathologies otherwise. In five patients data was incomplete and therefore insufficient to assign them to any category.
The median score of the BPH in the entire cohort was 0.14 (0–0.67; 25th percentile = 0, 75th percentile = 0.25; n = 125). In patients who were found to have (most likely) osteopathologies by the expert opinion, the BPH score was elevated compared to the patients labeled as (most likely) healthy (0.32 ± 0.17 vs. 0.06 ± 0.07; P < 0.001, Figure 1). To evaluate specificity and sensitivity of the BPH score for the study cohort, it was matched to the expert opinion. The resulting receiver operating curve features an area under the curve of 0.97. A cutoff of 0.16 for the BPH accounts for a sensitivity of 82 % and a specificity of 90 %.
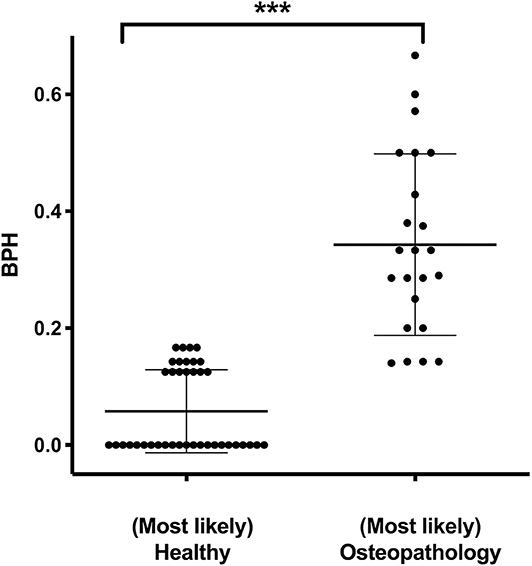
Figure 1. Expert opinion and the Bone Pathology Harbinger (BPH). BPH scores in patients who were assigned to the categories (most likely) healthy (n = 39) or (most likely) osteopathology (n = 23) by independent expert opinion. Lines indicate mean and standard deviation. Statistically significant differences between the groups, determined via Mann-Whitney test, are indicated with asterisks (***P < 0.001).
Bone Health and Calcium/Vitamin D Metabolism
To evaluate the role of a 25-OH vitamin D deficiency for bone health in this cohort, we compared BPH scores in survivors with severe vitamin D deficiency [as defined by Holick, (35)], to those with sufficient levels. BPH scores did not differ between the groups (0.16 ± 0.14 vs. 0.17 ± 0.20, P = 0.35, Figure 2A). However, in the group of patients with sufficient vitamin D levels the calcium:creatinine ratios in urine were higher, indicating adequate calcium stores, compared to the group of patients who displayed severe vitamin D deficiency (0.13 ± 0.09 vs. 0.08 ± 0.08 mg/mg, P = 0.01, Figure 2B).
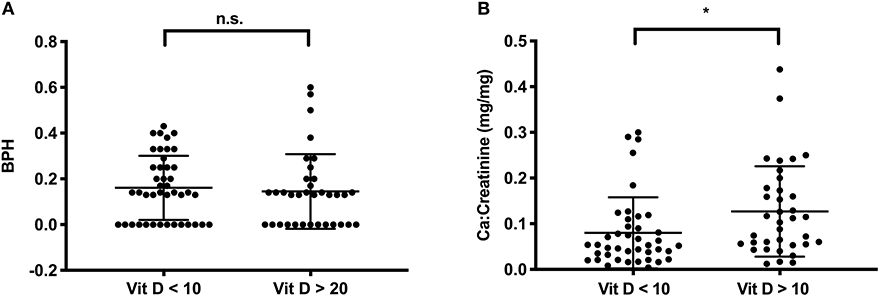
Figure 2. Vitamin D (in ng/ml) and calcium metabolism in survivors. (A) The BPH score is not different in survivors with severe Vitamin D deficiency (Vit D < 10 ng/ml, n = 43) or with sufficient vitamin D levels (Vit D > 20 ng/ml, n = 35); (B) Urinary Ca/Creatinine in the same group of patients. Lines indicate mean and standard deviation. Statistically significant differences between the groups, determined via Mann-Whitney test, are indicated with asterisks (*P < 0.05).
Bone Health and Risk Groups/Treatments Groups and Age at Diagnosis
Treatment stratification (standard and medium vs. high risk) did not affect the BPH score outcome (0.15 ± 0.15 vs. 0.19 ± 0.16, P = 0.25). However, the score was significantly higher in survivors after BMT compared to other survivors (0.22 ± 0.18 vs. 0.14 ± 0.15, P = 0.04, Figure 3A). This corresponds to a higher score in patients after total body irradiation compared with patients without irradiation (0.24 ± 0.19 vs. 0.14 ± 0.14, P = 0.03, Figure 3B).
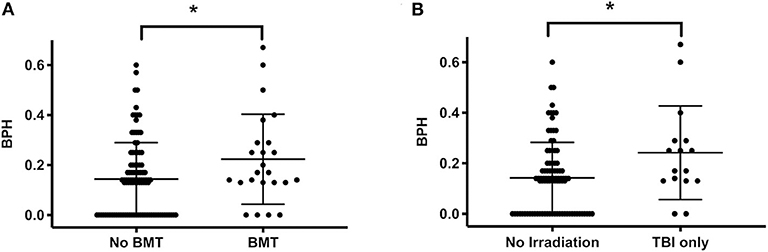
Figure 3. BPH score and bone marrow transplantation or total body irradiation. (A) The score in patients subjected to BMT (n = 24) is higher than in patients without BMT (n = 101, indicating impaired bone health following BMT). (B) Also, the score was higher in patients who underwent TBI (n = 16) vs. those without any irradiation (n = 92). Lines indicate mean and standard deviation. Statistically significant differences between the groups, determined via Mann-Whitney test, are indicated with asterisks (*P < 0.05).
The age at diagnosis differed between the groups labeled as (most likely) healthy vs. (most likely) osteopathologies. The healthy group being significantly younger at initial diagnosis (4.75 ± 3.36 vs. 7.91 ± 4.51 years, P = 0.001).
Prednisone equivalent dosages varied markedly between patients, depending on intensity of treatment, occurrence of GvHD, etc. However, the cumulative dose of steroids did not correlate with score values (r = 0.08, P = 0.4, Figure 4). Also, the cumulative prednisone dose was not different between the group of patients labeled as affected by (most likely) osteopathology compared to the group labeled as (most likely) healthy (3,799 ± 2,384 mg vs. 3,829 ± 1,364 mg, P = 0.52). In addition, no correlation between bone health score and the dose of the putative bone damaging agent methotrexate (r = 0.01, P = 0.96) was found.
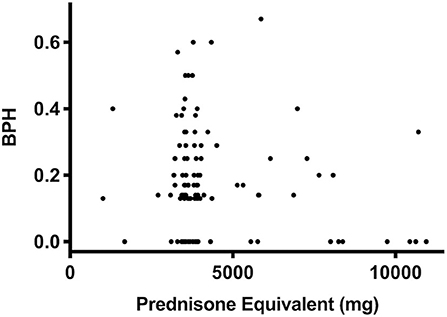
Figure 4. Prednisone equivalent dosage and BPH score as an indicator of bone health. In a bivariate regression the cumulative dose of prednisone equivalent did not correlate with score values (P = 0.4, r = 0.08, n = 123) in survivors of ALL.
Focusing on fractures, the total prednisone equivalent dosage in patients with any fractures (n = 33) vs. no fractures did not differ (4,317 ± 1,708 mg vs. 4,487 ± 2,103 mg, P = 0.52), neither was a difference for pathological fractures (n = 8) vs. no pathological fractures detectable (4,606 ± 3,303 mg vs. 4,347 ± 1,720 mg, P = 0.31).
Discussion
In juvenile survivors of ALL, a reduced bone mineral density and the occurrence of osteonecrosis have been previously described as indicators of impaired bone health (9, 37, 38). While the progression to overt osteopathologies may be preventable in many cases, sequelae from impaired bone health may contribute to increased morbidity and loss of quality of life in adulthood (as reviewed recently by 8). Therefore, efforts for detection and prevention of impaired bone health should be intensified in juvenile survivors. Identifying survivors at risk for impaired bone health requires not only the measurement of BMD but the evaluation of a spectrum of metabolic, hormonal, physical and lifestyle parameters. We aimed to describe the status of bone health thoroughly in this cross-sectional study in juvenile survivors of ALL.
To allow for a comprehensive assessment of bone health for the purpose of this study we used an expert opinion and compared it to a screening score (BPH). The score incorporates clinical, biochemical and radiographic parameters with defined reference values, which can easily be obtained in any follow-up setting. We found that the information of the score corresponded well to the evaluation of experienced experts, with a cutoff of 0.16 being highly specific and sensitively associated with impaired bone health. Such a score may become a helpful tool for pediatric oncologists to refer the patient to a pediatric bone specialist. Further modeling and validation in other cohorts of survivors and prospective long-term observation is therefore needed and well worth pursuing.
The hormone vitamin D and calcium as a nutritional component are known to be essential for bone metabolism (21, 39) and their deficiency is associated with impaired bone health in children and adults (40, 41). In this study, we detected a higher rate of severe vitamin D deficiency than expected given the known prevalence in healthy German children and adolescents (42). In this study, vitamin D deficiency was associated with a lower Ca/creatinine ratio and in 15% of survivors with (secondary) hyperparathyroidism, indicating a relevant negative calcium balance. Interestingly though, no difference in the BPH in patients with vitamin D deficiency or with vitamin D sufficient levels was observed. This is in line with the understanding that it is not the vitamin D deficiency itself, but the calcium deficiency, which is of relevance to bone health in children (39). Based on the results of this study, we have changed our routine clinical practice to a more frequent assessment of 25-OH vitamin D levels and a rigorous correction of Vitamin D deficiency in survivors of ALL.
However, in survivors of ALL, the finding of vitamin D deficiency and hyperparathyroidism is of importance in the context of the ongoing debate whether elevated PTH levels may increase the risk for relapse in hematological and non-hematological malignancies (43–45). One possible mechanism is a disruption of the bone marrow niche's homeostasis (46). Also, a recent study described lower overall survival of patients with suboptimal vitamin D levels after BMT (47). Although none of these findings prove causality, the vitamin D and PTH levels should be tightly controlled for in follow-up clinics and a routine vitamin D supplementation in survivors should be aimed for (48–50).
Fracture risk in this population was comparable to the report of te Winkel et al. who described a 17% 3 year risk in a population of 672 ALL survivors (51), but lower than that reported earlier by Nysom et al. who reported up to 55% fractures in a cohort over a median follow-up of 7.6 years after completion of therapy (52). DXA Z-scores were comparable to data, recently published by Inaba et al. (13). In general, studies reporting BMDs in closer proximity to the time of diagnosis describe lower BMDs (51). This indicates a time-dependent improvement of BMD after completion of oncological therapy (53). Lower Z-scores may also in part be explained by lower body height in the (CNS irradiated) population and could possibly be corrected by height-adapted re-calculation of the BMD [as discussed by Mäkitie et al. (15)]. The height- and age-adapted values for the Bone health Index (BHI-SDS) of BoneXpert derived from x-rays of the left hand (32) in this cross-sectional study do not indicate general impaired mineralization in the presented cohort. Nevertheless, a sufficient peak bone mass during and following pubertal development should be aimed for in all children with cancer (12).
The overall rate of osteonecroses in this cohort was not different from reported rates (54, 55). An increasing prevalence of osteonecroses with older age at diagnosis was observed in the present study like in others (56).
Frequent back pain and knee pain after exercise can be indicative of impaired bone health (57). About one third of the survivors in the present study reported recurrent bone pain in their questionaire.
Even though the intensity of therapy may vary markedly depending on the stratification into different risk groups, we could not attribute any differences in bone health status and BPH score on risk groups. In particular, it was surprising to see that neither the cumulative dose of prednisone nor the cumulative doses of methotrexate influenced the score values in this cohort. This is in contrast to the findings by Ward et al. (58) who showed that higher daily doses of prednisone proved a higher hazard for the development of vertebral fractures or non-vertebral fractures. However, the timing of these studies differs, since the present cohort exclusively consists of patients in follow-up clinic. It is likely that the vertebral fracture rate that is reported by Ward et al. as an early bone affection in ALL is present in the German population as well—however since no screening for vertebral fractures during intensive therapy is implemented, many of the lesser symptomatic fractures may not be reported, and may not be detectable at a later point in time due to healing/reshaping of the vertebrae. As expected, however, BMT as well as any irradiation was accompanied by higher BPH scores.
Limitations of this study are its cross-sectional design and the relatively young mean age of the cohort. With eight patients at the end of maintenance therapy, the cohort does not solely consist of survivors. Therefore, we are following up this group of survivors throughout puberty and through transition into adult follow up care. This will help to gain more insight into the development of the various outcome parameters of bone health with age.
The challenge to define the complex status of bone health led us to introduce the BPH score for the purpose of this study. In order to establish the score as a generalizable instrument for clinical use, however, it will have to be validated on at least one, best multiple, comparable data sets and preferably on a larger number of survivors. Parts of this larger dataset could be used as a training data set to improve the score. Here, a penalized regression model, like a Lasso model, could help selecting and weighing variables (59).
Conclusion
In this cross-sectional study 18% of the adolescent survivors of ALL displayed overt osteopathologies by clinical expert assessment and 77% of the study population displayed evidence of impaired bone health in at least one of the investigated parameters. Major contributing factors are BMT, irradiation and older age of initial diagnosis. Vitamin D deficiency itself likely accounts for secondary hyperparathyroidism in some patients but was not associated with aggravated impairment otherwise.
Patients with ALL need thorough surveillance in follow up clinics to investigate bone health, since bone morbidity is common but still poorly understood. Vitamin D deficiency and secondary hyperparathyroidism are easy to detect and to avoid by supplementation. Early detection of impaired bone health and appropriate intervention may improve bone health in survivors. A bone health score may be a suitable tool for this endeavor. More research and prospective studies on this subject are much-needed.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Ethics Statement
The studies involving human participants were reviewed and approved by Research Ethics Committee of the Medical Faculty, University of Duisburg-Essen # 12-4966-BO. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin and eligible patients.
Author Contributions
CG and MS: study conception and design. PH, MS, CK, and MM: acquisition of data. BH, CG, and MS: analysis and interpretation of data. MS, CG, and PH: drafting and revising the manuscript. All authors contributed to the article and approved the submitted version.
Funding
MS was supported by an IFORES stipend from the Medical Faculty of the University of Duisburg Essen.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank the survivors and families who participated in this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2020.00509/full#supplementary-material
Abbreviations
25-OH VD, Serum levels of 25-OH vitamin D; 1,25-(OH)2 VD, 1,25-(OH)2 vitamin D; BSAP, Bone-specific alkaline phosphatase; BMI, Body mass index; BMT, Allogeneic Bone Marrow Transplantation; BPH, Bone Pathology Harbinger; Ca:crea, Urinary calcium to creatinine ratio; DPD, Urinary deoxypyridinoline; FSH, Follicle-stimulating hormone; GvHD, Graft vs. Host Disease; IGF-1, Insulin-like growth factor-1; NTX, Urinary N-telopeptide; PTH, Parathyroid hormone; SD, Standard deviation; SDS, Standard deviation score; TSAP, Total serum alkaline phosphatase; TSH, Thyroid-stimulating hormone.
References
1. Pui C-H, Yang JJ, Hunger SP, Pieters R, Schrappe M, Biondi A, et al. Childhood acute lymphoblastic leukemia: progress through collaboration. J Clin Oncol. (2015) 33:2938–48. doi: 10.1200/JCO.2014.59.1636
2. Hiroto I, Greaves M, Mullighan CG. Acute lymphoblastic leukemia. Lancet. (2013) 381:62187–4. doi: 10.1016/S0140-6736(12)62187-4
3. Pui C-H, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. (2006) 354:166–78. doi: 10.1056/NEJMra052603
4. Brignardello E, Felicetti F, Castiglione A, Chiabotto P, Corrias A, Fagioli F, et al. Endocrine health conditions in adult survivors of childhood cancer: the need for specialized adult-focused follow-up clinics. Eur J Endocrinol. (2013) 168:465–72. doi: 10.1530/EJE-12-1043
5. Sklar CA, Antal Z, Chemaitilly W, Cohen LE, Follin C, Meacham LR, et al. Hypothalamic-Pituitary and growth disorders in survivors of childhood cancer: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. (2018) 103:1–24. doi: 10.1210/jc.2018-01175
6. Jensen MV, Rugbjerg K, De Fine Licht S, Johansen C, Schmiegelow K, Andersen KK, et al. Endocrine late effects in survivors of cancer in adolescence and young adulthood a Danish population-Based cohort study + invited commentary + supplemental content. JAMA Netw Open JAMA Netw Open. (2018) 11:180349–180349. doi: 10.1001/jamanetworkopen.2018.0349
7. Barnes N, Chemaitilly W. Endocrinopathies in survivors of childhood neoplasia. Front Pediatr. (2014) 2:1–12. doi: 10.3389/fped.2014.00101
8. Marcucci G, Beltrami G, Tamburini A, Body JJ, Confavreux CB, Hadji P, et al. Bone health in childhood cancer: review of the literature and recommendations for the management of bone health in childhood cancer survivors. Ann Oncol. (2019) 30:908–20. doi: 10.1093/annonc/mdz120
9. Mostoufi-Moab S, Halton J. Bone morbidity in childhood leukemia: epidemiology, mechanisms, diagnosis, and treatment. Curr Osteoporos Rep. (2014) 12:300–12. doi: 10.1007/s11914-014-0222-3
10. Halton J, Gaboury I, Grant R, Alos N, Cummings EA, Matzinger M, et al. Advanced vertebral fracture among newly diagnosed children with acute lymphoblastic leukemia: results of the canadian steroid-associated osteoporosis in the pediatric population (STOPP) research program. J Bone Miner Res. (2009) 24:1326–34. doi: 10.1359/jbmr.090202
11. Kawedia JD, Kaste SC, Pei D, Panetta JC, Cai X, Cheng C, et al. Pharmacokinetic, pharmacodynamic, and pharmacogenetic determinants of osteonecrosis in children with acute lymphoblastic leukemia. Blood. (2011) 117:2340–7. doi: 10.1182/blood-2010-10-311969
12. van der Sluis I, van den Heuvel-Eibrink M. Osteoporosis in children with cancer. Pediatr Blood Cancer. (2008) 50:474–8. doi: 10.1002/pbc.21407
13. Inaba H, Cao X, Han AQ, Panetta JC, Ness KK, Metzger ML, et al. Bone mineral density in children with acute lymphoblastic leukemia. Cancer. (2018) 124:1025–35. doi: 10.1002/cncr.31184
14. Thomas IH, Donohue JE, Ness KK, Dengel DR, Baker KS, Gurney JG. Bone mineral density in young adult survivors of acute lymphoblastic leukemia. Cancer. (2008) 113:3248–56. doi: 10.1002/cncr.23912
15. Mäkitie O, Heikkinen R, Toiviainen-Salo S, Henriksson M, Puukko-Viertomies L-R, Jahnukainen K. Long-term skeletal consequences of childhood acute lymphoblastic leukemia in adult males: a cohort study. Eur J Endocrinol. (2013) 168:281–8. doi: 10.1530/EJE-12-0702
16. Brennan BMD, Mughal Z, Roberts SA, Ward K, Shalet SM, Eden TOB, et al. Bone mineral density in childhood survivors of acute lymphoblastic leukemia treated without cranial irradiation. J Clin Endocrinol Metab. (2005) 90:689–94. doi: 10.1210/jc.2004-1476
17. Halton JM, Atkinson SA, Fraher L, Webber C, Gill GJ, Dawson S, et al. Altered mineral metabolism and bone mass in children during treatment for acute lymphoblastic leukemia. J Bone Miner Res. (1996) 11:1774–83. doi: 10.1002/jbmr.5650111122
18. Argüelles B, Barrios V, Pozo J, Muñoz MT, Argente J. Modifications of growth velocity and the insulin-like growth factor system in children with acute lymphoblastic leukemia: a longitudinal study. J Clin Endocrinol Metab. (2000) 85:4087–92. doi: 10.1210/jc.85.11.4087
19. Davies JH, Evans BAJ, Jenney MEM, Gregory JW. Skeletal morbidity in childhood acute lymphoblastic leukaemia. Clin Endocrinol (Oxf). (2005) 63:1–9. doi: 10.1111/j.1365-2265.2005.02263.x
20. Watsky MA, Carbone LD, An Q, Cheng C, Lovorn EA, Hudson MM, et al. Bone turnover in long-term survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. (2014) 61:1451–6. doi: 10.1002/pbc.25025
21. Saggese G, Vierucci F, Boot AM, Czech-Kowalska J, Weber G, Camargo CA, et al. Vitamin d in childhood and adolescence: an expert position statement. Eur J Pediatr. (2015) 174:565–76. doi: 10.1007/s00431-015-2524-6
22. Jackmann N, Mäkitie O, Harila-Saari A, Gustafsson J, Nezirevic Dernroth D, Frisk P. Vitamin d status in children with leukemia, its predictors, and association with outcome. Pediatr Blood Cancer. (2020) 67:1–10. doi: 10.1002/pbc.28163
23. Möricke A, Zimmermann M, Valsecchi MG, Stanulla M, Biondi A, Mann G, et al. Dexamethasone vs prednisone in induction treatment of pediatric aLL: results of the randomized trial aIEOP-BFM aLL (2000). Blood. (2016) 127:2101–12. doi: 10.1182/blood-2015-09-670729
24. Schündeln MM, Goretzki SC, Hauffa PK, Wieland R, Bauer J, Baeder L, et al. Impairment of bone health in pediatric patients with hemolytic anemia. PLoS One. (2014) 9:e108400. doi: 10.1371/journal.pone.0108400
25. van Buuren S, Ooms J. Stage line diagram : an age-conditional reference diagram for tracking development. Stat Med. (2009) 28:1569–79. doi: 10.1002/sim.3567
26. Mul D, Fredriks AM, van Buuren S, Oostdijk W, Verloove-Vanhorick SP, Wit JM. Pubertal development in the netherlands 1965-1997. Pediatr Res. (2001) 50:479–86. doi: 10.1203/00006450-200110000-00010
27. Greulich W, Pyle S. Radiographic Atlas of Skeletal Development of the Hand and Wrist. 2nd ed. Stanford, CA: Stanford University Press (1959). doi: 10.1097/00000441-195909000-00030
28. Henzen C. Therapie mit glukokortikoiden : risiken und nebenwirkungen. Schweizerisches Medizin-Forum. (2003) 19:442–6. doi: 10.4414/smf.2003.04866
30. Ellis KJ, Shypailo RJ, Hardin DS, Perez MD, Motil KJ, Wong WW, et al. Z score prediction model for assessment of bone mineral content in pediatric diseases. J Bone Miner Res. (2001) 16:1658–64. doi: 10.1359/jbmr.2001.16.9.1658
31. GE_Healthcare. Lunar enCORE Pediatrics Reference Data. Available online at: http://www3.gehealthcare.com/en/products/categories/bone_health/pediatrics. (2010).
32. Schündeln MM, Marschke L, Bauer JJ, Hauffa PK, Schweiger B, Führer-Sakel D, et al. A piece of the puzzle: the bone health index of the boneXpert software reflects cortical bone mineral density in pediatric and adolescent patients. PLoS ONE. (2016) 11:e0151936. doi: 10.1371/journal.pone.0151936
33. Thodberg HH, van Rijn RR, Tanaka T, Martin DD, Kreiborg S. A paediatric bone index derived by automated radiogrammetry. Osteoporos Int. (2010) 21:1391–400. doi: 10.1007/s00198-009-1085-9
35. Holick MF. High prevalence of vitamin d Inadequacy and implications for health - proQuest. Mayo Clin Proc. (2006) 81:353–73. doi: 10.4065/81.3.353
36. Matos V, Melle G Van, Boulat O, Markert M, Bachmann C, Guignard J. Urinary phosphate/creatinine, calcium/creatinine, and magnesium/creatinine ratios in a healthy pediatric population. j pediatr. (1997) 131:252–7. doi: 10.1016/S0022-3476(97)70162-8
37. Mandel K, Atkinson S, Barr RD, Pencharz P. Skeletal morbidity in childhood acute lymphoblastic leukemia. J Clin Oncol. (2004) 22:1215–21. doi: 10.1200/JCO.2004.04.199
38. Boot AM, van den Heuvel-Eibrink MM, Hählen K, Krenning EP, de Muinck Keizer-Schrama SM. Bone mineral density in children with acute lymphoblastic leukaemia. Eur J Cancer. (1999) 35:1693–7. doi: 10.1016/S0959-8049(99)00143-4
39. Gou GH, Tseng FJ, Wang SH, Chen PJ, Shyu JF, Pan RY. Nutritional factors associated with femoral neck bone mineral density in children and adolescents. BMC Musculoskelet Disord. (2019) 20:1–10. doi: 10.1186/s12891-019-2901-9
40. Greene DA, Naughton GA. Calcium and vitamin-D supplementation on bone structural properties in peripubertal female identical twins: a randomised controlled trial. Osteoporos Int. (2011) 22:489–98. doi: 10.1007/s00198-010-1317-z
41. Kalkwarf HJ, Khoury JC, Lanphear BP. Milk intake during childhood and adolescence, adult bone density, and osteoporotic fractures in uS women 1 - 3. (2003). (July). doi: 10.1093/ajcn/77.1.257
42. Dortschy R, Schaffrath Rosario A, Scheidt-Nave C, Thierfelder W, Thamm M, Gutsche J, et al. Bevölkerungsbezogene verteilungswerte ausgewählter laborparameter aus der studie zur gesundheit von kindern und jugendlichen in deutschland [KiGGS]. Beiträge zur Gesundheitsberichtserstattung des Bundes. (2009) 92–104. doi: 10.25646/3144
43. Radujkovic A, Kordelas L, Krzykalla J, Beelen DW, Benner A, Lehners N, et al. Pretransplant vitamin d Deficiency is associated with higher relapse rates in patients allografted for myeloid malignancies. J Clin Oncol. (2017) 35:JCO.2017.73.008. doi: 10.1200/JCO.2017.73.0085
44. Michels KB, Xue F, Brandt L, Ekbom A. Hyperparathyroidism and subsequent incidence of breast cancer. Int J Cancer. (2004) 110:449–51. doi: 10.1002/ijc.20155
45. Li M, Chen P, Li J, Chu R, Xie D, Wang H. Review: the impacts of circulating 25-Hydroxyvitamin d Levels on cancer patient outcomes: a Systematic review and meta-Analysis. J Clin Endocrinol Metab. (2014) 99:2327–36. doi: 10.1210/jc.2013-4320
46. Reagan MR, Rosen CJ. Navigating the bone marrow niche: translational insights and cancer-driven dysfunction. Nat Rev Rheumatol. (2015) 12:154–68. doi: 10.1038/nrrheum.2015.160
47. Beebe K, Magee K, McNulty A, Stahlecker J, Salzberg D, Miller H, et al. Vitamin d deficiency and outcomes in pediatric hematopoietic stem cell transplantation. Pediatr Blood Cancer. (2018) 65:2. doi: 10.1002/pbc.26817
48. Keum N, Giovannucci E. Vitamin d supplements and cancer incidence and mortality: a meta-analysis. Br J Cancer. (2014) 111:976–80. doi: 10.1038/bjc.2014.294
49. Chowdhury R, Kunutsor S, Vitezova A, Oliver-Williams C, Chowdhury S, Kiefte-De-Jong JC, et al. Vitamin d and risk of cause specific death: systematic review and meta-analysis of observational cohort and randomised intervention studies. BMJ. (2014) 348:1–13. doi: 10.1136/bmj.g1903
50. Munns CF, Shaw N, Kiely M, Specker BL, Thacher TD, Ozono K, et al. Global consensus recommendations on prevention and management of nutritional rickets. J Clin Endocrinol Metab. (2016) 101:83–106. doi: 10.1159/000443136
51. te Winkel ML, Pieters R, Hop WCJ, Roos JC, Bökkerink JPM, Leeuw J a, et al. Bone mineral density at diagnosis determines fracture rate in children with acute lymphoblastic leukemia treated according to the dCOG-ALL9 protocol. Bone. (2014) 59:223–8. doi: 10.1016/j.bone.2013.11.017
52. Nysom K, Holm K, Michaelsen KF, Hertz H, Müller J, Mølgaard C. Bone mass after treatment for acute lymphoblastic leukemia in childhood. J Clin Oncol. (1998) 16:3752–60. doi: 10.1200/JCO.1998.16.12.3752
53. Mostoufi-Moab S, Brodsky J, Isaacoff EJ, Tsampalieros A, Ginsberg JP, Zemel B, et al. Longitudinal assessment of bone density and structure in childhood survivors of acute lymphoblastic leukemia without cranial radiation. J Clin Endocrinol Metab. (2012) 97:3584–92. doi: 10.1210/jc.2012-2393
54. Kunstreich M, Kummer S, Laws H-J, Borkhardt A, Kuhlen M. Osteonecrosis in children with acute lymphoblastic leukemia. Haematologica. (2016) 28:90–107. doi: 10.3324/haematol.2016.147595
55. Kuhlen M, Kunstreich M, Krull K, Meisel R, Borkhardt A. Osteonecrosis in children and adolescents with acute lymphoblastic leukemia: a therapeutic challenge. Blood Adv. (2017) 1:981–94. doi: 10.1182/bloodadvances.2017007286
56. Bürger B, Beier R, Zimmermann M, Beck JD, Reiter A, Schrappe M. Osteonecrosis: a treatment related toxicity in childhood acute lymphoblastic leukemia (ALL)–experiences from trial aLL-BFM 95. Pediatr Blood Cancer. (2005) 44(September 2004):220–5. doi: 10.1002/pbc.20244
57. Neville KA, Cohn RJ. Bone health in survivors of childhood cancer. Lancet Diabetes Endocrinol. (2015) 8587:10–1. doi: 10.1016/S2213-8587(15)00029-7
58. Ward LM, Ma J, Lang B, Ho J, Alos N, Matzinger MA, et al. Bone morbidity and recovery in children with acute lymphoblastic leukemia: results of a six-Year prospective cohort study. J Bone Miner Res. (2018) 33:1435–43. doi: 10.1002/jbmr.3447
Keywords: acute lymphoblastic leukemia, bone health, childhood malignancies, osteopathologies, survivorship, vitamin D
Citation: Schündeln MM, Hauffa PK, Munteanu M, Kiewert C, Unger N, Bauer JJ, Hauffa BP and Grasemann C (2020) Prevalence of Osteopathologies in Children and Adolescents After Diagnosis of Acute Lymphoblastic Leukemia. Front. Pediatr. 8:509. doi: 10.3389/fped.2020.00509
Received: 12 May 2020; Accepted: 20 July 2020;
Published: 26 August 2020.
Edited by:
Wassim Chemaitilly, St. Jude Children's Research Hospital, United StatesReviewed by:
Sogol Mostoufi-Moab, Children's Hospital of Philadelphia, United StatesNathalie Alos, University of Montreal, Canada
Copyright © 2020 Schündeln, Hauffa, Munteanu, Kiewert, Unger, Bauer, Hauffa and Grasemann. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael M. Schündeln, bWljaGFlbC5zY2h1ZW5kZWxuQHVrLWVzc2VuLmRl
 Michael M. Schündeln
Michael M. Schündeln Pia K. Hauffa
Pia K. Hauffa Martin Munteanu2
Martin Munteanu2 Corinna Grasemann
Corinna Grasemann